Abstract
The local application of bone morphogenetic protein-7 (BMP-7) in combination with the transplantation of autologous bone graft improves the outcome in nonunion treatment; however, the specific reasons remain unclear. In this study, we sought to determine if the local application of BMP-7 contributes to improved bone regeneration in nonunion therapy by modulation of the angiogenic and inflammable cytokine expression patterns of the early inflammation response. Therefore, we utilized the analysis of serological cytokine expression patterns. As a matched pair analysis, best-fitting patients who were treated with transplantation of autologous bone graft (G1, n=10) were compared with patients who were treated with additional application of BMP-7 (G2, n=10). The changes in the cytokine expression patterns were monitored and correlated to clinical data of bone healing. Significant differences in angiogenesis potential (vascular endothelial growth factor [VEGF] serum levels) could be found in the first days after surgery (P<0.05). Furthermore, the increase and absolute amount of VEGF levels in the BMP-7 group were considerably higher than in the control group during the first 2 weeks after surgery. The expression pattern of inflammable cytokines showed noticeable differences in the time point of significant elevated levels, in particular, inflammable cytokines showed an earlier peak in G2. Furthermore, interleukin-6 was significantly elevated within the first week only, comparing G2 to G1 (P<0.05). Our findings indicate that BMP-7 induces an early and more intense expression of VEGF via a direct and postulated indirect pathway, thereby providing a favorable environment for bone healing. Moreover, application of BMP-7 leads to an earlier expression of known proinflammatory cytokines. The results of this study show that application of BMP-7 leads to costimulatory effect on both angiogenic and inflammable cytokine expression patterns that may serve as a possible stimulus for bone regeneration.
Introduction
Despite modern treatment concepts, the therapy of nonunions remains a challenge in orthopedic and trauma surgery. According to the diamond concept, mechanical stability and biological factors such as angiogenesis, differentiated bone cells, and growth factors must be provided.Citation1–Citation9 Although the gold standard in nonunion therapy is the transplantation of autologous bone graft, missing bone regeneration subsequent to transplantation of autologous bone graft poses a serious disadvantage for the concerned patients.Citation2,Citation10–Citation12 Therefore, in the last few years, this therapy was combined with the application of bone morphogenetic protein-7 (BMP-7) more and more frequently, and thereby, the use of BMP-7 in the current nonunion therapy was established. Studies show a better outcome after nonunion treatment if BMP-7 was applied.Citation7,Citation13–Citation15 The specific reasons still remain unresolved.
Previous studies reported local signs of inflammation after the application of BMP-7, such as swelling or soft-tissue inflammationCitation13,Citation16 without signs of systemic inflammation; in particular, no persistent increase in serological infectious parameters (such as C-reactive protein [CRP] and leukocytes) has been shown. However, patients and caregivers remain disconcerted by this local infectious reaction.Citation14 The inflammatory response marks the start of the fracture healing cascade.Citation17–Citation19 Important inflammatory mediators are the vascular endothelial growth factor (VEGF), tumor necrosis factor-α (TNF-α), and several interleukins (ILs), such as interleukin-6 (IL-6) and interleukin-8 (IL-8). The serum levels of the proinflammatory cytokines, such as IL-6 and TNF-α, initially increase within the first 24 hours after trauma.Citation12 These cytokines play a key role in the inflammatory response.Citation20 VEGF is of particular interest for its impact on not only inflammation but also angiogenesis.Citation21 However, the effect of BMP-7 on the early inflammatory response has hardly been explored and is the subject of the current research.
In previous studies, we could show that the serum analysis of the cytokine expression pattern is a validate tool in investigating the underlying principles of bone regeneration.Citation22,Citation23 Hence, in this study, we sought to improve the understanding of the modulating effect of the adjunct application of BMP-7 on the angiogenic and inflammable cytokine expression patterns, in particular the early inflammatory response by utilizing this established protocol.
Patients and methods
Study design
Between 2009 and 2014, 109 patients of the Berufsgenossenschaftliche Unfallklinik Ludwigshafen (BG Trauma Centre) and the Center for Orthopaedics and Trauma Surgery of the University Hospital Heidelberg were enrolled in this prospective clinical observer study, subsequent to a written declaration of consent given by all of them. In the current study, the cytokine serum levels of VEGF, TNF-α, and other ILs were compared between two groups: patients who were treated with a transplantation of autologous bone graft (G1) and patients who were treated with an additional application of BMP-7 (G2). The changes in the cytokine expression patterns were monitored during the preoperative examination and additionally at standardized intervals after surgery. Furthermore, all enrolled patients were invited to attend follow-up examinations both clinically and radiologically. The patients included in our study were examined radiologically and clinically at the following time points: 1, 2, 4, and 6 weeks as well as 3, 6, and 12 months after surgery; 6 months after surgery, the patients could be declared responders or nonresponders due to radiological signs of consolidation and clinical signs of mechanical stability and full weight bearing. The serum levels of CRP, leukocytes, VEGF, TNF-α, IL-1, IL-6, IL-8, and IL-10 were analyzed and correlated to clinical data of bone healing. The study was conducted in accordance with the Declaration of Helsinki, and it was approved by the ethics committee of the Ruprechts-Karls-Universität Heidelberg (S-636/2011 and 157/2002).
Patient characteristics and surgical procedure
In this clinical observer study, two groups of patients were compared. In total, we acquired blood samples of 59 patients from Heidelberg and 50 patients from Ludwigshafen with one-step procedure after nonunion. Out of the 109 patients, BMP-7 was used in 33 patients. Eleven of these patients did not show up consistency for follow-up examination as well as blood sample collection and had to be eliminated from the study. One patient had a nonunion close to the ankle joint and three patients did not heal in time and thus were also excluded from this study, leaving 18 patients with the matching criteria. Of the 76 patients without the application of BMP-7, 25 had a nonunion in a bone other than tibia or femur. Eighteen patients either got a subsequent operative procedure or were treated aberrantly to the sole application of autologous bone graft. Of 22 patients, we had less blood samples than needed. This left a remaining eleven patients fitting this study’s criteria. From these patients, we were able to form a group including five men and five women who were treated with solely the transplantation of autologous cancellous bone graft, serving as control (G1). As a matched pair analysis, we took the best-fitting five men and five women out of the patients fitting criteria, who were treated with a transplantation of autologous cancellous bone graft and with an additional application of BMP-7 to form the study group (G2; ).
Table 1 Patient’s characteristics and demographics comparing the BMP-7 group to the control group
G2 group in detail
Five female and 5 male patients were assigned to this group. The mean age at the time of surgery was 51.5±9.91 years. The mean body mass index (BMI) was 30.59±6.91 with a range of 21.48–43.21. Five patients suffered from a femoral nonunion, and the others had a tibial nonunion. Four patients were smoker, one patient was a former smoker (within the last 10 years), and five patients were nonsmokers.Citation24 Arterial hypertension was diagnosed in five patients. Nonunion fixation was done by nailing in eight cases and by plate in two cases ().
G1 group in detail
The control group also consisted of five female and five male patients. The mean age was 52.5±10.05 years. The mean BMI was 26.94±5.65 with a range of 21.55–39.45. The BMI of one patient could not be determined; thus, the mean BMI was calculated from the data of the remaining patients (n=9). There were five tibial and five femoral nonunions. Three patients were smokers, three were former smokers (within the last 10 years), and four were nonsmokers.Citation24 Four patients suffered from arterial hypertension. Plate fixation was used in nine cases and fixation by nailing in one case ().
Herewith, both groups were comparable in sex, age, BMI, location of fracture, smoking habits, and diseases. Furthermore, for each patient, the surgical treatment involved open reduction and internal fixation with fixation either by nailing or plate fixation.
Sample acquisition and analysis of serum cytokine levels
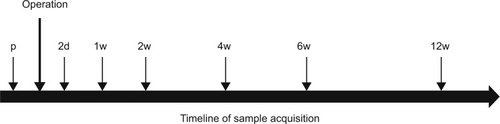
First blood samples were taken during the preoperative examination. Additional samples were taken postoperatively at standardized intervals, on days 2 and 7 and on 1, 2, 4, 6, and 12 weeks after surgery (). The samples were collected between 8 am and 11 am. In all, 3×7.5 mL serum samples (S-Monovette 7.5 mL; Sarstedt AG & Co., Nümbrecht, Germany) and 1×9 mL ethylenediaminetetraacetic acid were taken, centrifuged, aliquoted into Eppendorf tubes and stored at −80°C. Furthermore, C reactive protein (CRP) and leukocyte serum values were assessed during our clinical routine in the first 2 weeks to analyze systemic inflammation. Herewith, we assessed if application of BMP-7 and autologous cancellous bone graft influences systemic CRP and leukocyte levels and thereby a systemic unspecific inflammation that might modulate the cytokine serum values. Furthermore, a possible bias by infection or postoperative ongoing elevated CRP and leukocyte blood levels could be detected. Luminex Performance Human High Sensitivity Assays (Quantikine®,; R&D Systems, Inc., Minneapolis, MN, USA) were used to measure the cytokine serum levels of VEGF, TNF-α, IL-1, IL-6, IL-8, and IL-10 as described in the factory manual. The lab technician performing the Luminex assays was blinded to patient data and kept to manufacturer’s instructions during the measurements.
Statistical analysis
The serum levels were expressed as absolute mean concentrations ± standard deviation. To show significant differences between time points in one study group, the nonparametric Wilcoxon signed rank test was used. The Mann–Whitney U test was used to show significant differences between both study groups. Statistical significance was determined as P=0.05. Statistical analyses were carried out with the SPSS Statistics version 2.0 (SPSS Inc., Chicago, IL, USA). The graphs were created with the SigmaPlot software (Systat Software Inc., San Jose, CA, USA).
Results
In this prospective clinical observer study, we examined patients with long bone fractures that were treated with transplantation of autologous cancellous bone graft (G1) and adjunct local application of BMP-7 (G2) and compared their specific time course of cytokine expression (VEGF, TNF-α, IL-1, IL-6, IL-8, and IL-10) to their control group.
Differences in VEGF serum-level pattern
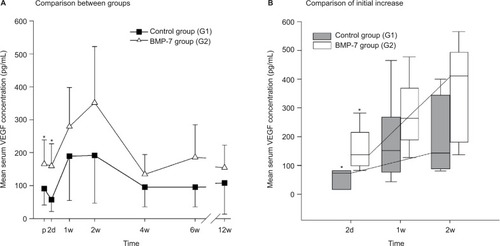
Significant differences in angiogenesis potential (VEGF serum levels) could be found in the first days after surgery comparing G2 and the control groups. In detail, the VEGF serum levels in the BMP-7 group rose from day 2 after surgery to a peak on day 14 and were finally comparable to those at admission 4 weeks after surgery (). In contrast, the VEGF serum levels in our control group decreased after surgery on day 2 after surgery and afterward showed a VEGF time course that was comparable to the G2 group, but in contrast to this, the absolute serum levels of VEGF showed significant differences before surgical treatment (P=0.035) and on day 2 after surgery (P=0.009) comparing the study group and control group. Furthermore, the absolute increase in the VEGF serum levels between preoperative values and values at week 2 was considerably higher in the G2 group compared to the control group (absolute increase 2 weeks vs preoperative: G2, 186.60 pg/mL and G1, 100.53 pg/mL). In summary, the increase () and absolute amount of VEGF levels in the BMP-7 group were considerably higher than in the control group during the first 2 weeks after surgery (G2 after 2 weeks: 351.95 pg/mL; G1 after 2 weeks: 191.57 pg/mL) as shown in .
Figure 2 Evaluation of the expression pattern of VEGF after nonunion treatment.
Notes: (A) Serum concentrations of VEGF in pg/mL 1d before and 2d, 1w, 2w, 4w, 6w, and 12w after surgery. Significant differences are indicated by a star (P<0.05, whiskers are SD). (B) Box plot after 2d to 2w of surgery. Whiskers demonstrate one-quarter of the sample. Stars indicate significant differences comparing the study and control groups at the time point (P<0.05).
Abbreviations: VEGF, vascular endothelial growth factor; p, preoperative; d, day(s); w, week(s); SD, standard deviation; BMP-7, bone morphogenetic protein-7; G1, patients who were treated with transplantation of autologous bone graft; G2, patients who were treated with additional application of BMP-7.
Differences in TNF-α serum-level pattern
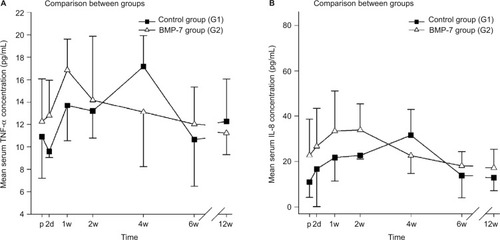
TNF-α was similarly elevated after surgery in both groups (control and study). The mean concentrations of TNF-α in the BMP-7 group peaked 1 week after treatment and returned to preoperative serum levels of 12.0±3.3 pg/mL after 6 weeks (). In contrast to the study group, an initial decrease in the TNF-α serum level was observed in the control group 2 days after surgery. Interestingly, the maximum TNF-α concentration was measured not merely at week 1 but rather 4 weeks after surgery (17.2±2.8 pg/mL). No significant differences could be found in comparing both groups at each time point. However, noticeable differences could be detected in the time point of significant elevated TNF-α levels. In the G2 group, peak values of TNF-α serum levels could be detected on week 1 comparing to peak values of TNF-α serum levels in the G1 group at week 4 (). These data imply an earlier peak in the TNF-α plasma levels of the BMP-7 group than those of the control group by more than 2 weeks.
Figure 3 Evaluation of the expression pattern of TNF-α and IL-8 after nonunion treatment.
Notes: (A) Serum concentrations of TNF-α in pg/mL 1d before and 2d, 1w, 2w, 4w, 6w, and 12w after surgery. (B) Serum concentrations of IL-8 in pg/mL 1d before and 2d, 1w, 2w, 4w, 6w, and 12w after surgery. Peak of IL-8 in the control group could be found after 4w of surgery. Peak of IL-8 in the study group was found within the first 2w after surgery.
Abbreviations: TNF-α, tumor necrosis factor-α; p, preoperative; d, day(s); w, week(s); SD, standard deviation; IL-8, interleukin-8; BMP-7, bone morphogenetic protein-7; G1, patients who were treated with transplantation of autologous bone graft; G2, patients who were treated with additional application of BMP-7.
Differences in IL-8 serum-level pattern
IL-8 did not show any significant differences comparing both groups at each measured time point. However interestingly, the highest values in both groups could be found at different time points during the measured time course after surgery with an earlier peak in the IL-8 serum levels of the BMP-7 group. In detail, the BMP-7 group showed IL-8 peak serum values after 2 weeks and a decrease in values near to admission after 4 weeks (22.6±7.8 pg/mL; ). Compared to this, the levels of admission were lower in the control group, followed by a constant increase until a peak value at week 4. The decrease to levels of admission was reached 6 weeks after surgery (13.9±9.8 pg/mL). No statistical significances could be found comparing both groups at each time point, but an earlier peak was observed in the BMP-7 group (week 2) compared to the control group (week 4; ).
Differences in IL-6 serum-level pattern
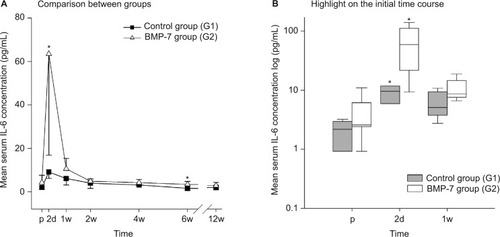
IL-6 was significantly elevated within the first week only, comparing G2 group to the G1 group. In detail, the IL-6 levels in the BMP-7 group increased to a peak mean concentration 2 days after surgery. Afterward, IL-6 plasma values decreased to similar levels at admission (4.9±1.0 pg/mL) within the first 2 weeks after surgical therapy (). Compared to this, in the control group, the levels of IL-6 at admission were similar to those in the G2 group. Furthermore, the IL-6 serum levels in the control group after 1 week of surgery were similar (6.1±3.0 pg/mL) to those at admission or to those in the BMP-7 group after 2 weeks of surgery. However in contrast, the peak value on day 2 was significantly lower (9.1±3.0 pg/mL) in the control group than any other value in the BMP-7 group within the first week after surgery. Additionally, during the following time course, a constant decrease was observed within a minimum 6 weeks after surgery (1.6±0.8 pg/mL, P=0.014 compared to the BMP-7 group). Additionally, the absolute increase on day 2 compared to the admission level was higher in the BMP-7 group (absolute increase: 59.2 pg/mL) than in the control group (absolute increase: 7.1 pg/mL; ). This increase and peak value is significant on day 2 comparing both study groups (P=0.049), suggesting an increased process of inflammation in the BMP-7 group.
Figure 4 Evaluation of the expression pattern of IL-6 after nonunion treatment.
Notes: (A) Serum concentrations of IL-6 in pg/mL 1d before and 2d, 1w, 2w, 4w, 6w, and 12w after surgery. Significant differences are indicated by a star (P<0.05, whiskers are SEM). (B) Box plot in a logarithmic scale on the y-axis of day before to 1w after surgery. Whiskers demonstrate one-quarter of the sample. Stars indicate significant differences comparing the study and control group at the time point (P<0.05).
Abbreviations: IL-6, interleukin-6; p, preoperative; d, day(s); w, week(s); SEM, standard error of the mean; BMP-7, bone morphogenetic protein-7; G1, patients who were treated with transplantation of autologous bone graft; G2, patients who were treated with additional application of BMP-7.
Pattern of IL-1 and IL-10 serum levels
In this study, we thoroughly assessed the influence of BMP-7 application on the IL serum pattern. However, no measurable values of IL-1 and IL-10 could be found using plasma samples as described earlier.
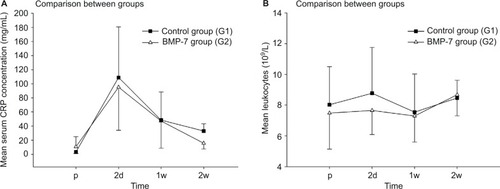
Differences in CRP and leukocyte serum-level patterns
During our follow-up, we assessed CRP and leukocytes in the initial 2 weeks only. As expected, preoperatively, both groups showed similar values (study group 10.72±14.248 mg/L compared to control group 3.19±2.094 mg/L), and subsequent to surgery, both groups showed an expected increase that showed similar peak values at the second day after surgery (study group 95.02±60.996 mg/L compared to control group 108.86±71.908 mg/L; ). Interestingly, a decrease in the serum values of CRP after 2 weeks of surgery was significant in the control group (study group 15.67±8.167 mg/L compared to control group 33.07±10.221 mg/L, P=0.037). The serum values of leukocytes showed a similar pattern during the complete study period, but values of both groups stayed in the physiological range (range 4,500–13,000/nL; ).
Figure 5 Analysis of the serological inflammable parameters after nonunion treatment.
Notes: (A) Serum concentrations of CRP in mg/L 1d before and 2d, 1w, and 2w after surgery. (B) Serum concentrations of leukocytes in l,000/nL 1d before and 2d, 1w, and 2w after surgery.
Abbreviations: CRP, C-reactive protein; p, preoperative; d, day(s); w, week(s); BMP-7, bone morphogenetic protein-7; G1, patients who were treated with transplantation of autologous bone graft; G2, patients who were treated with additional application of BMP-7.
Discussion
In this clinical observer study, we analyzed the serum concentrations of VEGF, TNF-α, IL-6, and IL-8 after nonunion treatment with the transplantation of autologous bone graft (control group) and adjunct application of BMP-7 (control group). A better bone healing after the additional application of BMP-7 to the standard nonunion therapy has already been characterized.Citation7,Citation13,Citation14,Citation25 We sought to determine if the adjunct application of BMP-7 modulates the cytokine expression pattern of angiogenic and inflammable cytokines, in particular the early inflammatory response. After the application of BMP-7, an earlier expression of all measured cytokines was observed, but only a higher peak value of VEGF and IL-6 was detected after the application of BMP-7 compared to the matched control group. The strengths of this study are its prospective approach, the clear inclusion and exclusion criteria, and the matching of patients. This allowed us to eliminate factors that could bias the evaluation of these patient groups.
Angiogenesis
Large defect sizes present great challenges in nonunion therapy. Therapy options are the transplantation of allo- and autografts or implants. The transplanted material has no chance of survival or integration into the fracture gap if no adequate vascular supply is given.Citation26 A sufficient angiogenesis is needed shortly after fracture to secure the following bone regeneration.Citation27 The expression of VEGF promotes angiogenesis; in particular, angiogenesis is the formation of new blood vessels in a regulated mechanism that is still not completely characterized. It is known that a total inhibition of angiogenesis causes a failure of fracture healing.Citation28 The most important mediator of angiogenesis is VEGF.Citation29 VEGF induces the inflammatory response and contributes to the transformation of soft-to-hard callus.Citation30 Analyses of VEGF levels in the human serum samples showed an increase in the VEGF levels up to 2 weeks after fracture.Citation31 These findings were confirmed by our results, but furthermore, our results did show a tendency for higher VEGF plasma levels in the study group. In our study, the VEGF levels in the BMP-7 group were elevated prior to local application of BMP-7 and the expression pattern of VEGF was similar in both groups. However, subsequent to adjunct application of BMP-7, VEGF levels showed both a steeper and faster increase in the first weeks of nonunion treatment and a higher absolute increase compared to the baseline value. Thereby, our data indicate a modulation of the VEGF expression after the application of BMP-7 that might be favorable for angiogenesis. In this study, we were able to postulate two different pathways that might achieve this modulation. Further studies are needed to confirm our postulations.
Direct stimulation of the VEGF expression by BMP-7
In recent years, multiple studies found a considerable role played by VEGF next to BMPs in osteogenesis. Therefore, a number of studies investigated the relationship of VEGF and BMP and found synergistic actions of VEGF and BMP-2 on bone healing. Furthermore, they could show that BMPs increased VEGF expression in osteoblasts.Citation32 Animal studies showed better bone regeneration after nonunion therapy with additional VEGF application.Citation33–Citation35 Additionally, a stimulation of angiogenesis via BMP-signaling was demonstrated in in vitro studies.Citation36–Citation38 Akiyama et alCitation39 pointed out that BMP-7 induces VEGF expression in granulosa cells as well as umbilical cord endothelium. Thereby, the results of our study indicate that the local application of BMP-7 increases the serum levels of VEGF by direct stimulation of the VEGF expression during nonunion therapy. The local application of BMP-7 let to a longer and higher VEGF expression.
Indirect stimulation of the VEGF expression by costimulatory effects of inflammable cytokines
Angiogenesis is a complex and multistep process that is regulated by many different growth factors and cytokines (eg, platelet-derived growth factor and basic fibroblast growth factor). VEGF is a very potent angiogenic agent that acts as a specific mitogen for endothelial cells through specific cell surface receptors. However, the molecular mechanism regulating the expression of VEGF is not characterized, and it is likely that other cytokines might promote the expression of VEGF as well. Cohen et al reported that the induction of IL-6 promotes the expression of VEGF during hypoxia, wound healing, and ovulation and should be considered as an indirect angiogenic factor. Furthermore, they reported that TNF-α has been implicated as an indirect angiogenic factor most probably by induction of the expression of VEGF.Citation40,Citation41 In other studies, IL-8 has been shown to be a promoting factor for angiogenesis and to interact with VEGF.Citation42,Citation43 The results of our study showed an earlier peak of IL-8 and TNF-α correlating with the steeper initial increase in the VEGF expression pattern. Furthermore, the IL-6 expression pattern showed a significant increase in the serum concentration immediately after surgery, which might contribute to a steeper increase in the VEGF concentration due to a costimulatory effect.
We could show that the local application of BMP-7 leads to a modulation of the VEGF expression pattern by a complex costimulatory response. In this clinical observer study, we were able to confirm results solely gathered in a previously published in vitro study.Citation38 Furthermore, our data indicate that the biological impact of BMP-7 leads to an increased and earlier VEGF expression pattern by an additional indirect stimulation of VEGF expression by a timely stimulation of the IL-6, IL-8, and TNF-α expressions.
Inflammation
Physiological fracture healing requires a sufficient angiogenesis as well as an adequate stimulus to trigger the beginning of bone healing. Multiple studies have shown that an early inflammatory response serves as a stimulus for bone healing. The inflammatory response is characterized by several cytokines. The proinflammatory cytokines IL-6, TNF-α, and IL-8 are expressed as important initiators of the acute-phase response.Citation44 An increase in the serum values can be observed shortly after fracture.Citation12,Citation45 It is known that the local application of BMP-7 may lead to local signs of inflammation, such as soft-tissue swelling, edema, and reddening of the skin. Although this BMP-mediated soft-tissue swelling is not fully understood, it has been associated with recent studies on the increased incident of proinflammatory cytokines (IL-6, IL-8, and TNF-α).Citation46–Citation48 A recent study found that the application of BMP-7 modulates the microenvironment at the fracture side, in particular by altering the cytokine expression, and thereby, osteoclastogenesis is inhibited.Citation47 Furthermore, as confirmed by our study, no signs of systemic inflammation were detected (both clinically and assessed by the comparison of CRP and leukocyte serum levels in-between groups). Triggered by these cytokines, inflammatory cells such as leukocytes and macrophages migrate into the hematoma caused by injuries of vessels by the fracture.Citation49,Citation50 The interfragmentary hematoma establishes a cellular foundation for the following bone healing.Citation18,Citation51 During this initial inflammatory response, various proinflammatory signals and growth factors are released. Among these proinflammatory cytokines, IL-6 and TNF-α play an important role.
In particular, the inflammatory mediator IL-6 has been shown to be upregulated in early fracture healing.Citation52,Citation53 IL-6 causes mesenchymal stem cells to differentiate to osteoblasts,Citation54,Citation55 thereby promoting bone regeneration. A delayed callus formation, mineralization, and remodeling were found in IL-6 knockout mice.Citation56 Additionally, IL-6 is responsible for the immigration of leukocytes into the fracture site.Citation49 Significant differences in the IL-6 serum levels between the study group and control group were detected after 2 days and 6 weeks. The BMP-7 group showed an increase in the peak serum levels immediately after the local application of BMP-7, whereas the peak serum levels in the control group were significantly lower. Especially, the stimulation of the expression of IL-6 on day 2 is relevant as it represents the initial inflammatory response. BMP-7 is therefore capable of inducing the expression of IL-6, which may induce an increased inflammatory response. In conclusion, application of BMP-7 leads to a significantly higher expression of IL-6 during the initial inflammatory response.
TNF-α is released by macrophages and inflammatory cells.Citation20 The initial release of TNF-α stimulates further expression of several inflammatory mediators and causes immigration of cells into the fracture site.Citation45 According to demand, TNF-α triggers apoptosis or secures the survival of cells.Citation57 The TNF-α-associated receptors are TNFR-1 and TNFR-2, which are located on cellular surfaces.Citation45 While TNFR-1 is permanently present, TNFR-2 is only expressed after fracture. TNF-α stimulation via TNFR-1 causes the differentiation of osteoclasts and therefore stimulates bone resorption. TNF-α stimulation via TNFR-2 leads to a contrary reaction and is a stimulus for bone regeneration.Citation20,Citation58 Our data showed an earlier and higher expression of TNF-α during the initial phase of bone healing after the local application of BMP-7. This may contribute to an earlier and higher influence on TNFR-2, resulting in favorable bone regeneration. However, the differences in the TNF-α expression level between both groups were at a nonsignificant extend; therefore, further studies are needed to prove this postulation.
IL-8 induces an acute inflammatory response via activation and immigration of neutrophile granulocytes.Citation59 Fuller et alCitation60 reported that expression of IL-8 inhibits bone resorption in isolated rat osteoclasts by a decreased proportion of osteoclasts resorbing bone. A recent study showed that IL-8 is important for the regulation of bone resorption by direct stimulation of IL-8 on osteoclastogenesis.Citation61 IL-8 expression depends on a variety of factors, such as the presence and effects of TNF-α and IL-6.Citation62 The results of our study show similar expression pattern of both TNF-α and IL-8. The control group showed an IL-8 peak after 4 weeks of surgery, while IL-8 levels in the BMP-7 group peaked after 2 weeks. Thereby, the results of our study indicate a complex interaction between multiple cytokines subsequent to the application of BMP-7. Despite existing studies, the impact of IL-8 on bone regeneration at the fracture site is not yet fully understood.Citation60–Citation62 In our study, the increase in the IL-8 expression during the initial inflammatory response subsequent to the application of BMP-7 indicates the role of IL-8 as a proinflammatory cytokine in bone regeneration. However, further studies are needed to prove this contribution to bone healing.
Limitations
Nonunions are a severe but an infrequent complication; this is why, clinical studies concerning nonunion therapy are complex and it explains the small size of our patient collective. Despite the large patient collective, just a small number of patients could be included in the study due to our strict inclusion and exclusion criteria. In the context of the current literature and our recent studies,Citation22,Citation23 the collective size of our patients is still sufficient. Another limiting factor is the treatment of two patients with plate fixation and eight patients with nail fixation in the BMP-7 group, whereas patients in the control group received plate fixation in nine cases and nail fixation in one case. The reason for different fixation methods in our study groups is the collaboration between two clinical centers. A comparison of both therapy methods characterized an equal inflammatory response after plate fixation or intramedullar nailing as a treatment for tibial fractures. Furthermore, the responder rate, the development of nonunions, and the number of following surgeries were similar.Citation63 The results of our study may be influenced by a systemic inflammation; therefore, we assessed the CRP and leukocyte serum patterns in the initial 2 weeks subsequent to the procedure. Our data show that the CRP values, after an expected peak after 2 days of surgery, decreased in both groups; furthermore, the CRP values in the control group were even higher than in the study group. Leukocytes stayed in a physiological range during the whole time, thereby indicating that no systemic inflammation was present and reducing the risk of influences on our study results. The preoperative serum levels of VEGF showed differences between both groups. This might influence the analysis of the BMP-7 effects. However, in our statistical analysis, we could see a steeper and faster increase in the VEGF serum levels. Furthermore, despite an overall similar VEGF expression pattern in both groups, VEGF levels rose higher and faster in the BMP-7 group. Therefore, the differences in the baseline do not interfere with the scientific results of our study. Another limitation of our study is a missing fracture group, in which cytokine expression patterns of physiological bone healing are analyzed. However, it is known that the microbiology in patients with delayed and failed bone healing is different. Therefore, we believe that the missing fracture group is not interfering with the results of our study.
Conclusion
The patients with failed fracture healing are known to have diminished concentrations of BMP. The local application of BMP served as substitution, thereby modulating the microenvironment in the fracture gap and increasing local concentrations of BMP-7. In this study, we were able to analyze the influence of BMP-7 application on the expression pattern of inflammable and angiogenic cytokines. Up-to-date multiple studies utilized animal models in order to assess the biological impact of BMP-7, leaving the bias of differences in the reaction of the immune system. Therefore, quantitative measurement of human serum cytokine expression provides a valid instrument in evaluating the biological impact of BMP-7.Citation7,Citation23,Citation64
The local application of BMP-7 in addition to autologous bone graft transplantation provides better bone healing.Citation13,Citation14 Our findings state that BMP-7 via a direct and a postulated indirect pathway by costimulatory effects on proinflammatory cytokine expression leads to an increased VEGF expression. Moreover, the application of BMP-7 leads to an earlier and higher expression of inflammable cytokines; thereby, the application of BMP-7 may contribute to an inflammatory response functioning as a possible stimulus for bone regeneration. Significant higher IL-6 levels and an earlier peak of IL-6, IL-8, and TNF-α were observed in the BMP-7 group. In conclusion, the application of BMP-7 modulates both angiogenic and inflammable cytokine expression patterns.
Acknowledgments
We would like to thank Martina Kutsche-Bauer for performing the Luminex assays.
Disclosure
The authors report no conflicts of interest in this work.
References
- GiannoudisPVEinhornTAMarshDFracture healing: the diamond conceptInjury200738suppl 4S3S6
- TakemotoRFormanJTaorminaDPEgolKANo advantage to rhBMP-2 in addition to autogenous graft for fracture nonunionOrthopedics2014376e525e53024972432
- SchmidmaierGSchwabePWildemannBHaasNPUse of bone morphogenetic proteins for treatment of non-unions and future perspectivesInjury200738suppl 4S35S4118224735
- GiannoudisPVEinhornTASchmidmaierGMarshDThe diamond concept – open questionsInjury200839suppl 2S5S818804574
- ZimmermannGMoghaddamAReumannMTGF-β1 als pathophysiologischer Faktor bei der Frakturheilung [TGF-beta1 as a pathophysiological factor in fracture healing]Unfallchirurg20071102130136 German17160396
- DoneganDJScolaroJMatuszewskiPEMehtaSStaged bone grafting following placement of an antibiotic spacer block for the management of segmental long bone defectsOrthopedics20113411e730e73522049954
- MoghaddamAZietzschmannSBrucknerTSchmidmaierGTreatment of atrophic tibia non-unions according to ‘diamond concept’: results of one- and two-step treatmentInjury201546suppl 4S39S5026542865
- SchmidmaierGMoghaddamAPseudarthrosen langer Röhrenknochen [Long Bone Nonunion]Z Orthop Unfall20151536659676 German26670151
- ChristouCOliverRAYuYWalshWRThe Masquelet technique for membrane induction and the healing of ovine critical sized segmental defectsPLoS One2014912e11412225461340
- GautschiOPFreySPZellwegerRBone morphogenetic proteins in clinical applicationsANZ J Surg200777862663117635273
- BorrelliJJrPrickettWDRicciWMTreatment of nonunions and osseous defects with bone graft and calcium sulfateClin Orthop Relat Res200341124525412782881
- ChoTJGerstenfeldLCEinhornTADifferential temporal expression of members of the transforming growth factor beta superfamily during murine fracture healingJ Bone Miner Res200217351352011874242
- GiannoudisPVKanakarisNKDimitriouRGillIKolimaralaVMontgomeryRJThe synergistic effect of autograft and BMP-7 in the treatment of atrophic nonunionsClin Orthop Relat Res2009467123239324819396502
- Moghaddam-AlvandiAZimmermannGBuchlerAErgebnisse der Pseudarthrosenbehandlung mit “bone morphogenetic protein 7” (BMP-7) [Results of nonunion treatment with bone morphogenetic protein 7 (BMP-7)]Unfallchirurg20121156518526 German22476375
- DaiJLiLJiangCWangCChenHChaiYBone morphogenetic protein for the healing of tibial fracture: a meta-analysis of randomized controlled trialsPLoS One20151010e014167026509264
- LeeKBTaghaviCEMurraySSSongKJKeorochanaGWangJCBMP induced inflammation: a comparison of rhBMP-7 and rhBMP-2J Orthop Res201230121985199422674456
- PhillipsAMOverview of the fracture healing cascadeInjury200536suppl 3S5S716188551
- PapeHCMarcucioRHumphreyCColnotCKnobeMHarveyEJTrauma-induced inflammation and fracture healingJ Orthop Trauma201024952252520736786
- CroesMOnerFCKruytMCProinflammatory mediators enhance the osteogenesis of human mesenchymal stem cells after lineage commitmentPLoS One2015107e013278126176237
- KonTChoTJAizawaTExpression of osteoprotegerin, receptor activator of NF-kappaB ligand (osteoprotegerin ligand) and related proinflammatory cytokines during fracture healingJ Bone Miner Res20011661004101411393777
- StreetJBaoMdeGuzmanLVascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnoverProc Natl Acad Sci U S A200299159656966112118119
- MoghaddamAMullerURothHJWentzensenAGrutznerPAZimmermannGTRACP 5b and CTX as osteological markers of delayed fracture healingInjury201142875876421168135
- WesthauserFZimmermannGMoghaddamSReaming in treatment of non-unions in long bones: cytokine expression course as a tool for evaluation of non-union therapyArch Orthop Trauma Surg201513581107111626085339
- BenderDHaubruckPBoxrikerSKorffSSchmidmaierGMoghaddamAValidity of subjective smoking status in orthopedic patientsTher Clin Risk Manag2015111297130326345646
- ZimmermannGWagnerCSchmeckenbecherKWentzensenAMoghaddamATreatment of tibial shaft non-unions: bone morphogenetic proteins versus autologous bone graftInjury200940suppl 3S50S5320082792
- KanczlerJMOreffoROOsteogenesis and angiogenesis: the potential for engineering boneEur Cell Mater20081510011418454418
- LuCMarcucioRMiclauTAssessing angiogenesis during fracture healingIowa Orthop J200626172616789443
- HausmanMRSchafflerMBMajeskaRJPrevention of fracture healing in rats by an inhibitor of angiogenesisBone200129656056411728927
- StreetJBaoMdeGuzmanLVascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnoverProc Natl Acad Sci U S A200299159656966112118119
- BeamerBHettrichCLaneJVascular endothelial growth factor: an essential component of angiogenesis and fracture healingHSS J201061859419763695
- SarahrudiKThomasABraunsteinerTWolfHVecseiVAharinejadSVEGF serum concentrations in patients with long bone fractures: a comparison between impaired and normal fracture healingJ Orthop Res200927101293129719402151
- YoungSPatelZSKretlowJDDose effect of dual delivery of vascular endothelial growth factor and bone morphogenetic protein-2 on bone regeneration in a rat critical-size defect modelTissue Eng Part A20091592347236219249918
- OgilvieCMLuCMarcucioRVascular endothelial growth factor improves bone repair in a murine nonunion modelIowa Orthop J201232909423576927
- EckardtHDingMLindMHansenESChristensenKSHvidIRecombinant human vascular endothelial growth factor enhances bone healing in an experimental nonunion modelJ Bone Joint Surg Br200587101434143816189323
- TarkkaTSipolaAJamsaTAdenoviral VEGF-A gene transfer induces angiogenesis and promotes bone formation in healing osseous tissuesJ Gene Med20035756056612825195
- RamoshebiLNRipamontiUOsteogenic protein-1, a bone morphogenetic protein, induces angiogenesis in the chick chorioallantoic membrane and synergizes with basic fibroblast growth factor and transforming growth factor-beta1Anat Rec200025919710710760748
- LangenfeldEMLangenfeldJBone morphogenetic protein-2 stimulates angiogenesis in developing tumorsMol Cancer Res20042314114915037653
- SuzukiYMontagneKNishiharaAWatabeTMiyazonoKBMPs promote proliferation and migration of endothelial cells via stimulation of VEGF-A/VEGFR2 and angiopoietin-1/Tie2 signallingJ Biochem2008143219920618006519
- AkiyamaIYoshinoOOsugaYBone morphogenetic protein 7 increased vascular endothelial growth factor (VEGF)-a expression in human granulosa cells and VEGF receptor expression in endothelial cellsReprod Sci201421447748224023033
- RegaGKaunCDemyanetsSVascular endothelial growth factor is induced by the inflammatory cytokines interleukin-6 and oncostatin m in human adipose tissue in vitro and in murine adipose tissue in vivoArterioscler Thromb Vasc Biol20072771587159517525365
- CohenTNahariDCeremLWNeufeldGLeviBZInterleukin 6 induces the expression of vascular endothelial growth factorJ Biol Chem199627127367418557680
- HeidemannJOgawaHDwinellMBAngiogenic effects of interleukin 8 (CXCL8) in human intestinal microvascular endothelial cells are mediated by CXCR2J Biol Chem2003278108508851512496258
- JohnsonKEWilgusTAVascular endothelial growth factor and angiogenesis in the regulation of cutaneous wound repairAdv Wound Care (New Rochelle)201431064766125302139
- GruysEToussaintMJMNiewoldTAKoopmansSJAcute phase reaction and acute phase proteinsJ Zhejiang Univ Sci B20056111045105616252337
- MountziarisPMMikosAGModulation of the inflammatory response for enhanced bone tissue regenerationTissue Eng Part B Rev200814217918618544015
- LeeKBTaghaviCESongKJInflammatory characteristics of rhBMP-2 in vitro and in an in vivo rodent modelSpine2011363E149E15421242879
- MaurerTZimmermannGMaurerSStegmaierSWagnerCHanschGMInhibition of osteoclast generation: a novel function of the bone morphogenetic protein 7/osteogenic protein 1Mediators Inflamm2012201217120923132958
- RobinBNChaputCDZeitouniSRahmMDZerrisVASampsonHWCytokine-mediated inflammatory reaction following posterior cervical decompression and fusion associated with recombinant human bone morphogenetic protein-2: a case studySpine20103523E1350E135420938385
- BastianOPillayJAlblasJLeenenLKoendermanLBlokhuisTSystemic inflammation and fracture healingJ Leukoc Biol201189566967321208896
- CampbellDJKimCHButcherECChemokines in the systemic organization of immunityImmunol Rev2003195587112969310
- KolarPGaberTPerkaCDudaGNButtgereitFHuman early fracture hematoma is characterized by inflammation and hypoxiaClin Orthop Relat Res2011469113118312621409457
- RundleCHWangHYuHMicroarray analysis of gene expression during the inflammation and endochondral bone formation stages of rat femur fracture repairBone200638452152916321582
- GerstenfeldLCCullinaneDMBarnesGLGravesDTEinhornTAFracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulationJ Cell Biochem200388587388412616527
- KudoOSabokbarAPocockAItonagaIFujikawaYAthanasouNAInterleukin-6 and interleukin-11 support human osteoclast formation by a RANKL-independent mechanismBone20033211712584029
- HeymannDRousselleAVgp130 Cytokine family and bone cellsCytokine200012101455146811023660
- YangXRicciardiBFHernandez-SoriaAShiYPleshko CamachoNBostromMPCallus mineralization and maturation are delayed during fracture healing in interleukin-6 knockout miceBone200741692893617921078
- LocksleyRMKilleenNLenardoMJThe TNF and TNF receptor super-families: integrating mammalian biologyCell2001104448750111239407
- BalgaRWetterwaldAPortenierJDolderSMuellerCHofstetterWTumor necrosis factor-alpha: alternative role as an inhibitor of osteoclast formation in vitroBone200639232533516580896
- HaradaASekidoNAkahoshiTWadaTMukaidaNMatsushimaKEssential involvement of interleukin-8 (IL-8) in acute inflammationJ Leukoc Biol19945655595647964163
- FullerKOwensJMChambersTJMacrophage inflammatory protein-1 alpha and IL-8 stimulate the motility but suppress the resorption of isolated rat osteoclastsJ Immunol199515411606560727751648
- BendreMSMontagueDCPeeryTAkelNSGaddyDSuvaLJInterleukin-8 stimulation of osteoclastogenesis and bone resorption is a mechanism for the increased osteolysis of metastatic bone diseaseBone2003331283712919697
- DeForgeLEPrestonAMTakeuchiEKenneyJBoxerLARemickDGRegulation of interleukin 8 gene expression by oxidant stressJ Biol Chem19932683425568255768244994
- VallierHACuretonBAPattersonBMRandomized, prospective comparison of plate versus intramedullary nail fixation for distal tibia shaft fracturesJ Orthop Trauma2011251273674121904230
- MoghaddamAChildCBrucknerTGernerHJDanielVBiglariBPosttraumatic inflammation as a key to neuroregeneration after traumatic spinal cord injuryInt J Mol Sci20151647900791625860946