Abstract
Hepatocellular carcinoma (HCC) is one of the male-dominant liver diseases with poor prognosis, although treatments for HCC have been progressing in the past decades. Androgen receptor (AR) is a member of the nuclear receptor superfamily. Previous studies reported that AR was expressed in human HCC and non-HCC tissues. AR is activated both ligand-dependently and ligand-independently. The latter is associated with a mitogen-activated protein kinase–, v-akt murine thymoma viral oncogene homolog 1–, or signal-transducer and activator of transcription–signaling pathway, which has been implicated in the development of HCC. It has been reported that more than 200 RNA expression levels are altered by androgen treatment. In the liver, androgen-responsive genes are cytochrome P450s, transforming growth factor β, vascular endothelial growth factor, and glucose-regulated protein 78 kDa, which are also associated with human hepatocarcinogenesis. Recent studies also revealed that AR plays a role in cell migration and metastasis. It is possible that cross-talk among AR-signaling, endoplasmic reticulum stress, and innate immune response is important for human hepatocarcinogenesis and HCC development. This review shows that AR could play a potential role in human HCC and represent one of the important target molecules for the treatment of HCC.
Introduction
Hepatocellular carcinoma (HCC) is one of the male-dominant cancers with poor prognosis, although treatments are being developed.Citation1–Citation4 HCC usually occurs after the age of 40 years, reaching a peak at approximately 70 years of age.Citation5 Irrespective of their etiology, rates of HCC among men are two to four times higher than those among women.Citation5 HCC derived from hepatitis B virus (HBV) or hepatitis C virus infection and virus-unrelated HCC are male-dominant disorders.Citation6,Citation7 Similar sex difference is also observed in mice given a chemical carcinogen, diethylnitrosamine.Citation8
In humans, androgen and estrogen are essential sex steroid hormones involved in many cellular processes such as cell metabolism and cell differentiation, as well as sex development.Citation9 Both androgen receptor (AR) and estrogen receptor α, for androgen and estrogen, respectively, seem to be involved in hepatocellular carcinogenesis.Citation8,Citation10–Citation12 There have been many reports concerning the expression of AR in HCC and its surrounding liver tissues.Citation13–Citation31 The association between AR and liver diseases is shown in . In this review article, we provide comprehensive insights regarding the association between ARs and HCC. We have been expecting that AR would become an emerging therapeutic target in HCC.
Table 1 Androgen receptor (AR) and liver diseases from different etiologies
ARs and androgen action
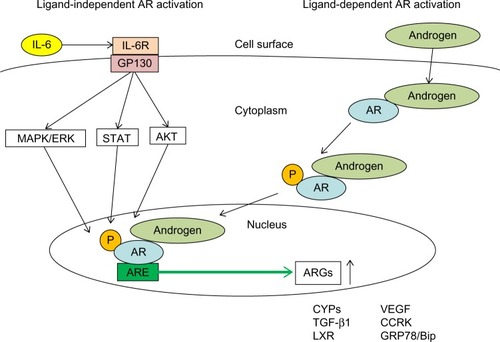
Human AR is a member of the nuclear steroid receptor super-family and AR gene is located on Xq11-12, indicating that males have a single copy of the gene.Citation32 AR is a ligand-activated transcriptional factor with three domains: DNA-binding domain, C-terminal ligand-binding domain, and N-terminal transactivation domain.Citation33 Unliganded AR is inactivate and is bound to cytoplasmic chaperones such as heat shock protein 90 (Hsp90).Citation34 Testosterone is produced in the testes and is converted to dihydrotestosterone. Ligands bind to AR and activate AR, inducing conformational change. Then, the AR dimerizes and the AR-dimers translocate to the nucleus, where AR binds to consensus-binding sequences (androgen-responsive elements [AREs]) in the DNA to regulate target gene expression.Citation34 Mitogen-activated protein kinase (MAPK)/extracellular signal–regulated protein kinase signaling increases the stability of AR.Citation34 Interleukin-6 is sufficient to activate AR in vitro, and steroid receptor coactivator-1 has been shown in interleukin-6–dependent signaling.Citation35 Steroid receptor coactivator-1 has been shown to interact with human AR and to modulate ligand-dependent AR transactivation, and it is regulated by phosphorylation by MAPK.Citation35 Growth and survival pathways such as MAPK, v-akt murine thymoma viral oncogene homolog 1 (AKT), and signal-transducer and activator of transcription (STAT) signaling are involved in the ligand-independent activation of AR of prostate cancer, pancreatic cancer, and HCC.Citation12,Citation35–Citation40 It is also known that MAPK, AKT, and STAT could activate AR signaling, and they also are involved in human hepatocarcinogenesis.Citation41–Citation48 In several human cancers, AR seems to be activated in an androgen-dependent and/or androgen-independent manner ().
Figure 1 Ligand-dependent and ligand-independent androgen receptor (AR)-activation in hepatocytes.
Target genes of AR in the liver
AR and its target genes
Androgens and steroid hormones bind to the AR and, in turn, AR associates with genomic AREs ().Citation49 In LNCap prostate cancer cells, more than 200 RNA expression levels are altered by androgen treatment.Citation49–Citation51 Androgen plays a critical role for the cytoskeleton and extracellular matrix in transducing signals for growth, differentiation, and secretion in normal and cancerous prostate cellsCitation50,Citation52 Upregulation of NF-κB and several DNA repair or stress-response gene expressions may be a secondary response to oxidative stress rather than a direct response to AR signaling.Citation50,Citation53 Bolton et alCitation49 reported that most androgen-responsive genes (ARGs) were associated with two or more AREs and that ARGs were sometimes themselves linked in gene clusters containing up to 13 AREs and 12 ARGs. Primary ARGs seem to produce effects on secondary target genes.Citation50
Table 2 Representative ARGs in the liver
Androgen and the liver
Human liver microsomes and cytochrome P450s (CYP) are major sites of metabolism of drugs and hormones. The liver could have an impact on the metabolism of androgen and the activation of AR or on the metabolism of anti-androgenic drugs such as flutamide.Citation54,Citation55 It was reported that downregulation of AR activity correlates with the severity of alcoholic liver injury.Citation56 Hepatocyte nuclear factor-1 and CCAAT/enhancer-binding protein are responsible for liver specificity of the rat dehydroepiandrosterone sulfotransferase gene, which catalyzes sulfonation of androgenic steroids and certain aromatic procarcinogens.Citation57
AR and transforming growth factor β1 (TGF-β1) in the liver
TGF-β1 expression increases during progression of HCC,Citation58,Citation59 hepatic cirrhosis,Citation60,Citation61 hepatic damage,Citation62,Citation63 and hepatic regeneration.Citation64,Citation65 Yoon et alCitation66 found that the promoter region of TGF-β1 includes putative androgen response sequence and also in vivo and in vitro evidence of activation of TGF-β1 expression by androgen and AR. They reported that androgen might regulate hepatocarcinogenesis by increasing transcription of TGF-β1 through direct interactions with AR and ARE in the TGF-β1 gene.Citation66
AR and cholesterol homeostasis in the liver
AR signaling plays a role in the development and progression of several liver diseases, including HCC and nonalcoholic fatty liver disease. Androgen control of growth hormone secretion also induces male-specific genes in the liver.Citation67 AR activation results in obesity and altered lipid metabolism in orchidectomized mice,Citation68 suggesting that the activation of AR might be involved in HCC development in patients with nonalcoholic steatohepatitis, although there is also a contrary opinion.Citation69 But these studies showed that hepatic AR may play a role in the development of insulin resistance and hepatic steatosis.Citation68,Citation69 CYP27A1 is a key enzyme in cholesterol homeostasis and vitamin D3 metabolism. AR could induce CYP27A1, which is a target for the JNK/c-Jun pathway. The JNK/c-Jun pathway is thought to be involved in AR-mediated upregulation of human CYP27A1.Citation70 Krycer and BrownCitation71 showed that liver-X-receptor activity is down-regulated by AR. The cross-talk between AR and liver-X-receptor is important for cholesterol homeostasis.
AR and hepatocarcinogenesis
Vascular endothelial growth factor (VEGF) is a target gene of ARCitation49 and plays an important role in angiogenesis in the liver.Citation12 Hepatitis C virus core protein enhances AR signaling, upregulates VEGF expression in hepatocytes, and facilitates angio-genesis.Citation12 VEGF is one of the key molecules of treatment of HCC.Citation72,Citation73 Of interest, female sex was associated with better response to sorafenib in patients with unresectable HCC in Japan.Citation74 Feng et al reported that cell cycle–related kinase is a direct AR transcriptional target and that cell cycle–related kinase promotes hepatocarcinogenesis through the upregulation of β-catenin/TCF signaling.Citation75
AR and aryl hydrocarbon (or dioxin) receptor
Both aryl hydrocarbon (or dioxin) receptor and aryl hydrocarbon (or dioxin) receptor nuclear translocator are known to interact with AR.Citation39,Citation76,Citation77 AR might also be involved in hepatocarcinogenesis through aryl hydrocarbon (or dioxin) receptor pathways.Citation39 Li et alCitation78 reported that the vertebrae forkhead box A factors and their targets estrogen receptor α and AR play an important role in the sex difference of HCC. Nuclear receptors including AR and estrogen receptor and their related signaling pathways play a role in human hepatocarcinogenesis.Citation40
AR and cell migration
Recent studies revealed that AR is involved in cell migration and metastasis.Citation79 At present, it is not clear whether AR could promote cell migrationCitation81–Citation83 or not.Citation80,Citation84,Citation85 Although further studies will be needed regarding this point, AR is one of the important target molecules for treatment targeting metastasis or advanced HCC.
AR and endoplasmic reticulum stress
Dihydrotestosterone could induce RNA-dependent protein kinase/eukaryotic initiation factor-2α activation in human hepatocytes.Citation86 Dai et alCitation86 reported that RNA-dependent protein kinase/eukaryotic initiation factor-2α activation is involved in dihydrotestosterone-induced cell cycle arrest and that the eukaryotic initiation factor-2α/GADD153 pathway, a branch of ER stress response, is enhanced. It is well known that the ER stress pathway is involved in human hepatocarcinogenesis.Citation87 Glucose-regulated protein 78 kDa (GRP78/Bip) is one of the androgen response genes in human prostate cells as well as in human hepatocytes.Citation39,Citation88–Citation92 We reported that stronger positive correlations between the expressions of AR mRNA and GRP78 mRNA in stage I/II HCC samples, compared with stage III/IV HCC samples, indicated that AR-controlling GRP78 activation plays a role in hepatocarcinogenesis in especially earlier-stage HCC patients.Citation92 We also observed that AR overexpression increased ER stress–responsive gene expression in human hepatocytes and that AR-knockdown led to the down-regulation of expression of ER stress molecules.Citation92 We also confirmed that the double-knockdown of AR and GRP78 enhanced sorafenib-induced apoptosis in human hepatoma cell lines.Citation92 The cross-talk between AR and ER stress response might be a potential target in the treatment of HCC.
AR and Toll-like receptor signaling pathways
Tissue expression of AR is associated with differential immune responsiveness.Citation93 Toll-like receptors (TLRs) are a family of transmembrane receptors and play central roles in innate immunity. TLR4 recognizes lipopolysaccharide, a cell wall component of gram-negative bacteria that activate innate immunity.Citation94 Lipopolysaccharide induced apoptosis in hepatocytes and reduced the hepatic expressions of ER stress–related proteins. ER stress response is important for hepatic cell damage from an innate immune response.Citation94
Testosterone downregulated the expression of several TLR genes, possibly resulting in the inhibition of the immune response.Citation95 MyD88, downstream of TLR4, may play a role in limiting prostate tumorigenesis by altering tumor-infiltrating immune populations.Citation96 We observed an increase of lipopolysaccharide-induced apoptosis (67%) in HepG2 stably expressing shAR as compared to that (47%) in HepG2 control cells.Citation92 AR and ER stress response may be involved in innate immune response of hepatocytes.
AR and other signaling pathways
Several reports indicated that insulin-like growth factor (IGF), fibroblast growth factor (FGF), and VEGF, as well as mammalian target of the rapamycin (mTOR) signaling pathways are involved in human hepatocarcinogenesis.Citation48,Citation97–Citation99 Cell surface receptors for IGF, FGF, and VEGF activate downstream signal transduction through the receptor-tyrosine kinases. These receptors are also important molecular targets for drugs against HCC such as sorafenib, brivanib, and everolimus.Citation48,Citation97–Citation99
IGF-1 and its binding proteins are also known as AR-targeting genes in prostate cancer cells.Citation100,Citation101 Tsuei et alCitation102 showed that downregulation of IGF-1 and its binding protein-3 were observed in the RNA-binding motif gene on the Y chromosome–knockdown HepG2 cells, suggesting the enhancing effect of RNA-binding motif gene on the Y chromosome on AR transactivation activity in human HCC.
AR could control FGF and FGF-binding protein production and affect FGF signaling pathway in prostate cancer cells.Citation103 AR may have an impact on FGF signaling pathway as well as VEGF signaling pathways in human hepatocarcinogenesis.Citation12 PI3K/phosphatase and tensin homologs deleted on chromosome 10 (PTEN)/Akt/mTOR pathway are involved in many cellular processes of human HCC.Citation48 Everolimus and sirolimusis could inhibit HCC growth through this signaling pathway.Citation48 Previous study in prostate cancer cell linesCitation104 showed AR-mTOR cross-talk is regulated by testosterone availability. Further study will be needed at a significance of AR-mTOR cross-talk in human hepatocarcinogenesis.
HBV and AR
Chen and Yehs’ group has extensively studied the association between HBV infection and AR, or the association between HBx protein and AR, and reported that AR is involved in human hepatocarcinogenesis.Citation38,Citation39,Citation105–Citation107 shows several mechanisms of the effects of AR in HBV-associated HCC. Many studies with human liver tissuesCitation13–Citation31 also support this concept. These data support the idea that AR could be one of the important molecular targets for the treatment of HCC with or without HBV infection.
Conclusion
AR could play critical roles in human HCC and be one of the important target molecules for the treatment of HCC. The previous controlled study shows the lack of efficacy of androgen treatment in unresectable HCC ().Citation108–Citation115 However, the present review clearly suggests that AR but not androgen could be an important target of hepatocarcinogenesis and HCC development, and more specific inhibitors of AR would shed light on the treatment of HCC. Further studies concerning AR and ARGs in the liver should be carried out.
Table 3 Clinical trials targeting androgen in hepatocellular carcinoma
Acknowledgments
This work was supported by a KAKEN Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (24590955).
Disclosure
The authors report no conflicts of interest in this work.
References
- YamashitaYIYoshidaYKuriharaTSurgical results for recurrent hepatocellular carcinoma after curative hepatectomy: repeat hepatectomy vs salvage living donor liver transplantationLiver Transpl2015
- LlovetJMRicciSMazzaferroVSHARP Investigators Study GroupSorafenib in advanced hepatocellular carcinomaN Engl J Med2008359437839018650514
- FuruseJIshiiHNakachiKSuzukiEShimizuSNakajimaKPhase I study of sorafenib in Japanese patients with hepatocellular carcinomaCancer Sci200899115916517953709
- ShiinaSTateishiRAranoTRadiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factorsAm J Gastroenterol2012107456957722158026
- El-SeragHBHepatocellular carcinomaN Engl J Med2011365121118112721992124
- ShiratoriYShiinaSImamuraMCharacteristic difference of hepatocellular carcinoma between hepatitis B- and C-viral infection in JapanHepatology1995224 pt 1102710337557847
- TateishiROkanoueTFujiwaraNClinical characteristics, treatment, and prognosis of non-B, non-C hepatocellular carcinoma: a large retrospective multicenter cohort studyJ Gastroenterol201550335036024929638
- NauglerWESakuraiTKimSGender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 productionScience2007317583412112417615358
- ZhaoYLiZInterplay of estrogen receptors and FOXA factors in the liver cancerMol Cell Endocrinol2015
- ChiuCMYehSHChenPJHepatitis B virus X protein enhances androgen receptor-responsive gene expression depending on androgen levelProc Natl Acad Sci U S A200710482571257817259306
- ZhengYChenWLMaWLChangCOuJHEnhancement of gene transactivation activity of androgen receptor by hepatitis B virus X proteinVirology2007363245446117335866
- KandaTSteeleRRayRRayRBHepatitis C virus core protein augments androgen receptor-mediated signalingJ Virol20088222110661107218768969
- IqbalMJWilkinsonMLJohnsonPJWilliamsRSex steroid receptor proteins in foetal, adult and malignant human liver tissueBr J Cancer19834867917966317004
- WongLYChanSHOonCJRauffAImmunocytochemical localization of testosterone in human hepatocellular carcinomaHistochem J19841666876926330003
- NagasueNYukayaHChangYCOgawaYKohnoHItoAActive uptake of testosterone by androgen receptors of hepatocellular carcinoma in humansCancer19865711216221673008976
- NagasueNItoAYukayaHOgawaYAndrogen receptors in hepatocellular carcinoma and surrounding parenchymaGastroenterology19858936436472991072
- WilkinsonMLIqbalMJWilliamsRCharacterisation of high affinity binding sites of androgens in primary hepatocellular carcinomaClin Chim Acta19851521–21051132414040
- OhnishiSMurakamiTMoriyamaTMitamuraKImawariMAndrogen and estrogen receptors in hepatocellular carcinoma and in the surrounding noncancerous liver tissueHepatology1986634404433011631
- BannisterPMeystreCMLosowskyMSAndrogen receptor concentrations in needle biopsy specimens of human liverLiver19888128313367706
- NagasueNKohnoHChangYCAndrogen and estrogen receptors in hepatocellular carcinoma and the surrounding liver in womenCancer19896311121162535950
- NagasueNChangYCHayashiTAndrogen receptor in hepatocellular carcinoma as a prognostic factor after hepatic resectionAnn Surg198920944244272539062
- NagasueNKohnoHChangYHayashiTNakamuraTSpecificity of androgen receptors of hepatocellular carcinoma and liver in humansHepatogastroenterology19903754744792174826
- NagasueNKohnoHYamanoiAKimotoTChangYCNakamuraTProgesterone receptor in hepatocellular carcinoma. Correlation with androgen and estrogen receptorsCancer19916710250125051849788
- EagonPKFrancavillaADiLeoAQuantitation of estrogen and androgen receptors in hepatocellular carcinoma and adjacent normal human liverDig Dis Sci1991369130313081654243
- NagasueNYamanoiAKohnoHAndrogen receptor in cirrhotic liver, adenomatous hyperplastic nodule and hepatocellular carcinoma in the humanHepatogastroenterology19923954554601334038
- BoixLBruixJCastellsASex hormone receptors in hepatocellular carcinoma. Is there a rationale for hormonal treatment?J Hepatol19931721871918383159
- NegroFPapottiMPacchioniDGalimiFBoninoFBussolatiGDetection of human androgen receptor mRNA in hepatocellular carcinoma by in situ hybridisationLiver19941442132197968281
- BoixLCastellsABruixJAndrogen receptors in hepatocellular carcinoma and surrounding liver: relationship with tumor size and recurrence rate after surgical resectionJ Hepatol19952266166227560855
- TavianDDe PetroGPitozziAPortolaniNGiuliniSMBarlatiSAndrogen receptor mRNA under-expression in poorly differentiated human hepatocellular carcinomaHistol Histopathol20021741113111912371139
- VizosoFJRodriguezMAltadillALiver expression of steroid hormones and apolipoprotein D receptors in hepatocellular carcinomaWorld J Gastroenterol200713233221322717589901
- ZhangYShenYCaoBYanAJiHElevated expression levels of androgen receptors and matrix metalloproteinase-2 and -9 in 30 cases of hepatocellular carcinoma compared with adjacent tissues as predictors of cancer invasion and stagingExp Ther Med20159390590825667651
- HughesIADaviesJDBunchTIPasterskiVMastroyannopoulouKMacDougallJAndrogen insensitivity syndromeLancet201238098511419142822698698
- GelmannEPMolecular biology of the androgen receptorJ Clin Oncol200220133001301512089231
- ShafiAAYenAEWeigelNLAndrogen receptors in hormone-dependent and castration-resistant prostate cancerPharmacol Ther2013140322323823859952
- UedaTMawjiNRBruchovskyNSadarMDLigand-independent activation of the androgen receptor by interleukin-6 and the role of steroid receptor coactivator-1 in prostate cancer cellsJ Biol Chem200227741380873809412163482
- CuligZHobischACronauerMVAndrogen receptor activation in prostatic tumor cell lines by insulin-like growth factor-I, keratinocyte growth factor, and epidermal growth factorCancer Res19945420547454787522959
- OkitsuKKandaTImazekiFInvolvement of interleukin-6 and androgen receptor signaling in pancreatic cancerGenes Cancer20101885986721779469
- KandaTYokosukaOOmataMAndrogen receptor and hepatocellular carcinomaJ Gastroint Dig Syst2013S12
- KandaTJiangXYokosukaOAndrogen receptor signaling in hepatocellular carcinoma and pancreatic cancersWorld J Gastroenterol201420289229923625071315
- WuSKandaTImazekiFNakamotoSShirasawaHYokosukaONuclear receptor mRNA expression by HBV in human hepatoblastoma cell linesCancer Lett20113121334221903321
- TanakaSMohrLSchmidtEVSugimachiKWandsJRBiological effects of human insulin receptor substrate-1 overexpression in hepatocytesHepatology19972635986049303488
- ItoYSasakiYHorimotoMActivation of mitogen-activated protein kinases/extracellular signal-regulated kinases in human hepatocellular carcinomaHepatology19982749519589537433
- SuzukiAHayashidaMKawanoHSugimotoKNakanoTShirakiKHepatocyte growth factor promotes cell survival from fas-mediated cell death in hepatocellular carcinoma cells via Akt activation and Fas-death-inducing signaling complex suppressionHepatology2000324 pt 179680211003625
- FujimakiSMatsudaYWakaiTBlockade of ataxia telangiectasia mutated sensitizes hepatoma cell lines to sorafenib by interfering with Akt signalingCancer Lett201231919810822265862
- SatoMMatsudaYWakaiTP21-activated kinase-2 is a critical mediator of transforming growth factor-β-induced hepatoma cell migrationJ Gastroenterol Hepatol20132861047105523425030
- MatsudaYWakaiTKubotaMValproic acid overcomes transforming growth factor-β-mediated sorafenib resistance in hepatocellular carcinomaInt J Clin Exp Pathol2014741299131324817927
- YoshikawaHMatsubaraKQianGSSOCS-1, a negative regulator of the JAK/STAT pathway, is silenced by methylation in human hepatocellular carcinoma and shows growth-suppression activityNat Genet2001281293511326271
- KandaTImazekiFKanaiFTadaMYokosukaOOmataMSignaling pathways involved in molecular carcinogenesis of HCCQiaoLYanXLiYGeorgeYMolecular Aspect of Hepatocellular CarcinomaSharjahBentham Science Publishers20123955
- BoltonECSoAYChaivorapolCHaqqCMLiHYamamotoKRCell- and gene-specific regulation of primary target genes by the androgen receptorGenes Dev200721162005201717699749
- DePrimoSEDiehnMNelsonJBTranscriptional programs activated by exposure of human prostate cancer cells to androgenGenome Biol200237RESEARCH003212184806
- NelsonPSCleggNArnoldHThe program of androgen-responsive genes in neoplastic prostate epitheliumProc Natl Acad Sci U S A20029918118901189512185249
- GetzenbergRHPientaKJWardWSCoffeyDSNuclear structure and the three-dimensional organization of DNAJ Cell Biochem19914742892991795013
- RippleMOHenryWFSchwarzeSRWildingGWeindruchREffect of antioxidants on androgen-induced AP-1 and NF-kappaB DNA-binding activity in prostate carcinoma cellsJ Natl Cancer Inst199991141227123210413424
- ShetMSMcPhaulMFisherCWStallingsNREstabrookRWMetabolism of the antiandrogenic drug (Flutamide) by human CYP1A2Drug Metab Dispos19972511129813039351907
- GehlhausMSchmittNVolkBMeyerRPAntiepileptic drugs affect neuronal androgen signaling via a cytochrome P450-dependent pathwayJ Pharmacol Exp Ther2007322255055917505019
- EagonPKElmMSTadicSDNanjiAADownregulation of nuclear sex steroid receptor activity correlates with severity of alcoholic liver injuryAm J Physiol Gastrointest Liver Physiol20012812G342G43911447013
- SongCSJungMHKimSCHassanTRoyAKChatterjeeBTissue-specific and androgen-repressible regulation of the rat dehydroepiandrosterone sulfotransferase gene promoterJ Biol Chem19982733421856218669705324
- ItoNKawataSTamuraSElevated levels of transforming growth factor beta messenger RNA and its polypeptide in human hepatocellular carcinomaCancer Res19915115408040831649698
- NagaharaHEzhevskySAVocero-AkbaniAMKaldisPSolomonMJDowdySFTransforming growth factor beta targeted inactivation of cyclin E:cyclin-dependent kinase 2 (Cdk2) complexes by inhibition of Cdk2 activating kinase activityProc Natl Acad Sci U S A19999626149611496610611320
- RobertsABSpornMBAssoianRKTransforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitroProc Natl Acad Sci U S A19868312416741712424019
- TsukamotoHMatsuokaMFrenchSWExperimental models of hepatic fibrosis: a reviewSemin Liver Dis199010156652110685
- YagerJDZurloJNiNSex hormones and tumor promotion in liverProc Soc Exp Biol Med199119826676741924403
- LinJKChouCKIn vitro apoptosis in the human hepatoma cell line induced by transforming growth factor beta 1Cancer Res19925223853881309441
- FaustoNMeadJEBraunLProto-oncogene expression and growth factors during liver regenerationSymp Fundam Cancer Res19863969863321311
- BraunLMeadJEPanzicaMMikumoRBellGIFaustoNTransforming growth factor beta mRNA increases during liver regeneration: a possible paracrine mechanism of growth regulationProc Natl Acad Sci U S A1988855153915433422749
- YoonGKimJYChoiYKWonYSLimIKDirect activation of TGF-beta1 transcription by androgen and androgen receptor complex in Huh7 human hepatoma cells and its tumor in nude miceJ Cell Biochem200697239341116187311
- RobinsDMAndrogen receptor and molecular mechanisms of male-specific gene expressionNovartis Found Symp2005268425216206874
- Movérare-SkrticSVenkenKAnderssonNDihydrotestosterone treatment results in obesity and altered lipid metabolism in orchidectomized miceObesity (Silver Spring)200614466267216741268
- LinHYYuICWangRSIncreased hepatic steatosis and insulin resistance in mice lacking hepatic androgen receptorHepatology20084761924193518449947
- NorlinMPetterssonHTangWWikvallKAndrogen receptor-mediated regulation of the anti-atherogenic enzyme CYP27A1 involves the JNK/c-jun pathwayArch Biochem Biophys2011506223624121134350
- KrycerJRBrownAJCross-talk between the androgen receptor and the liver X receptor: implications for cholesterol homeostasisJ Biol Chem201128623206372064721489984
- SchöffskiPDumezHClementPEmerging role of tyrosine kinase inhibitors in the treatment of advanced renal cell cancer: a reviewAnn Oncol20061781185119616418310
- KudoMUeshimaKPositioning of a molecular-targeted agent, sorafenib, in the treatment algorithm for hepatocellular carcinoma and implication of many complete remission cases in JapanOncology201078Suppl 115416620616599
- TakedaHNishikawaHOsakiYJapanese Red Cross Liver Study GroupClinical features associated with radiological response to sorafenib in unresectable hepatocellular carcinoma: a large multicenter study in JapanLiver Int20153551581158924836552
- FengHChengASTsangDPCell cycle-related kinase is a direct androgen receptor-regulated gene that drives β-catenin/T cell factor-dependent hepatocarcinogenesisJ Clin Invest201112183159317521747169
- KrügerTLongMBonefeld-JørgensenECPlastic components affect the activation of the aryl hydrocarbon and the androgen receptorToxicology20082462–311212318294747
- WuYBaumgartenSCZhouPStoccoCTestosterone-dependent interaction between androgen receptor and aryl hydrocarbon receptor induces liver receptor homolog 1 expression in rat granulosa cellsMol Cell Biol201333152817282823689136
- LiZTutejaGSchugJKaestnerKHFoxa1 and Foxa2 are essential for sexual dimorphism in liver cancerCell20121481–2728322265403
- ShenFYanCLiuMFengYChenYRACK1 promotes prostate cancer cell proliferation, invasion and metastasisMol Med Rep201384999100423912224
- MaWLHsuCLYehCCHepatic androgen receptor suppresses hepatocellular carcinoma metastasis through modulation of cell migration and anoikisHepatology201256117618522318717
- AoJMengJZhuLActivation of androgen receptor induces ID1 and promotes hepatocellular carcinoma cell migration and invasionMol Oncol20126550751522819717
- HuangCKLeeSOLaiKPTargeting androgen receptor in bone marrow mesenchymal stem cells leads to better transplantation therapy efficacy in liver cirrhosisHepatology20135741550156323150236
- NieHCaoQZhuLGongYGuJHeZAcetylcholine acts on androgen receptor to promote the migration and invasion but inhibit the apoptosis of human hepatocarcinomaPLoS One201384e6167823620780
- MaWLLaiHCYehSCaiXChangCAndrogen receptor roles in hepatocellular carcinoma, fatty liver, cirrhosis and hepatitisEndocr Relat Cancer2014213R165R18224424503
- MaWLJengLBLaiHCLiaoPYChangCAndrogen receptor enhances cell adhesion and decreases cell migration via modulating β1-integrin-AKT signaling in hepatocellular carcinoma cellsCancer Lett20143511647124944078
- DaiRYanDLiJActivation of PKR/eIF2α signaling cascade is associated with dihydrotestosterone-induced cell cycle arrest and apoptosis in human liver cellsJ Cell Biochem201211351800180822228470
- ShudaMKondohNImazekiNActivation of the ATF6, XBP1 and grp78 genes in human hepatocellular carcinoma: a possible involvement of the ER stress pathway in hepatocarcinogenesisJ Hepatol200338560561412713871
- TanSSAhmadIBennettHLGRP78 up-regulation is associated with androgen receptor status, Hsp70-Hsp90 client proteins and castrate-resistant prostate cancerJ Pathol20112231818721125667
- BennettHLFlemingJTO’PreyJRyanKMLeungHYAndrogens modulate autophagy and cell death via regulation of the endoplasmic reticulum chaperone glucose-regulated protein 78/BiP in prostate cancer cellsCell Death Dis20101e7221364676
- KimSSChoHJKangJYKangHKYooTKInhibition of androgen receptor expression with small interfering RNA enhances cancer cell apoptosis by suppressing survival factors in androgen insensitive, late stage LNCaP cellsScientific World Journal2013201351939723476140
- YangYCFuHCHsiaoBLAndrogen receptor inclusions acquire GRP78/BiP to ameliorate androgen-induced protein misfolding stress in embryonic stem cellsCell Death Dis20134e60723618905
- JiangXKandaTNakamotoSMiyamuraTWuSYokosukaOInvolvement of androgen receptor and glucose-regulated protein 78 kDa in human hepatocarcinogenesisExp Cell Res2014323232633624583399
- ButtsCLJonesYLLimJKSalterCEBelyavskayaESternbergEMTissue expression of steroid hormone receptors is associated with differ ential immune responsivenessBrain Behav Immun20112551000100721074604
- JiangXKandaTTanakaTLipopolysaccharide blocks induction of unfolded protein response in human hepatoma cell linesImmunol Lett2013152181523578665
- Sánchez-HernándezMChaves-PozoECabasIMuleroVGarcía-AyalaAGarcía-AlcázarATestosterone implants modify the steroid hormone balance and the gonadal physiology of gilthead seabream (Sparus aurata L) malesJ Steroid Biochem Mol Biol201313818319423743364
- PeekEMSongWZhangHHuangJChinAILoss of MyD88 leads to more aggressive TRAMP prostate cancer and influences tumor infiltrating lymphocytesProstate201575546347325597486
- HuynhHNgoVCKoongHNSorafenib and rapamycin induce growth suppression in mouse models of hepatocellular carcinomaJ Cell Mol Med2009138B2673268319220580
- HuynhHNgoVCFargnoliJBrivanib alaninate, a dual inhibitor of vascular endothelial growth factor receptor and fibroblast growth factor receptor tyrosine kinases, induces growth inhibition in mouse models of human hepatocellular carcinomaClin Cancer Res200814196146615318829493
- HuynhHChowKHSooKCRAD001 (everolimus) inhibits tumour growth in xenograft models of human hepatocellular carcinomaJ Cell Mol Med20091371371138018466352
- PengLMalloyPJWangJFeldmanDGrowth inhibitory concentrations of androgens up-regulate insulin-like growth factor binding protein-3 expression via an androgen response element in LNCaP human prostate cancer cellsEndocrinology20061474599460716825320
- PandiniGMineoRFrascaFAndrogens up-regulate the insulin-like growth factor-I receptor in prostate cancer cellsCancer Res2005651849185715753383
- TsueiDJLeePHPengHYMale germ cell-specific RNA binding protein RBMY: a new oncogene explaining male predominance in liver cancerPLoS One2011611e2694822073224
- RosiniPBonaccorsiLBaldiEAndrogen receptor expression induces FGF2, FGF-binding protein production, and FGF2 release in prostate carcinoma cells: role of FGF2 in growth, survival, and androgen receptor down-modulationProstate200253431032112430142
- WuYChhipaRRChengJZhangHMohlerJLIpCAndrogen receptor-mTOR crosstalk is regulated by testosterone availability: implication for prostate cancer cell survivalAnticancer Res201030103895390121036700
- WangSHYehSHChenPJThe driving circuit of HBx and androgen receptor in HBV-related hepatocarcinogenesisGut201463111688168924598130
- WangSHChenPJYehSHGender disparity in chronic hepatitis B: mechanisms of sex hormonesJ Gastroenterol Hepatol2015
- YehSHChenPJGender disparity of hepatocellular carcinoma: the roles of sex hormonesOncology201078Suppl 117217920616601
- Groupe d’Etude et de Traitement du Carcinome HépatocellulaireRandomized trial of leuprorelin and flutamide in male patients with hepatocellular carcinoma treated with tamoxifenHepatology20044061361136915565568
- ChaoYChanWKHuangYSPhase II study of flutamide in the treatment of hepatocellular carcinomaCancer19967746356398616754
- GrimaldiCBleibergHGayFEvaluation of antiandrogen therapy in unresectable hepatocellular carcinoma: results of a European Organization for Research and Treatment of Cancer multicentric double-blind trialJ Clin Oncol19981624114179469323
- ForbesAWilkinsonMLIqbalMJJohnsonPJWilliamsRResponse to cyproterone acetate treatment in primary hepatocellular carcinoma is related to fall in free 5 alpha-dihydrotestosteroneEur J Cancer Clin Oncol19872311165916642828073
- GuptaSKorulaJFailure of ketoconazole as anti-androgen therapy in nonresectable primary hepatocellular carcinomaJ Clin Gastroenterol19881066516542466073
- GuéchotJPeigneyNBalletFVaubourdolleMGiboudeauJPouponREffect of D-tryptophan-6-luteinizing hormone-releasing hormone on the tumoral growth and plasma sex steroid levels in cirrhotic patients with hepatocellular carcinomaHepatology19891033463482547704
- ManesisEKGiannoulisGZoumboulisPVafiadouIHadziyannisSJTreatment of hepatocellular carcinoma with combined suppression and inhibition of sex hormones: a randomized, controlled trialHepatology1995216153515427768497
- FalksonGAnsellSPhase II trial of buserelin in hepatocellular carcinomaEur J Cancer Clin Oncol1989259133913402553420
- YuMWChengSWLinMWAndrogen-receptor gene CAG repeats, plasma testosterone levels, and risk of hepatitis B-related hepatocellular carcinomaJ Natl Cancer Inst200092242023202811121465
- YuMWYangYCYangSYHormonal markers and hepatitis B virus-related hepatocellular carcinoma risk: a nested case-control study among menJ Natl Cancer Inst200193211644165111698569
- YuMWYangYCYangSYAndrogen receptor exon 1 CAG repeat length and risk of hepatocellular carcinoma in womenHepatology200236115616312085360
- YehSHChangCFShauWYDominance of functional androgen receptor allele with longer CAG repeat in hepatitis B virus-related female hepatocarcinogenesisCancer Res200262154346435112154039
- YehSHChiuCMChenCLSomatic mutations at the tri-nucleotide repeats of androgen receptor gene in male hepatocellular carcinomaInt J Cancer200712081610161717230529
- ZhuRZhangJSZhuYZHBx-induced androgen receptor expression in HBV-associated hepatocarcinoma is independent of the methylation status of its promoterHistol Histopathol201126233521117024
- LiuBWenXHuangCWeiYUnraveling the complexity of hepatitis B virus: from molecular understanding to therapeutic strategy in 50 yearsInt J Biochem Cell Biol2013451987199623819994
- YangWJChangCJYehSHHepatitis B virus X protein enhances the transcriptional activity of the androgen receptor through c-Src and glycogen synthase kinase-3beta kinase pathwaysHepatology20094951515152419205031
- WangSHYehSHLinWHWangHYChenDSChenPJIdentification of androgen response elements in the enhancer I of hepatitis B virus: a mechanism for sex disparity in chronic hepatitis BHepatology20095051392140219670412
- WuMHMaWLHsuCLAndrogen receptor promotes hepatitis B virus-induced hepatocarcinogenesis through modulation of hepatitis B virus RNA transcriptionSci Transl Med201023232ra35
- TianYKuoCFChenWLOuJHEnhancement of hepatitis B virus replication by androgen and its receptor in miceJ Virol20128641904191022156518
- YuZGaoYQFengHCell cycle-related kinase mediates viral-host signalling to promote hepatitis B virus-associated hepatocarcinogenesisGut201463111793180424440987
- HoggKWoodCMcNeillyASDuncanWCThe in utero programming effect of increased maternal androgens and a direct fetal intervention on liver and metabolic function in adult sheepPLoS One201169e2487721935484
- ZhangHLiuYWangLDifferential effects of estrogen/androgen on the prevention of nonalcoholic fatty liver disease in the male ratJ Lipid Res201354234535723175777
- KellyDMNettleshipJEAkhtarSTestosterone suppresses the expression of regulatory enzymes of fatty acid synthesis and protects against hepatic steatosis in cholesterol-fed androgen deficient miceLife Sci201410929510324953607
