Abstract
Therapeutic antibodies hold great promise for the treatment of cancer and autoimmune diseases, and developments in antibody–drug conjugates and bispecific antibodies continue to enhance treatment options for patients. Immunoglobulin (Ig) G antibodies are proteins with complex modifications, which have a significant impact on their function. The most important of these modifications is glycosylation, the addition of conserved glycans to the antibody Fc region, which is critical for its interaction with the immune system and induction of effector activities such as antibody-dependent cell cytotoxicity, complement activation and phagocytosis. Communication of IgG antibodies with the immune system is controlled and mediated by Fc gamma receptors (FcγRs), membrane-bound proteins, which relay the information sensed and gathered by antibodies to the immune system. These receptors are also glycoproteins and provide a link between the innate and adaptive immune systems. Recent information suggests that this receptor glycan modification is also important for the interaction with antibodies and downstream immune response. In this study, the current knowledge on FcγR glycosylation is discussed, and some insight into its role and influence on the interaction properties with IgG, particularly in the context of biotherapeutics, is provided. For the purpose of this study, other Fc receptors such as FcαR, FcεR or FcRn are not discussed extensively, as IgG-based antibodies are currently the only therapeutic antibody-based products on the market. In addition, FcγRs as therapeutics and therapeutic targets are discussed, and insight into and comment on the therapeutic aspects of receptor glycosylation are provided.
Therapeutic antibodies and glycosylation
Antibodies or immunoglobulins (Igs) are important components of the humoral immune system, which act as surveyors, sensing pathogens and transformed cells, communicating this information to the innate and adaptive immune systems. IgG antibodies provide the first line of defense against invading microorganisms, and due to their ability to detect tumor-associated antigens and neutralize inflammatory mediators such as tumor necrosis factor (TNF)-α this class of antibodies has been used with great success in treatments for cancer and autoimmunity conditions. Therapeutically, all the current monoclonal antibodies (Mabs) and Mab fusion proteins used in autoimmune diseases, inflammatory conditions and oncology use the IgG backbone. This is the most studied and best characterized of the Igs and is divided into four distinct subclasses (IgG1, IgG2, IgG3, IgG4), each with differences in sequence and structure, binding properties to cellular Fc gamma receptors (FcγRs) and effector functions ().Citation1,Citation2 Mab therapy was born in the 1970s with the major discoveries of the IgG structure by Edelman et alCitation3 and PorterCitation4 and the development of hybridoma technology by Kohler and Milstein.Citation5 Initially, Mab therapeutics were murine in nature, leading to significant problems such as inadequate serum retention, induction of IgE-specific allergic reactions and anaphylaxis due to the presence of murine-derived gal α(1,3)-gal and N-glycolylneuraminic acid glycan epitopes and failure to induce effector responses through impaired interaction with human FcγRs.Citation6 Developments in recombinant antibody technology and the production of chimeric, humanized and fully human antibodies have addressed many of these issues, most importantly the humanization of glycosylation to ensure productive interaction with FcγRs and prevention of anaphylaxis.
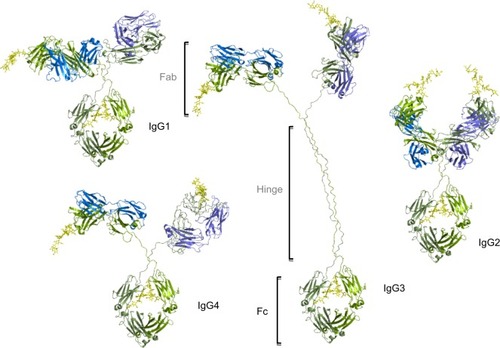
Figure 1 The IgG subtypes.
Notes: Four subtypes of IgG exist in humans: IgG1, IgG2, IgG3, IgG4, each with differences in sequence, structure, glycosylation and communication with FcγRs. The four subtypes are named based on their respective abundance in serum with IgG1 being the most abundant. IgG antibodies consist of two Fab regions that can bind both an antigen molecule and the Fc region which interact with the FcγR, joined by a highly flexible hinge region. IgG has a longer hinge region than the other IgG subtypes. Each IgG subtype has conserved Asn 297 amino acids in the Fc region with N-glycans attached (shown in yellow). Typically, the Fc glycans are bi-antennary galactosylated structures with varying amounts of core fucosylation and sialylation. Glycosylation is also found in the Fab regions with higher proportions of galactosylated and sialylated glycans. Heavy chains are shown in green, and light chains are shown in blue and purple.
Abbreviations: Fab, fragment antigen binding; FcγR, Fc gamma receptor; IgG, immunoglobulin G.
Glycans play an important role in IgG-mediated immunity, and crucially IgG-based therapeutics typically have glycan attributes that influence the interaction with FcγRs and downstream immune response.Citation7–Citation10 Therefore, glycans are important factors in the design of IgG-based therapeutics, particularly in the Fc region, which mediates the effector responses induced by IgG, as well as recycling and the anti-inflammatory activity of IgG.Citation2,Citation11,Citation12 Currently, the most important of these appears to be the α(1,6)-linked core fucose, which has been the subject of intensive pharmaceutical interest since it was discovered that IgG lacking this glycan characteristic had enhanced binding to activating FcγRs and improved antibody-dependent cell cytotoxicity (ADCC).Citation13–Citation18 The market approval of the glycoengineered form of the anti-CD20 Mab Gazyra (Genentech, San Francisco, CA, USA) with reduced core fucosylation highlights the success of this strategy (comprehensive reviews on the biopharmaceutical and therapeutic antibody markets are discussed by WalshCitation19 and Ecker et alCitation20). Terminal sialylation and mannosylation of antibody N-glycans are also important functional features of antibodies, which significantly impact their activity and serum retention. A high sialic acid content has been proposed to impact the IgG Fc structure and force it to acquire a closed conformation resulting in decreased binding to FcγRs; however, X-ray crystallographic data suggest that this is not the case, and no major Fc structural alterations were observed with increased sialylation.Citation21,Citation22 Sialylation can also impact the clearance rates of therapeutic antibodies with higher sialylation leading to longer serum retention timesCitation23,Citation24 and induction of the anti-inflammatory effects of intravenous immunoglobulin (IVIg).Citation12,Citation25 Terminal mannosylation, usually in the form of hypogalactosylated glycans (G0, G1), can also affect the serum retention of antibodies and binding to mannose-binding lectin (MBL) on macrophages.Citation26,Citation27 The glycan attributes of potential Mab therapeutics must therefore be carefully considered as the binding to cellular FcγRs, activation of the complement cascade and phagocytosis, serum retention, recycling and placental transport of the therapeutic can be greatly influenced by the Fc glycans.
FcγRs: the key to IgG biological activity
Emerging from the success of Mab therapy and glycoengineering is the importance of FcγRs for their success and therapeutic efficacy and the vast complexity in receptor biology. Therapeutically, FcγRs were once utilized solely for analyzing the efficacy and safety of therapeutic Mabs through biophysical binding experiments; however, this is no longer the case and these antibody receptors are now realizing their potential as anti-inflammatory therapies and in autoimmune conditions. IgG antibodies survey and communicate the information sensed to the immune system via interaction with these single-pass transmembrane receptors of the Ig superfamily. The family of receptors that are found almost ubiquitously throughout the body, from myeloid cells to lymphoid and neuronal cells, are broadly characterized into three groups: FcγRI, FcγRII and FcγRIII.Citation28 Differences exist between the groups of receptors, particularly in their structure, function, glycosylation and affinity for IgG.Citation28–Citation33 FcγRI, FcγRIII and FcγRIIa are activating receptors and induce effector activities in innate effector cells such as macrophages and natural killer (NK) cells. FcγRIIb is fundamentally different from the other activating receptors and acts as an inhibitory receptor. Signaling through this receptor induces inhibitory signals that decrease the activation/inhibition (A/I) ratio and bring the cells further from the threshold level required for activation.Citation34
Extensive variability at the genomic, transcriptomic and proteomic level exists among the human FcγRs, with multiple genes, transcripts, polymorphic variants and glycovariants adding to the complexity of these receptors. Polymorphic variants have been found for nearly all of the FcγRs, with significant effects on the interaction with IgG and downstream physiological response.Citation35–Citation38 Multimerization of antibodies and antigen and engagement of FcγRs lead to microclustering of receptors in the plasma membrane and activation of signaling cascades involving immunoreceptor tyrosine-based activation motif and immunoreceptor tyrosine-based inhibitory motif resulting in cellular activation or inhibition, activities that can be influenced by the glycosylation state and polymorphic variant of the receptor. It is also important to note that due to the high-affinity nature of FcγRI, it is believed to be constantly bound by monomeric IgG and it is the lower affinity receptor that participates in many of the proinflammatory activities of FcγRs. In addition, when Mabs are used therapeutically, due to the very high serum concentration of IgG (~15 mg/mL), practically all of the cellular FcγRs will be occupied and therefore higher concentrations of a therapeutic antibody are required, further increasing the need for glycoengineered antibodies with higher FcγR affinities than serum IgG.
Glycobiology of FcgRs
Glycosylation research into FcγRs began over 30 years ago, but still currently relatively little is known about how these receptors are glycosylated by cells of the immune system, in healthy and disease states (a review of Fc receptors and glycosylation is discussed by Hayes et alCitation39). Several recent structural and biophysical studies have pointed to important roles of FcγR glycosylation and have implicated it in the binding mechanism with IgG.Citation40–Citation43 The vast majority of glycan data and glycosylation information that is available is for the activating FcγRIIIa receptor, mainly due to its role in NK cell-mediated ADCC and its therapeutic relevance and importance to the pharmaceutical industry. Glycosylation, however, varies widely between the different receptors with different numbers of glycosylation sites and differential and cell type-specific glycosylation patterns ( and ; ). Seminal work by Edberg et al,Citation44 Edberg and KimberleyCitation45 and Kimberly et alCitation46 showed that FcγRIIIa exists as cell type-specific glycoforms on monocytes and macrophages with different affinities for IgG and different responses to an IgG stimulus due to the differently glycosylated FcγRs. This has intriguing implications for how immune cells respond to IgG based on the glycosylation status of the FcγR. Unfortunately, no further information on the natural glycosylation on these receptors exists in the literature, and there is therefore a lack of information regarding these receptors and the cellular activation or inhibition by IgG. The A/I ratio, for example, is likely to be skewed toward activation or inhibition depending on how a particular cell glycosylates its FcγRs in healthy and disease states. This also has important implications for the biopharmaceutical industry, and response to Mab therapy in patients may depend on how their FcγRs are glycosylated.
Table 1 Properties of human FcγRs
Table 2 Annotation of human FcγR glycosylation showing position and conservation of N-glycan sites
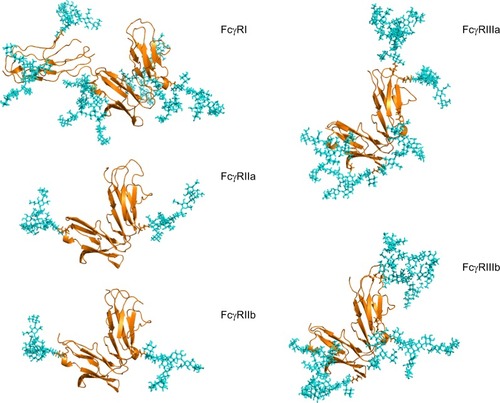
Figure 2 Human FcγRs are complex glycoproteins.
Notes: FcγR ectodomains were modeled with N-glycans on each glycosylation site for each receptor, taking into account glycan size and composition, torsion angles and free energies. The N-glycans (shown in cyan) were modeled based on identified glycans from FcγRs (shown in gold) produced in recombinant systems such as NS0, CHO and HEK293 systems.Citation43,Citation47,Citation50 In the case of FcγRIIIa, site-specific glycosylation studies were performed by Zeck et al,Citation47 and this information was used to build the glycans shown on the Asn 162 site located at the binding interface with IgG. For the remaining receptors, no site-specific analysis was available, and in these cases the most abundant glycans identified from the recombinant sources were used to model N-glycosylation for these receptors. FcγRI has an extra D3 domain, which contributes to its high-affinity nature, and this domain also contains two glycosylation sites. The glycan compositions modeled onto each N-glycosylation site for each FcγR are named according to the Oxford notation (https://glycobase.nibrt.ie/glycobase/show_nibrt.action)Citation86 and are as follows: FcγRI: Asn 59 (Man 5), Asn 78 (FA2G2S1), Asn 152 (FA2GN2S2), Asn 159 (Man 6), Asn 163 (FA2G2), Asn 195 (FA2G1GN1), Asn 240 (FA2BG2). FcγRIIa: Asn 64 (FA2G2S1), Asn 145 (FA2BG2). FcγRIIb: Asn 66 (FA2G2S1), Asn 147 (FA2BG2). FcγRIIIa: Asn 38 (FA2G2S1), Asn 45 (FA2G2), Asn 74 (FA4G4S4), Asn 162 (FA2G2), Asn 169 (FA2BG2). FcγRIIIb: Asn 35 (FA2GalNAc2S2), Asn 42 (Man 5), Asn 61 (FA2G2S1), Asn 71 (FA3G2), Asn 159 (FA2BG1), Asn 166 (FA2BG2). PDB accession numbers used to build the models were as follows: FcγRI: 4×4m, FcγRIIa: 1fcg, FcγRIIb: 2fcb, FcγRIIIa: 3ay4, FcγRIIIb: 1e4j.
Abbreviations: FcγR, Fc gamma receptor; IgG, immunoglobulin G; PDB, Protein Data Bank.
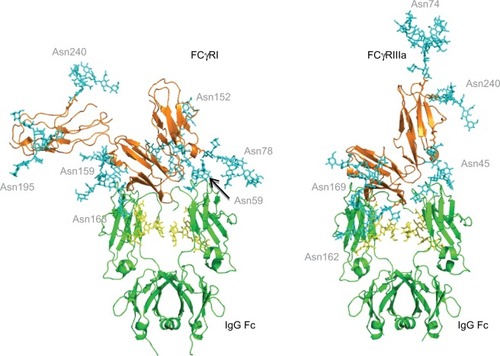
More recently, high-resolution biophysical and structural data collection has revealed the importance of FcγRIIIa glycosylation.Citation40,Citation43,Citation47 This low-affinity activating receptor is extremely homologous in its extracellular domain, in both amino acid sequence and three-dimensional structure to the related FcγRIIIb.Citation48,Citation49 Two N-linked sites of FcγRIIIa have been shown to regulate the binding of IgG; a glycan on Asn 45 has an inhibitory role and negative effect on IgG binding, whereas glycosylation at Asn 162, which is located at the IgG-binding interface in the three-dimensional structure, increases IgG interaction and binding affinityCitation40–Citation42 (). Glycan analysis of FcγRIIIa from recombinant systems followed by biophysical binding experiments showed that glycosylation is dependent on the source of the receptor and that specific glycans can be located on the Asn 162 site that influences and mediates IgG bindingCitation43,Citation47,Citation50 (). Structural studies have also shown that on a molecular level a unique carbohydrate–carbohydrate interface exists between afucosylated IgG1 and FcγRIIIa, which can explain the increase in affinity for therapeutic antibodies lacking core fucose.Citation40
Figure 3 FcγR–IgG complexes with modeled N-glycans show complexity of glycosylation and the potential roles of glycans in the binding interaction with IgG.
Notes: The Asn 162 glycan of FcγRIIIa has been shown to form carbohydrate–carbohydrate interactions with the bi-antennary glycan of IgG.Citation40 This asparagine residue is at the binding interface with IgG. FcγRI does not have a glycan in this position but does have glycans near the binding site such as Asn 78 (Asn 162 in FcγRIIIa), which is structurally conserved in each of the FcγRs. The glycan compositions modeled onto each N-glycosylation site for each FcγR are named according to the oxford notation (see https://glycobase.nibrt.ie/glycobase/show_nibrt.action)Citation86 and are as follows: FcgRI: Asn 59 (Man 5), Asn 78 (FA2G2S1), Asn 152 (FA2GN2S2), Asn 159 (Man 6), Asn 163 (FA2G2), Asn 195 (FA2G1GN1), Asn 240 (FA2BG2). FcgRIIIa: Asn 38 (FA2G2S1), Asn 45 (FA2G2), Asn 74 (FA4G4S4), Asn 162 (FA2G2), Asn 169 (FA2BG2). PDB accession numbers used to build the models were as follows: FcgRI: 4×4m, FcgRIIIa: 3ay4.
Abbreviations: FcγR, Fc gamma receptor; IgG, immunoglobulin G.
Human immune cells have different combinations of FcγRs with different numbers of N-glycosylation sites ( and ), and IgG immune complexes will interact with many different receptors on the same cell, creating an extremely complex series of interactions and signaling pathways/stimuli. Adding further complexity is the differential glycosylation of the FcγRs, which are found on the same cell. Previous studies have described the glycan compositions of recombinant FcγRs (FcγRI, FcγRIIa, FcγRIIb, FcγRIIIa and FcγRIIIb) from different sources and showed that the glycosylation is complex with multi-antennary structures and extensive outer-arm modifications ().Citation43,Citation50–Citation55 FcγRI, which has seven potential N-glycosylation sites, is structurally different from the other receptors with an extra D3 domain, which contributes to its high-affinity nature.Citation56,Citation57 There is little information available regarding the nature of FcγRI glycosylation or the glycosylation site occupancy; however, studies performed on recombinant FcγRI from NS0 and HEK293 cells showed that the receptor expressed significant amounts of high-mannose glycans and complex multi-antennary structures with large amounts of core-fucosylation and outer-arm modifications; glycan monosaccharide compositions which can influence the IgG-binding interaction ( and ).Citation43,Citation50 Crystal structures of FcγRI are available, and recently a crystal structure in complex with IgG was described (); however, even though the IgG glycans were shown to be important for the interaction, little information is available on the receptor glycans ( and ).Citation58,Citation59 Biologically and functionally FcγRIIa and FcγRIIb are significantly different as FcγRIIb is the inhibitory FcγR; however, despite the biological differences, they demonstrate significant homology in their extracellular domains with ~92% sequence identity. Data exist for the glycosylation of the receptors from NS0, HEK293, CHO and insect cells and demonstrate that the receptors display complex glycan structures with core fucosylation and minimal sialylation ().Citation43,Citation50–Citation52 In one report of glycosylation of FcγRIIa from insect cells, sialylation was not detected and glycosylation was not reported to influence IgG binding.Citation51 The only information on FcγRIIb glycosylation comes from reports describing recombinant sources from NS0 and HEK293 cells, and similar to other FcγRs this receptor presents with multi-antennary structures, which are core fucosylated and undersialylated ().Citation43,Citation50 FcγRs have a number of glycan characteristics in common, all contain complex multi-antennary structures, which are core fucosylated and undersialylated (). Oligomannose structures are also present and vary depending on the receptor with the largest amount observed for FcγRI (). Very little information exists as to the natural glycosylation state of any of the receptors in healthy or disease states, and little information exists as to the glycosylation site occupancy for any of the natural receptors and only for recombinant FcγRIIIa.Citation47
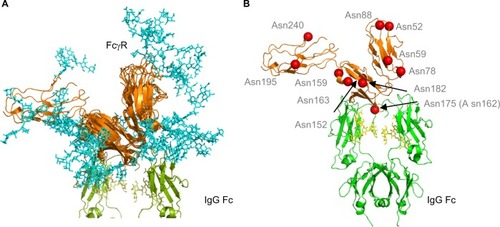
Figure 4 Human FcγRs show complex N-glycosylation in the protein ectodomain and around the IgG binding site.
Notes: (A) Structural overlay of FcγRs. FcγRs are very structurally homologous with the exception of the extra D3 domain in FcγRI. Complexity of glycans (cyan) is shown and potential interactions with IgG Fc and the IgG Fc glycans. The glycan compositions modeled onto each N-glycosylation site for each FcγR are named according to the oxford notation (see https://glycobase.nibrt.ie/glycobase/show_nibrt.action)Citation86 and are as follows: FcgRI: Asn 59 (Man 5), Asn 78 (FA2G2S1), Asn 152 (FA2GN2S2), Asn 159 (Man 6), Asn 163 (FA2G2), Asn 195 (FA2G1GN1), Asn 240 (FA2BG2). FcgRIIa: Asn 64 (FA2G2S1), Asn 145 (FA2BG2). FcgRIIb: Asn 66 (FA2G2S1), Asn 147 (FA2BG2). FcgRIIIa: Asn 38 (FA2G2S1), Asn 45 (FA2G2), Asn 74 (FA4G4S4), Asn 162 (FA2G2), Asn 169 (FA2BG2). FcgRIIIb: Asn 35 (FA2GalNAc2S2), Asn 42 (Man 5), Asn 61 (FA2G2S1), Asn 71 (FA3G2), Asn 159 (FA2BG1), Asn 166 (FA2BG2). PDB accession numbers used to build the models were as follows: FcgRI: 4×4m, FcgRIIa: 1fcg, FcgRIIb: 2fcb, FcgRIIIa: 3ay4, FcgRIIIb: 1e4j. (B) Position of N-glycan sites in human FcγRs. Asn 175 (Asn 162 in FcγRIIIa) is at the binding site with IgG and glycans in this position can participate in carbohydrate–carbohydrate interactions. A number of other glycan sites are present close to the binding interface with IgG and glycans in this position can potentially participate in glycan–glycan interactions and glycan–protein interactions with IgG Fc. Structure is based on FcγRI (PDB: 4×4m). N-glycosylation sites are named for FcγRI using the UniprotKB numbering scheme.
Abbreviations: FcγR, Fc gamma receptor; IgG, immunoglobulin G; PDB, Protein Data Bank.
FcgRs as therapeutic targets and a role for glycosylation
FcγRs mediate many of the biological functions of therapeutic Mabs, particularly when the induction of effector activities is desired. Manipulating the Fc glycan of Mabs is a successful strategy to prevent interaction with FcγRs when the activation of the immune system is not required for the efficacy of the antibody or is undesirable. In the primary mechanism of oncology Mabs such as rituximab and trastuzumab, FcγRIIIa is targeted to induce ADCC. Glycoengineering of the antibody Fc region has proved to be a highly successful strategy to target and improve FcγR binding and ADCC, and the next-generation glycoengineered afucosylated Mab Gazyva is now on the market. It is also worth noting that aglycosylated IgG variants with specific mutations in the Fc region have been shown to bind FcγRI with equal or greater affinity than wild-type IgG, presenting an alternative therapeutic strategy.Citation60 Various glycosylation modeling platforms have also added to our understanding of complex networks leading to specific glycoforms.Citation61–Citation65 Polymorphisms found in the extracellular domain of FcγRs further add to their variability and complexity, in particular, the Val 158/Phe 158 polymorphism of FcγRIIIa and Arg 131/His 131 polymorphism of FcγRIIa dictate how a patient responds to antibody therapy with the Val 158 and His 131 variants responding better to rituximab treatment in non-Hodgkins lymphoma.Citation66,Citation67 Furthermore, with detailed knowledge of the glycosylation of FcγRs in patients, there is the potential to manipulate and target an FcγR glyco-profile to improve the therapeutic effect. Prediction of how a patient will respond to antibody therapy or identification of biomarkers for nonresponders are important factors for developing a personalized medicine approach.
FcγR expression and regulation are important factors in antibody therapy for a range of clinical conditions. Receptor expression levels are shown to differ in patients with cancer, and the inhibitory FcγRIIb is shown to be upregulated in conditions such as malignant melanoma and lymphomas.Citation68–Citation71 Inhibitory receptor expression is also reported to be decreased on memory B-cells and plasma cells from patients with chronic inflammatory demyelinating neuropathy treated with IVIg, suggesting that in inflammatory or pro-inflammatory conditions FcγR activation prevails or is increased over inhibitory conditions.Citation72 In inflammatory bowel disease and systemic lupus erythematosus, conditions characterized by chronic inflammation, FcγRI upregulation has been reported.Citation73,Citation74 Higher expression levels of FcγRIIa and FcγRIIIa have also been reported in autoimmune conditions, and the anti-TNF Mab infliximab has been reported to decrease the expression of activating receptors.Citation75 Viral and bacterial infections also influence the surface numbers of activating and inhibitory FcγRs, and bacterial components such as lipopolysaccharide increase the expression of FcγRIII and FcγRIV in miceCitation76 and cytokines such as interferon-γ can regulate or alter FcγR expression, particularly in viral infections such as HIV.Citation77,Citation78 In these cases, an antibody or combination therapy to block a particular receptor such as the inhibitory receptor in cancer or activating receptors in inflammation could prove to be an effective strategy by skewing the A/I ratio toward cellular activation or inhibition. Furthermore, there are many examples of cancer-specific glycosylation changes, which promote metastasis, survival and immune evasion. It is therefore likely that the glycosylation of FcγRs present on cancer cells such as non-Hodgkin lymphoma will be affected in a way that will prevent productive antibody interactions. It is also likely that cancer cells will negatively influence the glycosylation of FcγRs on cytotoxic cells, such as NK cells to inhibit productive antibody interactions and promote cancer cell survival. Immunotherapies can be improved with the knowledge of the glycosylation profiles of FcγRs in healthy and disease states.
Glycosylated FcgRs as therapeutics
Targeting FcγRs with small molecule inhibitors or anti-receptor Mabs is an attractive strategy to prevent immune complex-driven activation of effector cells, a major driver of inflammation and autoimmunity. Since the 1990s, soluble FcγRs, formed by alternate splicing or proteolytic cleavage of the receptor ectodomain, have been identified in humans and mice and have been shown to inhibit B-cell proliferation and IgG production.Citation79 Recombinant forms of the soluble ectodomains of activating receptors (FcγRI, FcγRII and FcγRIII) and more recently the inhibitory FcγRIIb have been used to perform a similar therapeutic anti-inflammatory role in humans with significant success.Citation80–Citation84 These soluble domains are believed to function as decoy receptors to bind IgG immune complexes, decrease the A/I ratio and prevent inflammation and autoimmunity.Citation85 Although these soluble FcγRs have low affinity (except FcγRI) for IgG immune complexes, positive results in reducing inflammation have been shown in epidermolysis bullosa acquisita.Citation80,Citation84 In addition to the low-affinity nature of soluble FcγRs, another downside to their use as therapeutics is their relatively small size, ranging from 20 to 45 kDa in their aglycosylated state (). This leads to difficulties such as rapid excretion in vivo. A possible mechanism to increase the size of the FcγR therapeutic is to use recombinant glycosylated forms made in cells such as CHO cells or HEK293 cells (), as opposed to aglycosylated forms made in bacteria such as Escherichia coli. In addition to increasing the size of the therapeutic, the strategy of using glycosylated ectodomains has the added benefit of increasing the solubility of the receptor and may in addition help in the clearance of glycosylated FcγR immune complexes through interactions with lectins such as MBL on macrophages through phagocytosis. Glycosylated FcγR ectodomains have the potential to increase the clearance of immune complexes and further reduce inflammation through this glycosylated FcγR–lectin-based phagocytosis mechanism.
Glycosylation of FcgRs: therapeutic prospects
There is now clear evidence that FcγR glycosylation is an important factor in the interaction with therapeutic antibodies and could influence immune system activation or inhibition.Citation40,Citation43,Citation47 This has important implications for how Mab-based therapeutics and fusion proteins are designed. However, little is known about the glycosylation of these receptors in their natural environment and until detailed information on how each receptor is glycosylated by different cells of the immune system in healthy and disease states is available, complete understanding of how therapeutic antibodies interact with the immune system to activate or inhibit will remain incomplete. Although important information is available, which shows that immune cells such as macrophages and monocytes bind and respond to IgG differentially, most of the detailed information on glycosylation and its influence on the IgG–FcγR interaction come from recombinant systems and receptors expressed in NS0, HEK293 and CHO cells.Citation43–Citation47 This information is valuable and shows that receptor glycosylation is cell type specific and influences the IgG-binding kinetics and indicates that cells of the immune system such as NK cells, macrophages, neutrophils and B-cells will also glycosylate in a cell type-specific manner, which can result in each cell responding differently to IgG and IgG immune complexes. The particular glycans that the cell expresses on the FcγR, based on its own glycosylation machinery, can determine the antibody response and it is conceivable that in a state of inhibition or inactivation specific glycans can be expressed on the FcγR that can prevent a productive interaction with immune complexes or decrease the affinity for antibody. Conversely, in a state of inflammation or activation, different glycans can be expressed on the FcγR to promote a positive antibody interaction and induce effector responses. FcγR glycosylation can therefore be used as a mechanism by the immune system to fine-tune the antibody response. In inflammatory and autoimmune conditions, for example, FcγRs found on macrophages, monocytes and neutrophils are potentially glycosylated in a manner that facilitates excessive interaction with antibodies, immune complexes or self-antigens that promote inflammation and autoimmunity. In addition, in healthy states, glycans can potentially be used by FcγRs to inhibit or decrease antibody engagement, downregulate immune responses and prevent inflammation and autoimmunity. Glycans on FcγRs can therefore be used to alter the balance between activation and inhibition in a glycosylation-mediated control mechanism. In addition, until this detailed glycan information is available, the glycosylation of FcγRs cannot be fully understood with the aim of manipulating or optimizing therapeutics based on the glycosylation state of the FcγR. There is the potential to design therapeutics to specifically target known FcγR glycoforms in a particular disease such as cancer or inflammatory or autoimmune conditions, which are known to promote or inhibit productive antibody interactions. Information exists that monocyte FcγRIIIa does not contain high-mannose-type glycans whereas NK cell FcγRIIIa does, and this can explain the lower affinity of the monocyte/macrophage glycoform.Citation45 This information, together with further detailed glycan data, can be exploited to design therapeutics to differentially bind specific FcγR glycoforms such as an Mab with higher affinity for the high-mannose-type glycoform of macrophages if antibody-dependent phagocytosis is desired.
Detailed knowledge of FcγR glycobiology in inflammatory and autoimmune conditions and the availability of glycan information will allow for a deeper and more comprehensive knowledge and understanding of these conditions and how to treat them, the type of antibody isotype, glycoform or fusion protein to design and a much more targeted approach to an individual or condition. This raises the possibility of a personalized medicine approach, whereby patient’s FcγR glycoforms could be determined and the therapeutic approach tailored to suit a particular person. A patient’s FcγRs, including polymorphisms and glycoprofiles, could be used to predict the efficacy of a therapeutic Mab and identify responders and nonresponders to expensive and potentially dangerous biological therapies. There is evidence in the literature that the glycans present on FcγRs made in recombinant systems can modulate the binding interaction with antibody.Citation43 By knowing the natural glycoprofiles of these receptors, the interaction of therapeutic Mabs with their target cells such as NK cells in cancer treatment can be better understood and the clinical outcome can be better predicted.
Finally, the biopharmaceutical industry typically uses biophysical techniques such as surface plasmon resonance and recombinant forms of FcγRs expressed in NS0, HEK293 or CHO cells to determine the interactions of therapeutic antibodies with FcγRs, affinity constants and kinetic parameters. While these systems provide valuable information, the downside to this approach is that the physiological system is not fully represented and these recombinant receptors do not adequately predict the outcome of an antibody therapy or the interactions of a therapeutic antibody with cells and FcγRs of the immune system, partly because the FcγRs used in these analyses are glycosylated differently to the FcγRs of the immune system. There are examples in the literature, which show that the interaction kinetics of therapeutic antibodies is different in biophysical experiments depending on the source of the receptor and its glycosylation pattern.Citation47,Citation50 By understanding the glycosylation of the natural FcγRs, there is the potential to design and use recombinant forms of the receptors, which have glycoprofiles that physiologically resemble the natural FcγRs in biophysical evaluations to provide more accurate affinity determinations, analysis of the interaction and ultimately predictions of the physiological outcome of the antibody therapy.
Acknowledgments
The authors acknowledge the funding sources such as the EU Initial Training Network (Project No 608381) and Science Foundation Ireland (Grant No SFI-13/SP SSPC/I2893) for supporting this work.
Disclosure
The authors report no conflicts of interest in this work.
References
- AlzariPMLascombeMBPoljakRJThree-dimensional structure of antibodiesAnnu Rev Immunol198865555802454644
- BurtonDRWoofJMHuman antibody effector functionAdv Immunol1992511841502974
- EdelmanGMCunninghamBAGallWEGottliebPDRutishauserUWaxdalMJThe covalent structure of an entire gammaG immunoglobulin moleculeProc Natl Acad Sci U S A196963178855257969
- PorterRRStructural studies of immunoglobulinsScience197318040877137164122075
- KohlerGMilsteinCContinuous cultures of fused cells secreting antibody of predefined specificityNature197525655174954971172191
- ChungCHMirakhurBChanECetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactoseN Engl J Med2008358111109111718337601
- ArnoldJNWormaldMRSimRBRuddPMDwekRAThe impact of glycosylation on the biological function and structure of human immunoglobulinsAnnu Rev Immunol200725215017029568
- JefferisRGlycosylation as a strategy to improve antibody-based therapeuticsNat Rev Drug Discov20098322623419247305
- JefferisRLundJGoodallMRecognition sites on human IgG for Fc gamma receptors: the role of glycosylationImmunol Lett1995442–31111177797239
- ReuschDTejadaMLFc glycans of therapeutic antibodies as critical quality attributesGlycobiology201525121325133426263923
- KrappSMimuraYJefferisRHuberRSondermannPStructural analysis of human IgG-Fc glycoforms reveals a correlation between glycosylation and structural integrityJ Mol Biol2003325597998912527303
- KanekoYNimmerjahnFRavetchJVAnti-inflammatory activity of immunoglobulin G resulting from Fc sialylationScience2006313578767067316888140
- OkazakiAShoji-HosakaENakamuraKFucose depletion from human IgG1 oligosaccharide enhances binding enthalpy and association rate between IgG1 and FcgammaRIIIaJ Mol Biol200433651239124915037082
- IidaSMisakaHInoueMNonfucosylated therapeutic IgG1 antibody can evade the inhibitory effect of serum immunoglobulin G on antibody-dependent cellular cytotoxicity through its high binding to FcgammaRIIIaClin Cancer Res20061292879288716675584
- SatohMIidaSShitaraKNon-fucosylated therapeutic antibodies as next-generation therapeutic antibodiesExpert Opin Biol Ther20066111161117317049014
- ShieldsRLLaiJKeckRLack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicityJ Biol Chem200227730267332674011986321
- NiwaRNatsumeAUeharaAIgG subclass-independent improvement of antibody-dependent cellular cytotoxicity by fucose removal from Asn297-linked oligosaccharidesJ Immunol Methods20053061–215116016219319
- NatsumeAWakitaniMYamane-OhnukiNFucose removal from complex-type oligosaccharide enhances the antibody-dependent cellular cytotoxicity of single-gene-encoded bispecific antibody comprising of two single-chain antibodies linked to the antibody constant regionJ Biochem2006140335936816861252
- WalshGBiopharmaceutical benchmarks 2014Nat Biotechnol20143210992100025299917
- EckerDMJonesSDLevineHLThe therapeutic monoclonal antibody marketMAbs20157191425529996
- SondermannPPinceticAMaamaryJLammensKRavetchJVGeneral mechanism for modulating immunoglobulin effector functionProc Natl Acad Sci U S A2013110249868987223697368
- CrispinMYuXBowdenTACrystal structure of sialylated IgG Fc: implications for the mechanism of intravenous immunoglobulin therapyProc Natl Acad Sci U S A201311038E3544E354623929778
- LiuLAntibody glycosylation and its impact on the pharmacokinetics and pharmacodynamics of monoclonal antibodies and Fc-fusion proteinsJ Pharm Sci201510461866188425872915
- LiuLGomathinayagamSHamuroLThe impact of glycosylation on the pharmacokinetics of a TNFR2:Fc fusion protein expressed in glycoengineered Pichia pastorisPharm Res201330380381223135825
- AnthonyRMNimmerjahnFAshlineDJReinholdVNPaulsonJCRavetchJVRecapitulation of IVIG anti-inflammatory activity with a recombinant IgG FcScience2008320587437337618420934
- KandaYYamadaTMoriKComparison of biological activity among nonfucosylated therapeutic IgG1 antibodies with three different N-linked Fc oligosaccharides: the high-mannose, hybrid, and complex typesGlycobiology200717110411817012310
- GoetzeAMLiuYDZhangZHigh-mannose glycans on the Fc region of therapeutic IgG antibodies increase serum clearance in humansGlycobiology201121794995921421994
- DaeronMFc receptor biologyAnnu Rev Immunol1997152032349143687
- BruhnsPIannascoliBEnglandPSpecificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclassesBlood2009113163716372519018092
- PowellMSHogarthPMFc receptorsAdv Exp Med Biol2008640223419065781
- RavetchJVBollandSIgG Fc receptorsAnnu Rev Immunol20011927529011244038
- SondermannPKaiserJJacobUMolecular basis for immune complex recognition: a comparison of Fc-receptor structuresJ Mol Biol2001309373774911397093
- HulettMDHogarthPMMolecular basis of Fc receptor functionAdv Immunol19945711277872156
- NimmerjahnFRavetchJVFcgamma receptors as regulators of immune responsesNat Rev Immunol200881344718064051
- KoeneHRKleijerMAlgraJRoosDvon dem BorneAEde HaasMFc gammaRIIIa-158V/F polymorphism influences the binding of IgG by natural killer cell Fc gammaRIIIa, independently of the Fc gammaRIIIa-48L/R/H phenotypeBlood1997903110911149242542
- WuJEdbergJCRedechaPBA novel polymorphism of FcgammaRIIIa (CD16) alters receptor function and predisposes to autoimmune diseaseJ Clin Invest19971005105910709276722
- NietoACalizRPascualMMataranLGarciaSMartinJInvolvement of Fcgamma receptor IIIA genotypes in susceptibility to rheumatoid arthritisArthritis Rheum200043473573910765917
- DuitsAJBootsmaHDerksenRHSkewed distribution of IgG Fc receptor IIa (CD32) polymorphism is associated with renal disease in systemic lupus erythematosus patientsArthritis Rheum19953812183218368849356
- HayesJMCosgraveEFStruweWBGlycosylation and Fc receptorsCurr Top Microbiol Immunol201438216519925116100
- FerraraCGrauSJagerCUnique carbohydrate-carbohydrate interactions are required for high affinity binding between FcgammaRIII and antibodies lacking core fucoseProc Natl Acad Sci U S A201110831126691267421768335
- FerraraCStuartFSondermannPBrunkerPUmanaPThe carbohydrate at FcgammaRIIIa Asn-162. An element required for high affinity binding to non-fucosylated IgG glycoformsJ Biol Chem200628185032503616330541
- Shibata-KoyamaMIidaSOkazakiAThe N-linked oligosaccharide at Fc gamma RIIIa Asn-45: an inhibitory element for high Fc gamma RIIIa binding affinity to IgG glycoforms lacking core fucosylationGlycobiology200919212613418952826
- HayesJMFrostellACosgraveEFFc gamma receptor glycosylation modulates the binding of IgG glycoforms: a requirement for stable antibody interactionsJ Proteome Res201413125471548525345863
- EdbergJCBarinskyMRedechaPBSalmonJEKimberlyRPFc gamma RIII expressed on cultured monocytes is a N-glycosylated transmembrane protein distinct from Fc gamma RIII expressed on natural killer cellsJ Immunol199014412472947342141043
- EdbergJCKimberlyRPCell type-specific glycoforms of Fc gamma RIIIa (CD16): differential ligand bindingJ Immunol19971598384938579378972
- KimberlyRPTappeNJMerriamLTCarbohydrates on human Fc gamma receptors. Interdependence of the classical IgG and nonclassical lectin-binding sites on human Fc gamma RIII expressed on neutrophilsJ Immunol198914211392339302523939
- ZeckAPohlentzGSchlothauerTPeter-KatalinicJRegulaJTCell type-specific and site directed N-glycosylation pattern of FcgammaRIIIaJ Proteome Res20111073031303921561106
- SondermannPHuberROosthuizenVJacobUThe 3.2-A crystal structure of the human IgG1 Fc fragment-Fc gammaRIII complexNature2000406679326727310917521
- RadaevSMotykaSFridmanWHSautes-FridmanCSunPDThe structure of a human type III Fcgamma receptor in complex with FcJ Biol Chem200127619164691647711297532
- CosgraveEFStruweWBHayesJMHarveyDJWormaldMRRuddPMN-linked glycan structures of the human Fcgamma receptors produced in NS0 cellsJ Proteome Res20131283721373723777450
- SondermannPJacobUKutscherCFreyJCharacterization and crystallization of soluble human Fc gamma receptor II (CD32) isoforms produced in insect cellsBiochemistry199938268469847710387093
- PowellMSBartonPAEmmanouilidisDBiochemical analysis and crystallisation of Fc gamma RIIa, the low affinity receptor for IgGImmunol Lett1999681172310397151
- TakahashiNCohen-SolalJGalinhaAFridmanWHSautes-FridmanCKatoKN-glycosylation profile of recombinant human soluble Fcgamma receptor IIIGlycobiology200212850751512145191
- TakahashiNYamadaWMasudaKN-glycan structures of a recombinant mouse soluble Fcgamma receptor IIGlycoconj J199815990591410052594
- GalonJRobertsonMWGalinhaAAffinity of the interaction between Fc gamma receptor type III (Fc gammaRIII) and monomeric human IgG subclasses. Role of Fc gammaRIII glycosylationEur J Immunol1997278192819329295028
- AllenJMSeedBIsolation and expression of functional high-affinity Fc receptor complementary DNAsScience198924348893783812911749
- HarrisonPTAllenJMHigh affinity IgG binding by FcgammaRI (CD64) is modulated by two distinct IgSF domains and the transmembrane domain of the receptorProtein Eng19981132252329613847
- LuJChuJZouZHamacherNBRixonMWSunPDStructure of FcgammaRI in complex with Fc reveals the importance of glycan recognition for high-affinity IgG bindingProc Natl Acad Sci U S A2015112383383825561553
- LuJEllsworthJLHamacherNOakSWSunPDCrystal structure of Fcgamma receptor I and its implication in high affinity gamma-immunoglobulin bindingJ Biol Chem201128647406084061321965667
- JungSTReddySTKangTHAglycosylated IgG variants expressed in bacteria that selectively bind FcgammaRI potentiate tumor cell killing by monocyte-dendritic cellsProc Natl Acad Sci U S A2010107260460920080725
- KrambeckFJBetenbaughMJA mathematical model of N-linked glycosylationBiotechnol Bioeng200592671172816247773
- UmanaPBaileyJEA mathematical model of N-linked glycoform biosynthesisBiotechnol Bioeng199755689090818636599
- McDonaldAGHayesJMBezakTGalactosyltransferase 4 is a major control point for glycan branching in N-linked glycosylationJ Cell Sci2014127pt 235014502625271059
- McDonaldAGTiptonKFDaveyGPA knowledge-based system for display and prediction of O-glycosylation network behaviour in response to enzyme knockoutsPLoS Comput Biol2016124e100484427054587
- McDonaldAGHayesJMDaveyGPMetabolic flux control in glycosylationCurr Opin Struct Biol2016409710327620650
- WengWKLevyRTwo immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphomaJ Clin Oncol200321213940394712975461
- CartronGDacheuxLSallesGTherapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa geneBlood200299375475811806974
- CassardLCohen-SolalJFFournierEMSelective expression of inhibitory Fcgamma receptor by metastatic melanoma impairs tumor susceptibility to IgG-dependent cellular responseInt J Cancer2008123122832283918798552
- CassardLCohen-SolalJFGalinhaAModulation of tumor growth by inhibitory Fc(gamma) receptor expressed by human melanoma cellsJ Clin Invest2002110101549155712438452
- CallananMBLe BacconPMossuzPThe IgG Fc receptor, FcgammaRIIB, is a target for deregulation by chromosomal translocation in malignant lymphomaProc Natl Acad Sci U S A200097130931410618414
- Camilleri-BroetSCassardLBroetPFcgammaRIIB is differentially expressed during B cell maturation and in B-cell lymphomasBr J Haematol20041241556214675408
- TackenbergBJelcicIBaerenwaldtAImpaired inhibitory Fcgamma receptor IIB expression on B cells in chronic inflammatory demyelinating polyneuropathyProc Natl Acad Sci U S A2009106124788479219261857
- LiYLeePYSobelESIncreased expression of FcgammaRI/CD64 on circulating monocytes parallels ongoing inflammation and nephritis in lupusArthritis Res Ther2009111R619144150
- TillingerWJilchRJilmaBExpression of the high-affinity IgG receptor FcRI (CD64) in patients with inflammatory bowel disease: a new biomarker for gastroenterologic diagnosticsAm J Gastroenterol2009104110210919098857
- BelostockiKParkMSRedechaPBMasudaESalmonJEPricopLFcgammaRIIa is a target for modulation by TNFalpha in human neutrophilsClin Immunol20051171788616084773
- LunnonKTeelingJLTuttALCraggMSGlennieMJPerryVHSystemic inflammation modulates Fc receptor expression on microglia during chronic neurodegenerationJ Immunol2011186127215722421572034
- DugastASTonelliABergerCTDecreased Fc receptor expression on innate immune cells is associated with impaired antibody-mediated cellular phagocytic activity in chronically HIV-1 infected individualsVirology2011415216016721565376
- CapsoniFMinonzioFOngariAMFc receptors expression and function in mononuclear phagocytes from AIDS patients: modulation by IFN-gammaScand J Immunol199439145508290892
- de HaasMKleijerMMinchintonRMRoosDvon dem BorneAESoluble Fc gamma RIIIa is present in plasma and is derived from natural killer cellsJ Immunol199415229009078283059
- WerwitzkeSTrickDSondermannPTreatment of lupus-prone NZB/NZW F1 mice with recombinant soluble Fc gamma receptor II (CD32)Ann Rheum Dis200867215416117557887
- EllsworthJLHamacherNHarderBRecombinant soluble human FcgammaR1A (CD64A) reduces inflammation in murine collagen-induced arthritisJ Immunol2009182117272727919454724
- EllsworthJLMaurerMHarderBTargeting immune complex-mediated hypersensitivity with recombinant soluble human FcgammaRIA (CD64A)J Immunol2008180158058918097060
- MagnussonSEAndrenMNilssonKESondermannPJacobUKleinauSAmelioration of collagen-induced arthritis by human recombinant soluble FcgammaRIIbClin Immunol2008127222523318346938
- IwataHPipiEMockelNRecombinant soluble CD32 suppresses disease progression in experimental epidermolysis bullosa acquisitaJ Invest Dermatol2015135391691925330298
- NimmerjahnFTranslating inhibitory Fc receptor biology into novel therapeutic approachesJ Clin Immunol201636suppl 1838726957094
- CampbellMPRoyleLRadcliffeCMDwekRARuddPMGlycoBase and autoGU: tools for HPLC-based glycan analysisBioinformatics20082491214121618344517
