Abstract
Clostridioides difficile infection (CDI) has a serious impact on the healthcare system, and most of its pathogenic effects are mainly due to the activity of toxins A and B (TcdA and TcdB, respectively). The molecular mechanisms of their cytotoxic activity are well known, especially in the colon, where the infection occurs and normally remains localized. However, the mechanisms causing toxic effects on various systemic organs (extraintestinal manifestations) with frequent lethal outcomes in some patients affected by CDI are still poorly understood. Few studies are available that demonstrate low serum levels of Tcds in both experimental animal models and patients with CDI. Until now, it has remained unclear how low levels of circulating Tcds could lead to serious toxic effects. On the basis of our previous in vitro studies, in which the proinflammatory cytokines TNF-alpha and IFN-gamma strongly potentiated the toxic activity of low doses of TcdB, we hypothesize that the presence of both TcdB in the circulation and a systemic proinflammatory cytokine storm may be responsible for the selective severe effects of TcdB in some patients. This may occur in patients with severe CDI and systemic Tcds, in whom proinflammatory cytokines such as TNF-alpha and IFN-gamma reach a significant concentration in the circulation. This hypothesis could identify therapeutic interventions based on the reduction or neutralization of the indirect toxic action of these cytokines.
Introduction
ClostridioidesCitation1 difficile infection (CDI) represents an important burden on the healthcare systems of both Eastern and Western populationsCitation2,Citation3 as a major cause of iatrogenic diarrhoea, and it is increasingly present within the community.Citation4 The clinical picture of CDI is mutable and mainly involves the gastrointestinal tract, varying from asymptomatic carrier status to life-threatening diarrhoea.Citation5 However, the effects of CDI may sometimes become systemic with extraintestinal manifestations (liver, lung, kidney, cardiac, and neurological impairment) and multiorgan dysfunction syndrome with a clinical picture of toxaemia.Citation6–Citation15 Of interest, recurrent extraintestinal manifestations are also correlated with CDI relapse.Citation16
It is worth noting that to date, the mechanisms responsible for the systemic effects of CDI are poorly known, and data on this topic are relatively scarce. Here, we hypothesize that these effects may be due to a “systemic proinflammatory cytokine storm” occurring during CDI, which enhances the toxicity of C. difficile toxins (Tcds) once they reach the systemic circulation.
Pathophysiological Aspects of CDI
The pathological effects of C. difficile are mainly due to the production of two large glucosylating toxins, toxin A (TcdA) and toxin B (TcdB).Citation17–Citation20 TcdA and TcdB inactivate Rho-GTPases by monoglucosylation, with the following effects:
loss of the cytoskeletal structure, disassembly of focal adhesions and disruption of tight junctionsCitation17–Citation21 (in cultured cells, these effects cause cell rounding (cytopathic effect);Citation17–Citation20
arrest of the cell cycle, reduced expression of cyclins and activation of cyclin-dependent kinases involved in cell cycle phase progression;Citation17–Citation20,Citation22
cell death by apoptosis or necrosis (cytotoxic effect).Citation17–Citation20,Citation22
In vivo, TcdA and TcdB disrupt epithelial tight junctions and induce cell death, causing direct injury to the colonic epithelium. Furthermore, Tcds stimulate colonic epithelial cells to release proinflammatory cytokines and neutrophil chemoattractants, which in turn lead to an acute inflammatory response, a key characteristic of the clinical picture of CDI.Citation5,Citation17–Citation21 An impaired barrier within the context of active inflammation subsequently leads to enhanced intestinal and vascular permeability. Thus, the loss of a protective barrier favours the entry of Tcds and/or bacteria into the lamina propria, resulting in increased intestinal inflammation.Citation17–Citation21 The deepening of the lesion at the level of the submucosa could therefore favour the passage into the systemic circulation of Tcds.
TcdA primarily affects the intestinal epithelium, while TcdB has a broader cell tropism and represents the main virulence factor of C. difficile.Citation19 There is evidence suggesting that Tcds can reach the circulation during CDI, causing systemic effects.Citation6–Citation15 In experimental animal models (mouse and guinea pig), the presence of circulating and quantifiable amounts of Tcds has been related to the systemic effects of CDI and found to be associated with fatal diseases.Citation10–Citation13,Citation23 The presence of circulating Tcds has also been demonstrated in patients with CDI.Citation6,Citation8,Citation24
Of the two Tcds produced by C. difficile, TcdB is probably mainly responsible for the systemic effectsCitation12,Citation13,Citation23 due to its toxicity, which is approximately 1000 times higher than that of TcdA.Citation17–Citation21 The mechanism by which Tcds reach the systemic circulation in some individuals with CDI is likely due to the important tissue damage that profoundly alters the barrier effect of the intestinal mucosa.Citation10–Citation13,Citation25,Citation26 Thus, when the two Tcds enter the circulation, cells of various organs may be damaged. In fact, in vitro cytotoxic studies have demonstrated that apart from causing the death of epithelial cells and colonic myofibroblasts,Citation17–Citation22,Citation27–Citation29 these Tcds target hepatocytes,Citation30 cardiomyocytes,Citation31 lung fibroblasts,Citation32 immunocytes,Citation17–Citation22,Citation33 enteric neurons,Citation17–Citation22,Citation34–Citation36 and enteric glial cells.Citation22,Citation37–Citation39
In cell culture models, death induced by TcdA and TcdB occurs in a glucosylation-dependent or glucosylation-independent manner, mainly by apoptosis with caspase-dependent or caspase-independent mechanisms.Citation17–Citation20,Citation22,Citation39 Apoptosis is induced by activation of the executioner caspases-3 and −7, which can occur via a death receptor-dependent extrinsic or by a mitochondria-dependent intrinsic pathway.Citation17–Citation20,Citation40,Citation41 Apoptosis can also be activated in a caspase-independent manner by the cleavage and activation of pro-apoptotic Bcl-2 family proteins and non-caspase proteases such as cathepsins and calpains.Citation17–Citation20,Citation42,Citation43
Cytokines: Possible Accomplices of Systemic Effects During CDI
The inflammatory response to CDI is characterized by increased local and systemic levels of cytokines,Citation17–Citation21,Citation44–Citation48 some of which are associated with disease severity and prognosis.Citation46–Citation48 However, it is important to understand the relationship between circulating Tcds and proinflammatory cytokines in CDI.
Human studies have shown that increased serum concentrations of tumour necrosis factor-alpha (TNF-α) are associated with poor prognosis in patients with CDI,Citation48 and in experimental animal models, substances able to decrease proinflammatory cytokine levels exert a protective effect against CDI.Citation49 However, the production of proinflammatory cytokines during severe CDI cannot be the sole cause of the relevant systemic effects found in only some patients, since their production is high in all subjects with severe CDI.Citation45
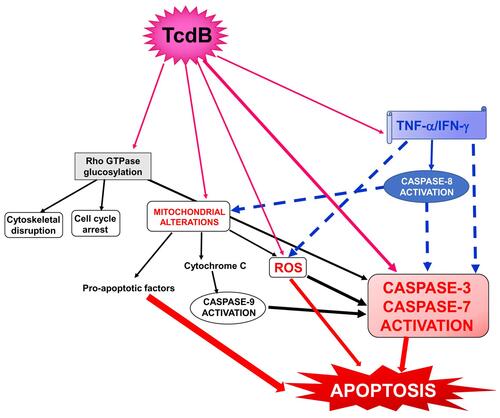
Susceptibility to the toxic action of Tcds varies between different cell types, and the systemic concentrations of Tcds can be so low as to cause only marginal toxic effects;Citation3,Citation10–Citation13,Citation22,Citation50 thus, we hypothesized that proinflammatory cytokines may strengthen the toxic effects of low doses of Tcds. This hypothesis stems from our recent in vitro studies in which TNF-α and interferon-gamma (IFN-γ) given before, concomitantly, or after low doses of TcdB (0.1 ng/mL or 1 ng/mL) strongly enhanced the apoptosis induced by TcdB in enteric glial cells.Citation22,Citation50 Apoptosis was characterized by an increase in caspase-3, caspase-7, caspase-9 and PARP activation without any change in the expression of Bcl-2 family members ().Citation22
Figure 1 Schematic of the possible signalling pathways involved in the cytotoxic synergism between TcdB and the proinflammatory cytokines TNF-α and IFN-γ in the induction of apoptosis. Full arrows indicate activation; dotted arrows indicate activation enhancement.
Discussion
According to our hypothesis, two conditions must exist to have toxic systemic effects during CDI:
The production of high levels of proinflammatory cytokines is necessary.Citation17–Citation21,Citation44–Citation48 In patients with severe CDI, the systemic inflammatory response (as documented in both patients and experimental animal models)Citation6,Citation10–Citation13,Citation48,Citation51 is due to not only local tissue damage but also the inflammatory action of the Tcds,Citation17–Citation21,Citation44–Citation48,Citation52 the structural and metabolic components released by C. difficile itself, and the modification of the intestinal microbial flora following C. difficile-specific therapy;Citation5,Citation23–Citation26,Citation53–Citation56
Tcds have to reach the systemic circulation (a characteristic limited to only some patients with CDI).Citation6–Citation13
In these conditions, the interaction between TcdB, TNF-α and IFN-γ can trigger an increase in the toxicity of TcdB and its systemic pathological effects (). Of interest, this enhanced toxicity in vivo might occur at extremely low concentrations of Tcds,Citation10 ones comparable to those that synergize in vitro with inflammatory cytokines to induce strong cell death.Citation22 Since some evidence suggest that TcdA can also reach the circulation in patients with severe CDI and cause extraintestinal manifestations,Citation6–Citation16 it is of great interest to know if proinflammatory cytokines can increase its toxicity as it happens for TcdB and if the two effects can further amplify the systemic effects of CDI.
Figure 2 Proposed mechanism by which C. difficile toxins, in particular TcdB, enter the circulation and cause toxic effects enhanced by the systemic proinflammatory cytokines TNF-α and IFN-γ on various systemic organs in severe CDI.
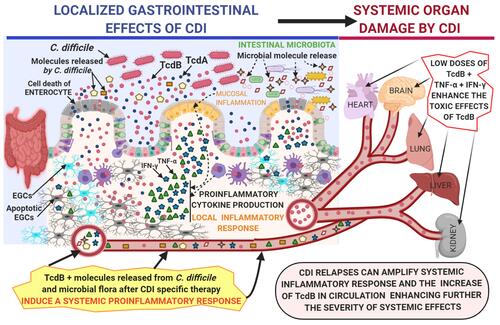
In conclusion, one of the pivotal mechanisms underlying the systemic effects of CDI could be the pathological alliance between C. difficile and proinflammatory cytokines.Citation50 This relationship could promote investigations aimed at antagonizing this mechanism by the use of selective drugs targeting proinflammatory cytokines.
Conclusions
In conclusion, here, we hypothesize that when TcdB enters the systemic circulation of patients affected by severe CDI, there can be a strong enhancement of its toxic action on various organs due to the presence of a systemic cytokine storm, in which proinflammatory cytokines such as TNF-α and IFN-γ reach a significant concentration in the circulation. This hypothesis could highlight new therapeutic interventions based on the reduction or neutralization of the indirect toxic action of these cytokines.
Abbreviations
CDI, Clostridioides difficile infection; Tcds, C. difficile toxins; TcdA, C. difficile toxin A; TcdB, C. difficile toxin B; TNF-α, tumour necrosis factor-alpha; IFN-γ, interferon-gamma.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest for this work.
Additional information
Funding
References
- Lawson PA, Citron DM, Tyrrell KL, Finegold SM. Reclassification of Clostridium difficile as Clostridioides difficile (Hall and O’Toole 1935) prévot 1938. Anaerobe. 2016;40:95–99. doi:10.1016/j.anaerobe.2016.06.008
- Malekzadegan Y, Halaji M, Hasannejad-Bibalan M, Jalalifar S, Fathi J, Ebrahim-Saraie HS. Burden of Clostridium (clostridioides) difficile infection among patients in western Asia: a systematic review and meta-analysis. Iran J Public Health. 2019;48(9):1589–1599.
- Marra AR, Perencevich EN, Nelson RE, et al. Incidence and outcomes associated with Clostridium difficile infections. JAMA Netw Open. 2020;3(1):e1917597. doi:10.1001/jamanetworkopen.2019.17597
- Guery B, Galperine T, Barbut F. Clostridioides difficile: diagnosis and treatments. BMJ. 2019;366:l4609. doi:10.1136/bmj.l4609
- Czepiel J, Dróżdż M, Pituch H, et al. Clostridium difficile infection: review. Eur J Clin Microbiol Infect Dis. 2019;38:1211–1221.
- Qualman SJ, Petric M, Karmali MA, Smith CR, Hamilton SR. Clostridium difficile invasion and toxin circulation in fatal pediatric pseudomembranous colitis. Am J Clin Pathol. 1990;94(4):410–416. doi:10.1093/ajcp/94.4.410
- Sensini A, Marroni M, Bassotti G, et al. Clostridium difficile-associated reactive arthritis in an HLA-B27 negative male. J Clin Gastroenterol. 1993;16(4):354–355. doi:10.1097/00004836-199306000-00020
- Jacobs A, Barnard K, Fishel R, Gradon JD. Extracolonic manifestations of Clostridium difficile infections. Presentation of 2 cases and review of the literature. Medicine (Baltimore). 2001;80(2):88–101. doi:10.1097/00005792-200103000-00002
- Dobson G, Hickey C, Trinder J. Clostridium difficile colitis causing toxic megacolon, severe sepsis and multiple organ dysfunction syndrome. Intensive Care Med. 2003;29(6):1030. doi:10.1007/s00134-003-1754-7
- Steele J, Chen K, Sun X, et al. Systemic dissemination of Clostridium difficile toxins A and B is associated with severe, fatal disease in animal models. J Infect Dis. 2012;205(3):384–391. doi:10.1093/infdis/jir748
- Gupta A, Patel R, Baddour LM, Pardi DS, Khanna S. Extraintestinal Clostridium difficile infections: a single-center experience. Mayo Clin Proc. 2014;89(11):1525–1536. doi:10.1016/j.mayocp.2014.07.012
- Carter GP, Chakravorty A, Pham Nguyen TA, et al. Defining the roles of TcdA and TcdB in localized gastrointestinal disease, systemic organ damage, and the host response during Clostridium difficile infections. mBio. 2015;6(3):e00551. doi:10.1128/mBio.00551-15
- Di Bella S, Ascenzi P, Siarakas S, Petrosillo N, Di Masi A. Clostridium difficile toxins A and B: insights into pathogenic properties and extraintestinal effects. Toxins (Basel). 2016;8(5):E134. doi:10.3390/toxins8050134
- Cimolai N. Are Clostridium difficile toxins nephrotoxic? Med Hypotheses. 2019;126:4–8. doi:10.1016/j.mehy.2019.03.002
- Yu H, Chen K, Wu J, et al. Identification of toxemia in patients with Clostridium difficile infection. PLoS One. 2015;10(4):e0124235. doi:10.1371/journal.pone.0124235
- Gardiner BJ, Thorpe CM, Pinkham NV, McDermott LA, Walk ST, Snydman DR. A repeat offender: recurrent extraintestinal Clostridium difficile infection following fecal microbiota transplantation. Anaerobe. 2018;51:68–72. doi:10.1016/j.anaerobe.2018.04.007
- Sun X, Savidge T, Feng H. The enterotoxicity of Clostridium difficile toxins. Toxins (Basel). 2010;2(7):1848–1880. doi:10.3390/toxins2071848
- Pruitt RN, Lacy DB. Toward a structural understanding of Clostridium difficile toxins A and B. Front Cell Infect Microbiol. 2012;2:28. doi:10.3389/fcimb.2012.00028
- Chandrasekaran R, Lacy DB. The role of toxins in Clostridium difficile infection. FEMS Microbiol Rev. 2017;41:723–750.
- Aktories K, Schwan C, Jank T. Clostridium difficile toxin biology. Annu Rev Microbiol. 2017;71(1):281–307. doi:10.1146/annurev-micro-090816-093458
- Voth DE, Ballard JD. Clostridium difficile toxins: mechanism of action and role in disease. Clin Microbiol Rev. 2005;18(2):247–263. doi:10.1128/CMR.18.2.247-263.2005
- Fettucciari K, Ponsini P, Gioè D, et al. Enteric glial cells are susceptible to Clostridium difficile toxin B. Cell Mol Life Sci. 2017;74(8):1527–1551. doi:10.1007/s00018-016-2426-4
- Awad MM, Johanesen PA, Carter GP, Rose E, Lyras D. Clostridium difficile virulence factors: insights into an anaerobic spore-forming pathogen. Gut Microbes. 2014;5(5):579–593. doi:10.4161/19490976.2014.969632
- Lee NY, Huang YT, Hsueh PR, Ko WC. Clostridium difficile bacteremia, Taiwan1. Emerg Infect Dis. 2010;16(8):1204–1210. doi:10.3201/eid1608.100064
- Smits WK, Lyras D, Lacy DB, Wilcox MH, Kuijper EJ. Clostridium difficile infection. Nat Rev Dis Primers. 2016;2:16020.
- Dicks LMT, Mikkelsen LS, Brandsborg E, Marcotte H. Clostridium difficile, the difficult “Kloster” fuelled by antibiotics. Curr Microbiol. 2019;76(6):774–782. doi:10.1007/s00284-018-1543-8
- Jafari NV, Allan E, Bajaj-Elliott M. Human intestinal epithelial response(s) to Clostridium difficile. Methods Mol Biol. 2010;646:135–146.
- Mullan N, Hughes KR, Mahida YR. Primary human colonic myofibroblasts are resistant to Clostridium difficile toxin A-induced, but not toxin b-induced, cell death. Infect Immun. 2011;79(4):1623–1630. doi:10.1128/IAI.00686-10
- Brito GA, Fujji J, Carneiro-Filho BA, Lima AA, Obrig T, Guerrant RL. Mechanism of Clostridium difficile toxin A–induced apoptosis in T84 cells. J Infect Dis. 2002;186(10):1438–1447. doi:10.1086/344729
- Grossmann EM, Longo WE, Kaminski DL, et al. Clostridium difficile toxin: cytoskeletal changes and lactate dehydrogenase release in hepatocytes. J Surg Res. 2000;88(2):165–172. doi:10.1006/jsre.1999.5736
- Krijnen PA, Sipkens JA, Molling JW, et al. Inhibition of Rho-ROCK signaling induces apoptotic and non-apoptotic PS exposure in cardiomyocytes via inhibition of flippase. J Mol Cell Cardiol. 2010;49(5):781–790. doi:10.1016/j.yjmcc.2010.07.017
- Florin I. Isolation of a fibroblast mutant resistant to Clostridium difficile toxins A and B. Microb Pathog. 1991;11(5):337–346. doi:10.1016/0882-4010(91)90019-7
- Solomon K, Webb J, Ali N, Robins RA, Mahida YR. Monocytes are highly sensitive to clostridium difficile toxin A-induced apoptotic and nonapoptotic cell death. Infect Immun. 2005;73(3):1625–1634. doi:10.1128/IAI.73.3.1625-1634.2005
- Xia Y, Hu HZ, Liu S, Pothoulakis C, Wood JD. Clostridium difficile toxin A excites enteric neurones and suppresses sympathetic neurotransmission in the guinea pig. Gut. 2000;46(4):481–486. doi:10.1136/gut.46.4.481
- Farthing MJ. Enterotoxins and the enteric nervous system-a fatal attraction. Int J Med Microbiol. 2000;290(4–5):491–496. doi:10.1016/S1438-4221(00)80073-9
- Neunlist M, Barouk J, Michel K, et al. Toxin B of Clostridium difficile activates human VIP submucosal neurons, in part via an IL-1β-dependent pathway. Am J Physiol Gastrointest Liver Physiol. 2003;285(5):G1049–G1055. doi:10.1152/ajpgi.00487.2002
- Bassotti G, Macchioni L, Corazzi L, Marconi P, Fettucciari K. Clostridium difficile-related postinfectious IBS: a case of enteroglial microbiological stalking and/or the solution of a conundrum? Cell Mol Life Sci. 2018;75(7):1145–1149. doi:10.1007/s00018-017-2736-1
- Fettucciari K, Macchioni L, Davidescu M, et al. Clostridium difficile toxin B induces senescence in enteric glial cells: a potential new mechanism of Clostridium difficile pathogenesis. Biochim Biophys Acta Mol Cell Res. 2018;1865(12):1945–1958. doi:10.1016/j.bbamcr.2018.10.007
- Macchioni L, Davidescu M, Fettucciari K, et al. Enteric glial cells counteract Clostridium difficile Toxin B through a NADPH oxidase/ROS/JNK/caspase-3 axis, without involving mitochondrial pathways. Sci Rep. 2017;7(1):45569. doi:10.1038/srep45569
- Strasser A, O’Connor L, Dixit VM. Apoptosis signaling. Annu Rev Biochem. 2000;69(1):217–245. doi:10.1146/annurev.biochem.69.1.217
- Kaufmann SH, Hengartner MO. Programmed cell death: alive and well in the new millennium. Trends Cell Biol. 2001;11(12):526–534. doi:10.1016/S0962-8924(01)02173-0
- Mathiasen IS, Jaattela M. Triggering caspase-independent cell death to combat cancer. Trends Mol Med. 2002;8(5):212–220. doi:10.1016/S1471-4914(02)02328-6
- Jaattela M, Tschopp J. Caspase-independent cell death in T lymphocytes. Nat Immunol. 2003;4(5):416–423. doi:10.1038/ni0503-416
- Johal SS, Solomon K, Dodson S, Borriello SP, Mahida YR. Differential effects of varying concentrations of Clostridium difficile toxin a on epithelial barrier function and expression of cytokines. J Infect Dis. 2004;189(11):2110–2119. doi:10.1086/386287
- El Feghaly RE, Bangar H, Haslam DB. The molecular basis of Clostridium difficile disease and host response. Curr Opin Gastroenterol. 2015;31(1):24–29. doi:10.1097/MOG.0000000000000131
- Solomon K, Martin AJ, O’Donoghue C, et al. Mortality in patients with Clostridium difficile infection correlates with host pro-inflammatory and humoral immune responses. J Med Microbiol. 2013;62(9):1453–1460. doi:10.1099/jmm.0.058479-0
- Yu H, Chen K, Sun Y, et al. Cytokines are markers of the Clostridium difficile-induced inflammatory response and predict disease severity. Clin Vaccine Immunol. 2017;24(8):e00037–17. doi:10.1128/CVI.00037-17
- Czepiel J, Biesiada G, Brzozowski T, et al. The role of local and systemic cytokines in patients infected with Clostridium difficile. J Physiol Pharmacol. 2014;65(5):695–703.
- Fachi JL, Felipe JS, Pral LP, et al. Butyrate protects mice from Clostridium difficile-induced colitis through an HIF-1-dependent mechanism. Cell Rep. 2019;27:750–761.e7.
- Bassotti G, Marchegiani A, Marconi P, Fettucciari K. The cytotoxic synergy between Clostridioides difficile toxin B and proinflammatory cytokines: an unholy alliance favouring the onset of Clostridioides difficile infection and relapses. MicrobiologyOpen. 2020;8:e1061.
- El Feghaly RE, Stauber JL, Deych E, Gonzalez C, Tarr PI, Haslam DB. Markers of intestinal inflammation, not bacterial burden, correlate with clinical outcomes in Clostridium difficile infection. Clin Infect Dis. 2013;56(12):1713–1721. doi:10.1093/cid/cit147
- Li Y, Xu S, Xu Q, Chen Y. Clostridium difficile toxin B induces colonic inflammation through the TRIM46/DUSP1/MAPKs and NF-κB signalling pathway. Artif Cells Nanomed Biotechnol. 2020;48(1):452–462. doi:10.1080/21691401.2019.1709856
- Sun X, Hirota SA. The roles of host and pathogen factors and the innate immune response in the pathogenesis of Clostridium difficile infection. Mol Immunol. 2015;63(2):193–202. doi:10.1016/j.molimm.2014.09.005
- He J, Lange J, Marinos G, et al. Advancing our functional understanding of host-microbiota interactions: a need for new types of studies. Bioessays. 2020;42:e1900211.
- Oliva A, Aversano L, De Angelis M, et al. Persistent systemic microbial translocation, inflammation, and intestinal damage during Clostridioides difficile infection. Open Forum Infect Dis. 2019;7(1):ofz507. doi:10.1093/ofid/ofz507
- Pirofski LA, Casadevall A. Antimicrobial therapy in the context of the damage-response framework: the prospect of optimizing therapy by reducing host damage. Antimicrob Agents Chemother. 2020;64(2):e01800–19. doi:10.1128/AAC.01800-19
