Abstract
Background:
Most islet transplant groups worldwide routinely use the TNFα inhibitor Etanercept in their peri-transplant protocols. Surprisingly, there have been no published dose-response studies on the effects of Etanercept on human islets. Our study aimed to address this by treating cultured human islets with increasing concentrations of Etanercept.
Materials and Methods:
Isolated human islets were cultured for 3–4 days in normoxic (21% oxygen) or in hypoxic (2% oxygen) atmosphere using Etanercept dissolved in a range of 2.5–40 µg/mL prior to islet characterisation.
Results:
In normoxic atmosphere, it was found that 5 µg/mL is the most efficient dose to preserve islet morphological and functional integrity during culture. Increasing the dose to 10 µg/mL or more resulted in detrimental effects with respect to viability and glucose-stimulated insulin release. When human islets were cultured for 3 to 4 days in clinically relevant hypoxia and treated with 5 µg/mL Etanercept, post-culture islet survival (P < 0.001) and in vitro function (P < 0.01) were significantly improved. This correlated with a substantially reduced cytokine production (P < 0.05), improved mitochondrial function (P < 0.01), and reduced production of reactive oxygen species (P < 0.001) in hypoxia-exposed islets.
Conclusion:
These findings suggest that the therapeutic window of Etanercept is very narrow and that this should be considered when optimising the dosage and route of Etanercept administration in islet-transplant recipients or when designing novel drug-delivering islet scaffolds.
Introduction
Clinical islet allotransplantation is now established as a successful and safe treatment for reversing life-threatening hypoglycaemia unawareness and restoring euglycemia in prone patients with unstable type 1 diabetes mellitus.Citation1 Indeed, the outcome of islet allotransplantation alone (ITA) for selected nonuremic patients is now similar to whole organ pancreas transplantation alone in some centres.Citation2 One important contributor to this success has been the continuous optimisation of the peri-transplant drug regimens over the last decades. Many different immunosuppressive substances have been trialled, and anti-inflammatory agents are also now routinely included in islet allotransplant protocols.Citation3 Etanercept (ETA) is a tumor necrosis factor alpha (TNFα) inhibitorCitation4 that is widely administered to treat autoimmune diseases such as rheumatoid arthritis or psoriasis.Citation5,Citation6 In more than 40% of the islet allotransplant recipients, ETA is used as an anti-inflammatory drug to address the massive release of cytokines after intraportal infusion of donor islets into the liver of the recipient.Citation7,Citation8 This adjunctive therapy has resulted in a highly significant improvement of all functional parameters of transplanted islets.Citation3,Citation9 Since its introduction for islet transplantation almost 15 years ago,Citation10 the protocol for peri-transplant administration (50 mg intravenously 1 hour pre-transplant, followed by 25 mg subcutaneously on days 3, 7 and 10 post-transplant) has not been substantially changed.Citation11 In contrast to immunosuppressive agents such as mycophenolate mofetil, tacrolimus, rapamycin or sirolimus, that have each been systematically assessed using a range of doses for isolated human islets,Citation12–Citation14 to date, no such detailed assessment has been conducted with ETA. An understanding of the impact of different ETA doses on human islet structure and function is not only important for systemic administration as part of the routine islet allotransplant protocol but is imperative for the development of novel islet scaffolds and islet encapsulation devices that comprise integrated local drug delivery systems.Citation15–Citation17 For the latter, it is not only important to know the influence of drugs on human islets under ideal normoxic conditions, but for meaningful clinical translation, the impact during hypoxia must also be determined. The aim of this study therefore was to define the optimal concentration of ETA with respect to human islet function and integrity considering both normoxic and hypoxic conditions.
Materials and Methods
Experimental Design
In the first part of the study, dose-response experiments were performed culturing islets for 3–4 days in normoxic atmosphere. Islets were suspended in CMRL 1066 supplemented as described below and treated with ETA (Chembest Research Laboratories, Shanghai, China) added at a concentration of 2.5, 5, 10, 20 or 40 µg/mL. This dose–response curve was defined after several pilot experiments essentially including a concentration of 5 µg/mL calculated and converted from the first published study with ETA-treated islets.Citation18 Vehicle-treated islets served as controls.
In the second series of experiments, islet culture was carried out in hypoxic atmosphere to create a pro-inflammatory environment similar to that found after transplantation.Citation19 Culture medium was finally supplemented with five µg/mL of Etanercept.
Human Islet Isolation and Culture
All donor pancreases were voluntarily donated with written informed consent according to the Declaration of Istanbul. The use of isolated human islets for research purposes had been proven by the NHS National Research Ethics Service (09/H0605/2).
Twenty human pancreases were processed after a mean cold ischaemia time of 5.7 ± 0.3 hours (mean ± standard error [SEM]) ranging from 4.0 to 8.5 hours exclusively utilising University of Wisconsin solution (UWS; Bridge to Life, London, UK) for organ preservation. The donors had a mean age of 48.7 ± 1.2 years (33–58) and a mean body mass index of 27.2 ± 1.1 kg/m2 (18–36). The male-to-female ratio was 13 to 7.
The islets were isolated using standard isolation techniques as previously described.Citation20 Briefly, dissected and trimmed pancreases were intraductally infused by manual technique with 2680 PZ-units of collagenase NB1 and 50 DMC-units of neutral protease NB (Serva/Nordmark Arzneimittel GmbH & Co. KG, Uetersen, Germany) dissolved in Hanks’s Balanced Salt Solution (PAN-Biotech GmbH, Aidenbach, Germany) supplemented with 3.2 mmol/L calcium chloride (Sigma-Aldrich, Gillingham, U.K.). Average recirculation time during pancreas digestion using manual agitation at 35°C was 21.1 ± 0.8 min. Released islets were washed with UWS and stored in the same medium until purification by means of continuous gradient centrifugation using a Biocoll (Biochrom GmbH, Berlin, Germany) – UWS gradient ranging from a density of 1.070 to 1.100 g/mL.Citation21 Mean purity and viability after purification was 67.8 ± 3.7% and 73.1 ± 1.3%, respectively.
After isolation and purification, aliquots of 300 islet equivalents (IEQ) were placed in 24-well plates (Greiner Bio-One, Stonehouse, UK) and suspended in 500 µL of CMRL 1066 supplemented with 20 mmol/L HEPES, 2 mmol/L L-glutamine, 200 units/mL penicillin, 200 µg/mL streptomycin (all reagents from Life Technologies, Paisley, United Kingdom) and 5% fetal calf serum (PAA Laboratories, Pasching Austria). Islet culture was performed for three to four days in either normoxic (21% oxygen) or hypoxic (2% oxygen) atmosphere prior to islet characterisation.
Islet Characterisation
All assays were performed in duplicate to generate a data point from two replicates per sample except qRT-PCR which was performed in triplicate for each sample. Before and after culture, islet number was quantified as islet particle number (IN) and number of IEQ as previously described in detail.Citation22 Islet morphological integrity was determined by calculating the islet size index (IEQ/IN).Citation23 Islet viability was assessed utilising 0.67 µmol/L of fluorescein diacetate (FDA, Sigma-Aldrich, Dorset, UK) and 4.0 µmol/L of propidium iodide (PI, Sigma-Aldrich) for staining of viable and dead cells, respectively.Citation24 The fluorescence intensity (FI) of FDA-PI was quantified utilising a fluorometric plate reader as previously described.Citation25 Islet overall survival was calculated considering the recovery of viable cells only. Activity of mitochondria was evaluated postculture by measuring the conversion of the tetrazolium compound 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) into formazan utilising a colorimetric assay (Promega, Southampton, UK) to measure the optical density (OD) as previously described in detail.Citation26 Production of reactive oxygen species (ROS) was determined by measuring the intra-islet conversion of dichlorofluorescein diacetate (DCFH-DA) into fluorescent dichlorodihydrofluorescein (DCFH) as previously described in detail.Citation27 In vitro function of 20 hand-picked islets of similar size was assessed in duplicate during static glucose incubation. Islets were seeded on 3 µm-pore size filter inserts, transferred into 24-well plates and sequentially incubated for 45 min in 1 mL of Krebs-Ringer buffer supplemented with 2.0 mmol/L glucose followed by 45 min at 20 mmol/L and finally re-incubated for a second period of 45 min at 2 mmol/L glucose. After glucose stimulation, islets were recovered and sonified in distilled water prior to insulin extraction in acid ethanol and for subsequent determination of DNA content using the Pico Green assay (Life Technologies, Paisley, U.K.).Citation28 Secreted insulin was determined utilizing an enzyme immunoassay specific for human insulin (Mercodia, Uppsala, Sweden) and expressed as percentage of intracellularly stored insulin.Citation29 The glucose stimulation index was calculated by dividing the insulin release at 20 mmol/L glucose by the mean of the two basal periods.
After hypoxic culture, islet-preconditioned supernatants were collected and assessed for production and secretion of hypoxia- and inflammation-related chemokines by ETA-treated islets. Release of interleukin-1 beta (IL-1β), IL-6, IL-8, interferon gamma-induced protein-10 (IP-10/CXCL-10), monocyte chemoattractant protein-1 (MCP-1), tumor necrosis factor alpha (TNFα) and vascular endothelial growth factor A (VEGF-A) was detected utilising enzymes immunoassays specific for human chemokines (Invitrogen/Thermo Fisher, Rochford, United Kingdom) and expressed as ng per IEQ.
Quantitative Real-Time Polymerase Chain Reaction
Gene expression of cultured islets (n = 5) was measured using Taqman-based quantitative real-time polymerase chain reaction (qRT-PCR). Briefly, total RNA was extracted from 100 cultured handpicked islets of similar size (150–200 µm) using the RNeasy Micro kit (Qiagen, Germany) before being run in triplicate for 35 cycles on a QuantStudio 7 (Applied Biosystems, CA, USA) using the CellsDirect One-Step qRT-PCR kit (Invitrogen, CA, USA). Duplex reactions were performed using TaqMan assays specific for the target genes BCL-2 associated X protein (BAX, Hs00180269_m1), B-cell lymphoma-2 (BCL-2, Hs00608023_m1), insulin (Hs00355773_m1), and pancreatic and duodenal homeobox-1 (PDX-1, Hs00236830_m1) normalized to 18S ribosomal RNA (rRNA) (18S rRNA, Hs99999901_s1). All primers were provided by Applied Biosystems (U.K.). Quantitative values were obtained using the threshold cycle number and the x-fold change in expression using the ΔΔCT method.Citation30
Statistical Analysis
Statistical analysis and graphical presentations were performed utilizing Prism 9.0.0 for MacIntosh (GraphPad, La Jolla, USA). Analysis of data was carried out by the nonparametric Friedman test followed by Dunn’s test for multiple comparisons vs vehicle-treated islets or by the Wilcoxon test for 5 vs 0 µg/mL ETA in the second part of this study. Correlation analysis was performed calculating nonparametric Spearman correlation coefficient (r). Differences were considered significant at P less than 0.05. P-values larger than 0.05 were termed nonsignificant (NS). Results are expressed as mean ± standard error (SEM) and are normalised to islet variables determined pre-culture if appropriate.
Results
Dose-Finding Study
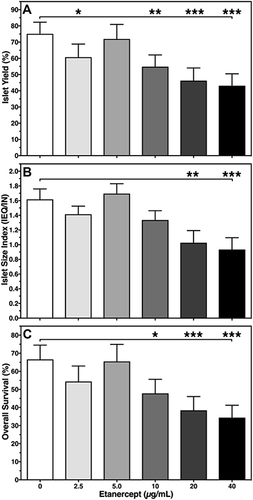
As shown in , a steady decline of the DNA content of islets treated with increased doses of ETA was noted. Although this decrease reached statistical significance only at 40 µg/mL of Etanercept when compared with vehicle-treated controls (P < 0.05 vs 0 µg/mL), it was decided to normalise all parameters to IEQ rather than to ng of DNA.
Table 1 Effect of Etanercept Concentration on Human Islet Characterisation After 3–4 Days of Culture in Normoxia (n = 8)
In contrast, the dose-dependent effect of ETA on yield (), size index (), and overall survival () of cultured islets followed an asymmetric distribution almost peaking at a concentration of 5 µg/mL. A similar observation was made for islet purity and viability (). At this particular concentration, islet size index (1.69 ± 0.14), purity (72.5 ± 2.7%), viability (90.9 ± 4.3%), and overall survival (65.5 ± 9.4%) were larger than or equal to compared with vehicle-treated islets. In contrast, as shown in , islets treated with an ETA concentration of 20 or 40 µg/mL had the significantly lowest values with respect to islet yield (46.2 ± 7.8%, 43.1 ± 7.4%), size index (1.03 ± 0.17, 0.93 ± 0.16), overall survival (38.5 ± 7.6%, 34.3 ± 6.8%), as well as viability and purity ().
Figure 1 Effect of Etanercept concentration on human islet (A) yield (%) normalised to preculture, (B) size index (IEQ/IN), and (C) overall survival (%). Islet characterisation was performed after 3–4 days of culture in normoxic atmosphere. *P < 0.05, **P < 0.01, ***P < 0.001 vs 0 µg/mL as indicated.
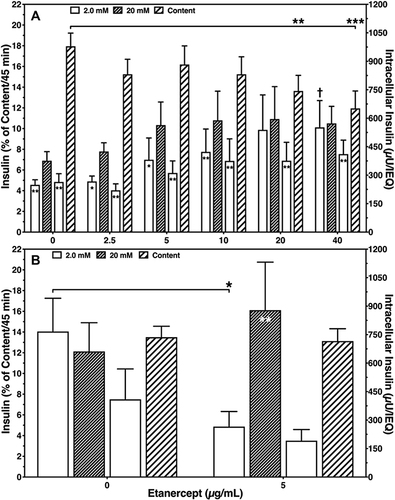
Moreover, ETA concentrations of 20 or 40 µg/mL substantially increased the basal insulin release at 2 mmol/L of glucose compared with vehicle-treated controls (P < 0.01 vs 40 µg/mL) as demonstrated in . This resulted in the lowest stimulation indices (1.36 ± 0.07, 1.25 ± 0.08) among all concentrations tested (). The highest stimulation indices were measured at a concentration of 2.5 (1.78 ± 0.13, P < 0.05 vs 0 µg/mL) and 5 µg/mL (1.71 ± 0.12, P < 0.05 vs 0 µg/mL). Islets treated with 20 or 40 µg/mL of ETA were also characterised by the lowest intracellular insulin content (743.7 ± 82.1 µU/IEQ, P < 0.01 vs 0 µg/mL; 651.8 ± 91.9 µU/IEQ, P < 0.001) as shown in .
Figure 2 Sequential glucose-stimulated insulin release after 3–4 days of human islet culture in (A) normoxic (n = 7) or (B) hypoxic atmosphere (n = 8). Insulin release of 20 IEQ is expressed as percentage of intracellularly stored insulin (striped bars). Symbols inside bars indicate *P < 0.05, **P < 0.01 for 2.0 vs 20 mmol/L of glucose. (A) †P < 0.05 for basal release at 40 µg/mL vs 0 µg/mL; **P < 0.01, ***P < 0.001 for intracellular insulin at 20 and 40 µg/mL vs 0 µg/mL. (B) *P < 0.05 for basal release at 5 µg/mL vs 0 µg/mL.
Islet Characterisation After Hypoxic Culture
In the second series of experiments, human islets were cultured for 3–4 days in hypoxic atmosphere at 2% O2 to create a pro-inflammatory environment. In this part of the study an ETA concentration of 5 µg/mL, determined as the most suitable one in the first part of the study, was applied. To evaluate the effect of decisive isolation-derived variables with a pro-inflammatory impact on islets, a correlation analysis was performed opposing islet purity and recirculation time to ROS production and chemokine release. The analysis did not reveal any significant correlation between islet purity and recirculation time with ROS production and with the release of TNF-α, IL-1β, IL-6, IL-8, IP-10, MCP-1 or VEGF-A (data not shown).
As expected, hypoxia had a detrimental effect on almost all variables of vehicle-treated controls assessed. Nevertheless, when 5 µg/mL of ETA were added, nearly all parameters of islet survival and function improved substantially. As shown in , the addition of ETA caused an increase of mitochondrial activity determined as formazan production (P < 0.01) and a reduced intra-islet generation of ROS (P < 0.001) which was associated with reduced islet fragmentation, as reflected by the size index (P < 0.001), and lesser formation of cell debris resulting in higher islet purity (P < 0.001). As a consequence, a significant increase in islet yield (P < 0.001) and viability (P < 0.001) was observed.
Table 2 Effect of Etanercept on Islet Characterisation After 3–4 Days of Culture in Hypoxic Atmosphere (n = 11)
The improved integrity of ETA-treated islets was also reflected by the glucose stimulation index (). On average, the stimulation index was 5.5 ± 2.1-fold higher (P < 0.01) when ETA had been present during hypoxic culture. Compared with ETA-treated islets, vehicle-treated controls were characterised by a substantially higher basal insulin release (P < 0.05) and failed to demonstrate an increased insulin release towards glucose challenge. Nevertheless, after switching back to low glucose concentrations, vehicle-treated islets were able to downregulate insulin secretion (). In contrast, the addition of 5 µg/mL ETA preserved the physiological insulin response towards different glucose concentrations in hypoxic atmosphere. Whilst overall survival increased substantially after ETA treatment (P < 0.001), no significant effect of ETA on key genes of apoptosis was found (). Although a slight reduction of the BAX-over-BCL-2 mRNA ratio could be detected, the decrease of pro-apoptotic BAX did not reach statistical significance.
In order to assess the TNF-α inhibitory potency of ETA at 5 µg/mL, the production of several chemokines during hypoxic culture was evaluated. As shown in , ETA significantly reduced the accumulation of TNF-α in the culture medium by approximately 30%. Nevertheless, ETA also had a significant impact on the release of several other islet chemokines. Despite the huge variability that was observed regarding the concentration of different chemokines, the inhibitory effect of ETA on the production of individual chemokines was remarkably similar and averaged approximately 30–40% compared with vehicle-treated controls (P < 0.05). The reduction of islet chemokine production did not only concern pro-inflammatory mediators such as TNF-α, IL-1β, IL-6 or MCP-1, it also applied for pro-angiogenic factors such as IL-8 and VEGF-A. As demonstrated in , a very close positive correlation was calculated between TNF-α and IL-1β, IL-6 or VEGF-A, varying between r = 0.72 (P < 0.05) and r = 0.95 (P < 0.001). The correlation coefficient of IL-8, IP-10 and MCP-1, not included in , was r = 0.81 (P < 0.01), r = 0.98 (P < 0.001) and r = 0.85 (P < 0.001), respectively. The correlation analysis also detected a correlation coefficient of r = 0.77 (P < 0.01) for ROS which seems to indicate an involvement of these oxidants in the chemokine network ().
Table 3 Effect of Etanercept on Islet Chemokine Production During 3–4 Days of Culture in Hypoxic Atmosphere (n = 6)
Figure 3 Effect of TNF-α production in hypoxic islets on (A) release of IL-1β (●), IL-6 (▲), VEGF-A (■)(left y-axis) and ROS generation (▼)(right y-axis) or (B) postculture islet yield (◆)(left y-axis) and viability (□)(right y-axis). The correlation coefficient (r) was calculated using Spearman’s rank correlation.
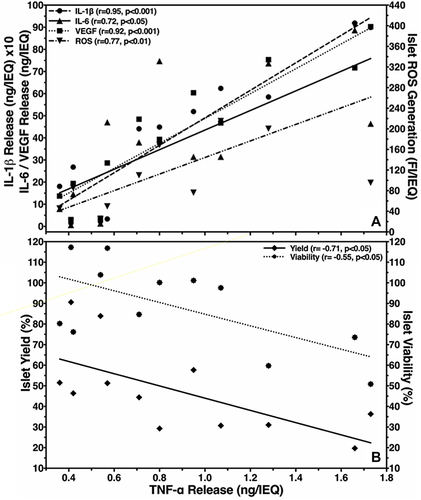
When TNF-α was correlated with islet yield and viability, a negative correlation coefficient was calculated (). Similar figures were calculated for the other chemokines assessed (data not shown). A tight inverse correlation was also observed when analysing ROS with yield (r = −0.72, P < 0.001) and islet viability (r = −0.57, P < 0.01).
Discussion
Considering the long period of time that has elapsed since ETA was introduced into islet transplant protocols,Citation10 it is quite surprising that, to the best of our knowledge, this is the first study to investigate the impact of ETA dose on the function and morphological integrity of isolated human islets. Assessing a dose range of 2.5 to 40 µg/mL, our study suggests that 5 µg/mL of ETA is the optimal concentration for maintaining the morphological and functional integrity of human islets. Using this relatively low concentration, ETA was highly effective to affect the cytokine production in hypoxia-exposed islets. Although ETA was formulated as an inhibitor specific for TNFα, a reduction of approximately 30% or more was noted also for other pro-inflammatory cytokines such as IL-1β, IL-6, IL-8, IP-10 and MCP-1. This observation is in accordance with previous studies performed in patients with rheumatoid arthritis or psoriasis.Citation31–Citation34 The findings of the present study support the hypothesis that TNFα plays a central role within the complex chemokine network.Citation35,Citation36 Surprisingly, VEGF-A, which is characterised as pro-angiogenic and protective chemokine,Citation37 was reduced in the same range as other the other pro-inflammatory chemokines. This is in keeping with previous data showing that TNFα and IL-1β can stimulate VEGF production under inflammation or hypoxic conditions as found in rheumatoid arthritisCitation38 or as observed in cultured cells isolated from varying tissues.Citation39,Citation40
Other pro-inflammatory mediators that are tightly associated with the chemokine network are ROS which have been identified as essential signal transducing agents for different chemokines.Citation41,Citation42 The present data demonstrate a strong correlation between ROS and all other chemokines assessed. A similar finding was made in a previous study with respect to MCP-1.Citation43 ROS are highly pro-oxidant molecules that are generated under hypoxic conditions by several enzymes located in different cellular compartments, such as mitochondria.Citation44,Citation45 Excessive ROS levels damage subcellular structures such as cell membranes,Citation46 protein structuresCitation47 or DNA.Citation48 These structural alterations result in decreased human islet viability and dysfunction.Citation49,Citation50 In the present study, we demonstrate in vitro that the detrimental effects of hypoxia can be substantially prevented by treating hypoxic human islet with 5 µg/mL ETA which reduced the intra-islet ROS production by approximately 45%.
The only prospective islet in vivo study we are aware of administered an ETA dose of 5 mg/kg body weight to treat diabetic immunodeficient mice transplanted with isolated human islets.Citation18 Translating this dosage to the clinical setting by using dose conversion tables,Citation51,Citation52 a recipient of 60 kg body weight would receive 25 mg ETA for the initial intravenous injection which is equivalent to a concentration of 5 µg/mL in whole blood. According to our findings and that of McCall et alCitation18, this concentration appears the most effective one to support highest survival of islets exposed to inflammation or hypoxia. However, the intravenous administration of the established initial standard dose of 50 mg would result in a doubling of the systemic level to approximately 10 µg ETA per mL of whole blood, which is close to the borderline of the toxic ETA range as defined by our study. Integrating the subsequent three subcutaneous injections of 25 mg into the calculation, the long half-life and low elimination rate of ETACitation53,Citation54 can easily result in at least two-fold increase to 20 µg/mL or even more. Consistent with our hypothesis, that high systemic peak values of ETA should be avoided, it was previously demonstrated in more than 240 islet allograft recipients that subcutaneously injected ETA induces a higher rate of insulin independence and a lower number of adverse events when compared with intravenous administration.Citation55 In support of this observation, absorption studies in 26 healthy subjects revealed that the initially measured peripheral serum level of intravenously injected ETA is at least twenty-fold higher compared with subcutaneous administration.Citation56 These findings underline again the necessity to identify an ETA dose that balances a maximum of anti-inflammatory potency and a minimum of adverse effects in islet-transplanted patients. As demonstrated by two previous studies in Asian and Caucasian patients suffering from rheumatoid arthritis, very similar cut-off ETA values were identified by using the receiver-operating characteristic (ROC) curve analysis.Citation57,Citation58 Because ETA serum samples are currently not available in islet transplant centres worldwide, this kind of analysis has not been performed for islet transplantation so far. In order to close this research gap in ETA pharmacokinetics in intraportally transplanted recipients, serum samples should be regularly collected during and after the human islet allo- and autotransplantation procedure. Furthermore, before our in vitro observations can be considered in clinical practice, it is absolutely mandatory to validate and confirm our findings in a subsequent transplant study using a small animal model such as the diabetic nude mouse.
Apart from its administration during pre-transplant culture and the peri-transplant period in the recipient, it can be discussed to broaden the use of ETA as protective reagent since non-physiological and detrimental conditions are virtually omnipresent at all levels of pancreas processing for subsequent islet isolation and islet transplantation.Citation59 One obvious option for an extended application may be ETA supplementation during islet shipment from the remote isolation facility to distant transplant centres. This step is characterised by a limited storage capacity resulting in a high islet seeding density which can cause a pro-inflammatory microenvironment as previously demonstrated.Citation25,Citation60 Nevertheless, the most harmful islet-specific responses are associated with the brain death-induced cytokine storm,Citation61–Citation63 cold storage of the pancreasCitation64 and the inflammatory blood-mediated immune reaction (IBMIR) occurring immediately after intraportal islet infusion into the patient.Citation7,Citation8,Citation65 While the cytokine release in the peri-transplant period is already antagonised by ETA administration, this anti-inflammatory compound has never been used during enzymatic pancreas digestion representing the central and most relevant intrinsic part of the islet isolation process. On the one hand, this step is essential to successfully release islets from within the acinar tissue. On the other, its severity impacts islet morphological integrity and functionality manifold by exposing islet tissue to mechanical shear forces, hypoxia and hyperosmolarity, by degrading the peri-islet extracellular matrix and abolishing anti-apoptotic and anti-inflammatory survival signals.Citation66 In contrast to human islets microdissected by laser-capture technique,Citation67,Citation68 enzyme-isolated islets are characterised by the enhanced activation of stress-signaling and pro-inflammatory pathwaysCitation69,Citation70 as well as pro-apoptotic cascadesCitation71 Moreover, enzymatic islet isolation acts as an amplifier for the brain death-induced expression and production of pro-inflammatory chemokines.Citation72 Amongst those, the tissue factor appears to be the most relevant one to trigger IBMIR as immediate innate immune response.Citation73
Conclusions
In conclusion, this initial study is the first one to define the protective and detrimental concentration ranges of ETA, an inflammatory agent that is routinely used in recipients of allogeneic human islets. Our findings suggest that the therapeutic window for ETA in islet allotransplantation is very narrow, ranging from 5 to 10 µg ETA per mL. These data are important for optimising the administration route and dosage of ETA in islet recipients, in order to avoid high systemic peak levels that can exceed the toxic threshold of ETA. The potency of ETA to protect islets from hypoxia-induced damage should also potentially be considered when shipping islets from remote islet isolation facilities to islet transplant centres. However, before these in vitro findings can be translated into clinical practice transplant studies in a small animal model are essentially required.
Abbreviations
Bax, BCL-2 associated X protein; BCL-2, B-cell lymphoma-2; CMRL, Connaught Medical Research Laboratories; DNA, deoxyribonucleic acid; ETA, Etanercept; FDA, fluorescein diacetate; IEQ, islet equivalents; IL-1β, interleukin-1 beta; IL-6, interleukin-6; IL-8, interleukin-8; IN, islet particle number; IP-10, interferon gamma-induced protein-10; MCP-1, monocyte chemoattractant protein-1; MTS, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium; NS, nonsignificant; PI, propidium iodide; qRT-PCR, quantitative real-time polymerase chain reaction; ROS, reactive oxygen species; rRNA, ribonucleic acid; SEM, mean ± standard error; TNF-α, tumor necrosis factor alpha; VEGF-A, vascular endothelial growth factor-A.
Acknowledgments
The authors would like to thank all past and present members of the DRWF Oxford Human Islet Isolation Facility for isolating and providing human islets for this study.
Funding
Isolation of human islets for research was supported by the Oxford NIHR Biomedical Research Centre and a Juvenile Diabetes Research Foundation (JDRF) award to P.R.V.J. (31-2008-617). The views expressed are those of the authors and not necessarily those of the NHS, NIHR or the Department of Health. Members of the Oxford islet isolation team are funded by the Diabetes Research and Wellness Foundation (DRWF). The study was supported by grants from the European Union’s Horizon 2020 (645991).
Disclosure
The authors of this manuscript declare no conflicts of interest.
References
- Hering BJ, Clarke WR, Bridges ND, et al. Phase 3 trial of transplantation of human islets in type 1 diabetes complicated by severe hypoglycemia. Diabetes Care. 2016;39(7):1230–1240. doi:10.2337/dc15-1988
- Moassesfar S, Masharani U, Frassetto LA, et al. A comparative analysis of the safety, efficacy, and cost of islet versus pancreas transplantation in nonuremic patients with type 1 diabetes. Am J Transplant. 2016;16(2):518–526. doi:10.1111/ajt.13536
- CITR. Tenths annual report of the Collaborative Islet Transplant Registry (CITR). 2017:1–328.
- Feldmann M, Maini RN. Lasker Clinical Medical Research Award. TNF defined as a therapeutic target for rheumatoid arthritis and other autoimmune diseases. Nat Med. 2003;9(10):1245–1250. doi:10.1038/nm939
- Jamnitski A, Krieckaert CL, Nurmohamed MT, et al. Patients non-responding to etanercept obtain lower etanercept concentrations compared with responding patients. Ann Rheum Dis. 2012;71(1):88–91. doi:10.1136/annrheumdis-2011-200184
- Mahil SK, Arkir Z, Richards G, Lewis CM, Barker JN, Smith CH. Predicting treatment response in psoriasis using serum levels of adalimumab and etanercept: a single-centre, cohort study. Br J Dermatol. 2013;169(2):306–313. doi:10.1111/bjd.12341
- Eriksson O, Eich T, Sundin A, et al. Positron emission tomography in clinical islet transplantation. Am J Transplant. 2009;9(12):2816–2824. doi:10.1111/j.1600-6143.2009.02844.x
- Kanak MA, Takita M, Kunnathodi F, Lawrence MC, Levy MF, Naziruddin B. Inflammatory response in islet transplantation. Int J Endocrinol. 2014;2014:451035. doi:10.1155/2014/451035
- Szempruch KR, Banerjee O, McCall RC, Desai CS. Use of anti-inflammatory agents in clinical islet cell transplants: a qualitative systematic analysis. Islets. 2019;11(3):65–75. doi:10.1080/19382014.2019.1601543
- Hering BJ, Kandaswamy R, Ansite JD, et al. Single-donor, marginal-dose islet transplantation in patients with type 1 diabetes. JAMA. 2005;293(7):830–835. doi:10.1001/jama.293.7.830
- Qi M, Kinzer K, Danielson KK, et al. Five-year follow-up of patients with type 1 diabetes transplanted with allogeneic islets: the UIC experience. Acta Diabetol. 2014;51(5):833–843. doi:10.1007/s00592-014-0627-6
- Polastri L, Galbiati F, Bertuzzi F, et al. Secretory defects induced by immunosuppressive agents on human pancreatic beta-cells. Acta Diabetol. 2002;39(4):229–233. doi:10.1007/s005920200039
- Johnson JD, Ao Z, Ao P, et al. Different effects of FK506, rapamycin, and mycophenolate mofetil on glucose-stimulated insulin release and apoptosis in human islets. Cell Transplant. 2009;18(8):833–845. doi:10.3727/096368909X471198
- Mita A, Ricordi C, Miki A, et al. Anti-proinflammatory effects of sirolimus on human islet preparations. Transplantation. 2008;86(1):46–53. doi:10.1097/TP.0b013e31817c79c0
- Buchwald P, Bocca N, Marzorati S, et al. Feasibility of localized immunosuppression: 1. Exploratory studies with glucocorticoids in a biohybrid device designed for cell transplantation. Pharmazie. 2010;65(6):421–428.
- Pinto E, Zhang B, Song S, Bodor N, Buchwald P, Hochhaus G. Feasibility of localized immunosuppression: 2. PLA microspheres for the sustained local delivery of a soft immunosuppressant. Pharmazie. 2010;65(6):429–435.
- Frei AW, Li Y, Jiang K, Buchwald P, Stabler CL. Local delivery of fingolimod from three-dimensional scaffolds impacts islet graft efficacy and microenvironment in a murine diabetic model. J Tissue Eng Regen Med. 2018;12(2):393–404. doi:10.1002/term.2464
- McCall M, Pawlick R, Kin T, Shapiro AM. Anakinra potentiates the protective effects of etanercept in transplantation of marginal mass human islets in immunodeficient mice. Am J Transplant. 2012;12(2):322–329. doi:10.1111/j.1600-6143.2011.03796.x
- Linn T, Schmitz J, Hauck-Schmalenberger I, et al. Ischaemia is linked to inflammation and induction of angiogenesis in pancreatic islets. Clin Exp Immunol. 2006;144(2):179–187. doi:10.1111/j.1365-2249.2006.03066.x
- Cross SE, Vaughan RH, Willcox AJ, et al. Key matrix proteins within the pancreatic islet basement membrane are differentially digested during human islet isolation. Am J Transplant. 2017;17(2):451–461. doi:10.1111/ajt.13975
- Barbaro B, Salehi P, Wang Y, et al. Improved human pancreatic islet purification with the refined UIC-UB density gradient. Transplantation. 2007;84(9):1200–1203. doi:10.1097/01.tp.0000287127.00377.6f
- Ricordi C, Gray DW, Hering BJ, et al. Islet isolation assessment in man and large animals. Acta Diabetol Lat. 1990;27(3):185–195. doi:10.1007/BF02581331
- Brandhorst H, Raemsch-Guenther N, Raemsch C, et al. The ratio between collagenase class I and class II influences the efficient islet release from the rat pancreas. Transplantation. 2008;85(3):456–461. doi:10.1097/TP.0b013e31816050c8
- London NJ, Contractor H, Lake SP, Aucott GC, Bell PR, James RF. A fluorometric viability assay for single human and rat islets. Horm Metab Res Suppl. 1990;25:82–87.
- Brandhorst D, Brandhorst H, Mullooly N, Acreman S, Johnson PR. High seeding density induces local hypoxia and triggers a proinflammatory response in isolated human islets. Cell Transplant. 2016;25(8):1539–1546. doi:10.3727/096368915X689929
- Brandhorst D, Iken M, Tanioka Y, Brendel MD, Bretzel RG, Brandhorst H. Influence of collagenase loading on long-term preservation of pig pancreas by the two-layer method for subsequent islet isolation. Transplantation. 2005;79(1):38–43. doi:10.1097/01.TP.0000146550.55596.48
- Meghana K, Sanjeev G, Ramesh B. Curcumin prevents streptozotocin-induced islet damage by scavenging free radicals: a prophylactic and protective role. Eur J Pharmacol. 2007;577(1–3):183–191. doi:10.1016/j.ejphar.2007.09.002
- Brandhorst H, Asif S, Andersson K, et al. A new oxygen carrier for improved long-term storage of human pancreata before islet isolation. Transplantation. 2010;89(2):155–160. doi:10.1097/TP.0b013e3181c9266c
- Korbutt GS, Elliott JF, Ao Z, Smith DK, Warnock GL, Rajotte RV. Large scale isolation, growth, and function of porcine neonatal islet cells. J Clin Invest. 1996;97(9):2119–2129. doi:10.1172/JCI118649
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–408. doi:10.1006/meth.2001.1262
- Chen DY, Chen YM, Chen HH, Hsieh CW, Lin CC, Lan JL. Increasing levels of circulating Th17 cells and interleukin-17 in rheumatoid arthritis patients with an inadequate response to anti-TNF-alpha therapy. Arthritis Res Ther. 2011;13(4):R126. doi:10.1186/ar3431
- Zhang F, Ding R, Li P, et al. Interleukin-34 in rheumatoid arthritis: potential role in clinical therapy. Int J Clin Exp Med. 2015;8(5):7809–7815.
- Gottlieb AB, Chamian F, Masud S, et al. TNF inhibition rapidly down-regulates multiple proinflammatory pathways in psoriasis plaques. J Immunol. 2005;175(4):2721–2729. doi:10.4049/jimmunol.175.4.2721
- Liu Y, Qin G, Meng Z, et al. IL-1beta, IL-17A and combined phototherapy predicts higher while previous systemic biologic treatment predicts lower treatment response to etanercept in psoriasis patients. Inflammopharmacol. 2018.
- Puren AJ, Fantuzzi G, Gu Y, Su MS, Dinarello CA. Interleukin-18 (IFNgamma-inducing factor) induces IL-8 and IL-1beta via TNFalpha production from non-CD14+ human blood mononuclear cells. J Clin Invest. 1998;101(3):711–721. doi:10.1172/JCI1379
- Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol. 2000;279(6):L1005–L1028. doi:10.1152/ajplung.2000.279.6.L1005
- Cross SE, Richards SK, Clark A, et al. Vascular endothelial growth factor as a survival factor for human islets: effect of immunosuppressive drugs. Diabetologia. 2007;50(7):1423–1432. doi:10.1007/s00125-007-0670-8
- Paleolog EM, Young S, Stark AC, McCloskey RV, Feldmann M, Maini RN. Modulation of angiogenic vascular endothelial growth factor by tumor necrosis factor alpha and interleukin-1 in rheumatoid arthritis. Arthritis Rheum. 1998;41(7):1258–1265. doi:10.1002/1529-0131(199807)41:7<1258::AID-ART17=3.0.CO;2-1
- Sun D, Matsune S, Ohori J, Fukuiwa T, Ushikai M, Kurono Y. TNF-alpha and endotoxin increase hypoxia-induced VEGF production by cultured human nasal fibroblasts in synergistic fashion. Auris Nasus Larynx. 2005;32(3):243–249. doi:10.1016/j.anl.2005.01.004
- Shin MR, Kang SK, Kim YS, Lee SY, Hong SC, Kim EC. TNF-alpha and LPS activate angiogenesis via VEGF and SIRT1 signalling in human dental pulp cells. Int Endod J. 2015;48(7):705–716. doi:10.1111/iej.12396
- Naik E, Dixit VM. Mitochondrial reactive oxygen species drive proinflammatory cytokine production. J Exp Med. 2011;208(3):417–420. doi:10.1084/jem.20110367
- Sverrisson K, Axelsson J, Rippe A, Asgeirsson D, Rippe B. Acute reactive oxygen species (ROS)-dependent effects of IL-1beta, TNF-alpha, and IL-6 on the glomerular filtration barrier (GFB) in vivo. Am J Physiol Renal Physiol. 2015;309(9):F800–F806. doi:10.1152/ajprenal.00111.2015
- Melzi R, Mercalli A, Sordi V, et al. Role of CCL2/MCP-1 in islet transplantation. Cell Transplant. 2010;19(8):1031–1046. doi:10.3727/096368910X514639
- Murphy MP. How mitochondria produce reactive oxygen species. Biochemical J. 2009;417(1):1–13. doi:10.1042/BJ20081386
- Forrester SJ, Kikuchi DS, Hernandes MS, Xu Q, Griendling KK. Reactive oxygen species in metabolic and inflammatory signaling. Circ Res. 2018;122(6):877–902. doi:10.1161/CIRCRESAHA.117.311401
- Rabinovitch A, Suarez WL, Thomas PD, Strynadka K, Simpson I. Cytotoxic effects of cytokines on rat islets: evidence for involvement of free radicals and lipid peroxidation. Diabetologia. 1992;35(5):409–413. doi:10.1007/BF02342435
- Stadtman ER, Levine RL. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids. 2003;25(3–4):207–218. doi:10.1007/s00726-003-0011-2
- Delaney CA, Pavlovic D, Hoorens A, Pipeleers DG, Eizirik DL. Cytokines induce deoxyribonucleic acid strand breaks and apoptosis in human pancreatic islet cells. Endocrinology. 1997;138(6):2610–2614. doi:10.1210/endo.138.6.5204
- Piemonti L, Leone BE, Nano R, et al. Human pancreatic islets produce and secrete MCP-1/CCL2: relevance in human islet transplantation. Diabetes. 2002;51(1):55–65. doi:10.2337/diabetes.51.1.55
- Armann B, Hanson MS, Hatch E, Steffen A, Fernandez LA. Quantification of basal and stimulated ROS levels as predictors of islet potency and function. Am J Transplant. 2007;7(1):38–47. doi:10.1111/j.1600-6143.2006.01577.x
- Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7(2):27–31. doi:10.4103/0976-0105.177703
- Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2007;22(3):659–661. doi:10.1096/fj.07-9574LSF
- Korth-Bradley JM, Rubin AS, Hanna RK, Simcoe DK, Lebsack ME. The pharmacokinetics of etanercept in healthy volunteers. Ann Pharmacother. 2000;34(2):161–164. doi:10.1345/aph.19126
- Lobo ED, Hansen RJ, Balthasar JP. Antibody pharmacokinetics and pharmacodynamics. J Pharm Sci. 2004;93(11):2645–2668. doi:10.1002/jps.20178
- Drogemuller C, Radford TM, Torpy DJ, Rainis HA, Barton FB, Coates PT. Effects of etanercept route of administration in clinical allogeneic islet transplantation: an analysis from the collaborative islet transplant registry (CITR). Xenotransplantation. 2015;22(S1):S185–S201.
- Zhou H. Clinical pharmacokinetics of etanercept: a fully humanized soluble recombinant tumor necrosis factor receptor fusion protein. J Clin Pharmacol. 2005;45(5):490–497. doi:10.1177/0091270004273321
- Chen DY, Chen YM, Tsai WC, et al. Significant associations of antidrug antibody levels with serum drug trough levels and therapeutic response of adalimumab and etanercept treatment in rheumatoid arthritis. Ann Rheum Dis. 2015;74(3):e16. doi:10.1136/annrheumdis-2013-203893
- Sanmarti R, Inciarte-Mundo J, Estrada-Alarcon P, et al. Towards optimal cut-off trough levels of adalimumab and etanercept for a good therapeutic response in rheumatoid arthritis. Results of the INMUNOREMAR study. Ann Rheum Dis. 2015;74(8):e42. doi:10.1136/annrheumdis-2015-207530
- Noguchi H. Activation of c-Jun NH2-terminal kinase during islet isolation. Endocr J. 2007;54(2):169–176. doi:10.1507/endocrj.KR-87
- Smith KE, Kelly AC, Min CG, et al. Acute ischemia induced by high-density culture increases cytokine expression and diminishes the function and viability of highly purified human islets of Langerhans. Transplantation. 2017;101(11):2705–2712. doi:10.1097/TP.0000000000001714
- Contreras JL, Eckstein C, Smyth CA, et al. Brain death significantly reduces isolated pancreatic islet yields and functionality in vitro and in vivo after transplantation in rats. Diabetes. 2003;52(12):2935–2942. doi:10.2337/diabetes.52.12.2935
- Toyama H, Takada M, Suzuki Y, Kuroda Y. Activation of macrophage-associated molecules after brain death in islets. Cell Transplant. 2003;12(1):27–32. doi:10.3727/000000003783985205
- Takada M, Toyama H, Tanaka T, Suzuki Y, Kuroda Y. Augmentation of interleukin-10 in pancreatic islets after brain death. Transplant Proc. 2004;36(5):1534–1536. doi:10.1016/j.transproceed.2004.05.019
- Lakey JR, Rajotte RV, Warnock GL, Kneteman NM. Human pancreas preservation prior to islet isolation. Cold ischemic tolerance. Transplantation. 1995;59(5):689–694. doi:10.1097/00007890-199503150-00008
- Delaune V, Berney T, Lacotte S, Toso C. Intraportal islet transplantation: the impact of the liver microenvironment. Transpl Int. 2017;30(3):227–238. doi:10.1111/tri.12919
- Brandhorst D, Brandhorst H, Linn T. Induction and amelioration of environmental stress in isolated islets until transplantation. Immun Endoc Metab Agents Med Chem. 2006;6:209–218. doi:10.2174/187152206776359957
- Marselli L, Thorne J, Ahn YB, et al. Gene expression of purified beta-cell tissue obtained from human pancreas with laser capture microdissection. J Clin Endocrinol Metab. 2008;93(3):1046–1053. doi:10.1210/jc.2007-0931
- Negi S, Jetha A, Aikin R, Hasilo C, Sladek R, Paraskevas S. Analysis of beta-cell gene expression reveals inflammatory signaling and evidence of dedifferentiation following human islet isolation and culture. PLoS One. 2012;7(1):e30415. doi:10.1371/journal.pone.0030415
- Abdelli S, Ansite J, Roduit R, et al. Intracellular stress signaling pathways activated during human islet preparation and following acute cytokine exposure. Diabetes. 2004;53(11):2815–2823. doi:10.2337/diabetes.53.11.2815
- Cowley MJ, Weinberg A, Zammit NW, et al. Human islets express a marked proinflammatory molecular signature prior to transplantation. Cell Transplant. 2012;21(9):2063–2078. doi:10.3727/096368911X627372
- Campbell PD, Weinberg A, Chee J, et al. Expression of pro- and antiapoptotic molecules of the Bcl-2 family in human islets postisolation. Cell Transplant. 2012;21(1):49–60. doi:10.3727/096368911X566262
- Saito Y, Goto M, Maya K, et al. Brain death in combination with warm ischemic stress during isolation procedures induces the expression of crucial inflammatory mediators in the isolated islets. Cell Transplant. 2010;19(6):775–782. doi:10.3727/096368910X508889
- Moberg L, Johansson H, Lukinius A, et al. Production of tissue factor by pancreatic islet cells as a trigger of detrimental thrombotic reactions in clinical islet transplantation. Lancet. 2002;360(9350):2039–2045. doi:10.1016/S0140-6736(02)12020-4
