Abstract
Introduction:
Numerous studies have elucidated adiponectin as a negative impact on inflammation and tissue fibrosis. However, little is known about the relevance between adiponectin and inflammatory factors in keloid.
Methods:
To clarify whether adiponectin plays a role in the inflammation and fibrosis of keloid, 50 patients with keloid and 50 healthy subjects were enrolled, We examined the serum and mRNA expression levels of adiponectin, TGF-β1, CTGF, IL-6 and TNF-α in normal skin tissues and keloid tissues by ELISA and qPCR, respectively. Correlation analysis between serum concentration of adiponectin with Vancouver Scar Scale (VSS) scores and the age of patients with keloid was evaluated, and the adiponectin concentrations in patients with keloid between different genders were measured. We further examined the effects of adiponectin on TGF-β1 mediated expression of collagen I, FN and MMP-1 in normal fibroblasts (NFs) and keloid fibroblasts (KFs).
Results:
We discovered that lower serum concentration and mRNA expression of adiponectin, but higher TGF-β1, CTGF, IL-6 and TNF-α levels were measured in patients with keloid compared with those in normal controls. Furthermore, there was a strong inverse correlation between the serum adiponectin levels and VSS scores in patients with keloid, but not in ages, and there was no statistically difference between different genders. Moreover, adiponectin attenuated TGF-β1 mediated expression of collagen I and FN, and upregulated the expression level of MMP-1 in KFs, but not in NFs. In addition, the inhibitory effect of adiponectin on TGF-β1 was attenuated by AMPK inhibitor Compound C, but not PI3K/Akt inhibitor LY294002.
Discussion:
Adiponectin may exert an anti-inflammation and anti-fibrosis role in the development of keloid. One of the underlying mechanisms may be the activation of the AMPK signaling pathway.
Introduction
Keloid is an inflammatory and fibroproliferative disease featured by overexpression of extracellular matrix (ECM), particularly collagen. Therefore, inflammation and fibrosis may be involved in the pathogenesis of keloid,Citation1 but the molecular mechanism underlying keloid formation remains unclear.
Multiple cytokines and inflammatory factors are participates in keloid formation. Transforming growth factor β (TGF-β)/Smad signaling pathway has been demonstrated to be a key player in the development of keloid. TGF-β1 can serve as a driver in wound healing, tissue repair and excessive ECM accumulation.Citation1 TGF-β1 can also directly activate Smad signaling and trigger inflammatory and fibrotic genes overexpression.Citation2 Connective tissue growth factor (CTGF) is a downstream factor of the TGF-β1/Smad signaling pathway and the overexpression of CTGF is critical for the maintenance of keloid fibrosis.Citation3 Inflammatory factors of interleukin (IL)-1, IL-6, Interferon (INF)-β and tumor necrosis factor-α (TNF-α) are upregulated in keloid, which promote fibroblast proliferation, migration, signal transmission of inflammatory response and subsequent uncontrolled collagen production.Citation4,Citation5 The existing data suggest that IL-6 mediated inflammation is a key player and may be considered as a common causative factor for the development of keloid.Citation6 TNF-α may be the cause of keloid formation.Citation7 Furthermore, IL-4 and IL-17 are also important mediators of keloid fibrosis and inflammation, and IL-10 was found to be able to inhibit the proliferation of keloid fibroblasts (KFs).Citation8–Citation10
Adiponectin, a cytokine that is predominantly secreted by adipose tissues, acts actually as an important biomarker in metabolism, vascular homeostasis, cell proliferation and tissue remodeling. Interestingly, the adiponectin serum concentration is far more than most other plasma cytokines, accounts for 0.01% of total serum proteins.Citation11 In addition to its role in tissue remodeling and cell proliferation, adiponectin is also a key regulator of controlling inflammation and anti-fibrosis in the fibrosis diseases.Citation12 Our previous research found that adiponectin suppressed the progression of CTGF-induced keloid fibrosis.Citation13 To our knowledge, no studies have yet to explore the relationship between adiponectin and inflammatory factors in keloid. In this research, we compared the expression of adiponectin and inflammatory factors TGF-β1, CTGF, IL-6 and TNF-α in patients with keloid and healthy control individuals, and detected whether adiponectin affects KFs fibrosis induced by TGF-β1, the upstream factor of CTGF, to further discuss the correlation between adiponectin and keloid pathogenesis.
Patients and Methods
Patients
Fifty patients with keloid (23 males, 27 females, aged 21–53 years, the average age is 31.88 years) were recruited from the Department of Dermatology, the First Affiliated Hospital, Zhejiang University School of Medicine. The lesions ranged from 2.5 to 36 cm2 in surface area. They were found on the ear (6 cases), mandible (3 cases), chest (18 cases), back (5 cases), upper arm (6 cases), shoulder blades (8 cases), umbilicus (4 cases), and vulva (6 cases). Furthermore, some patients may have multiple lesions, and 26 cases were caused by trauma, 10 cases by surgery, 3 cases by infections, 5 cases by folliculitis, 4 cases by ear piercing, and 2 cases had no obvious cause among the 50 cases. The course of illness is 1–12 years, with an average of 6.2 years. The inclusion criteria: 1. The 50 patients with keloid were recruited by the Department of Dermatology, the First Affiliated Hospital, Zhejiang University School of Medicine from January 2019 to January 2020. 2. Each of the 50 patients was diagnosed by two experienced doctors. 3. Prior to this research, no patient had received keloid treatment within 6 months, and no other autoimmune or chronic organic diseases. 4. Intervention measures. The keloid and part of the surrounding normal tissues were excised by surgery, and then radiotherapy combined with local steroid injection was performed. The exclusion criteria: 1. Before the study, the patients had local or systemic medication or treatment within half a year. 2. The patients suffered from severe heart, liver, kidney and blood system diseases, or tumor and immune-related diseases. As controls, 50 subjects (25 males, 25 females, aged 23–55 years, the average age is 32.27 years) were healthy individuals. All the cases and controls were collected using uniform criteria, and there was no statistically significant difference in the general data of the two groups (P>0.05). This study was approved by the Clinical Research Ethics Committee of the First Affiliated Hospital, Zhejiang University (Approval number 2019–464) and was conducted in accordance with the Declaration of Helsinki. Written informed consent was provided by all patients and healthy controls.
Reagents
Recombinant human TGF-β1 and recombinant human adiponectin were purchased from Sino Biological (Beijing, China). Adenosine 5ʹ-monophosphate-activated protein kinase (AMPK) inhibitor (Compound C) was obtained from Calbiochem (Merck Kgaa, Darmstadt, Germany), and phosphatidylinositol 3 kinase-protein kinase/protein kinase B (PI3K/Akt) inhibitor (LY294002) was purchased from Selleckchem (Houston, TX, USA).
Cell Source and Culture
Specimens were obtained from ten patients (5 males and 5 females, aged 25–48 years) with keloid and the adjacent normal skin tissues, cell cultures were established as described previously.Citation9 Briefly, the isolated skin specimen was placed in sterile petri dishes and washed with phosphate-buffered saline (PBS), then incubated with 2.5 mg/mL dispase II (Hoffman-La Roche, Indianapolis, IN, USA) overnight at 4°C. On the second day, the specimen was removed from the epidermis and subcutaneous tissues, and the dermis was manually cut as much as possible and then seeded in 6-well plates. The incubation conditions were at 37°C in 5% CO2. Fibroblasts were grown in DMEM with 10% fetal serum. The medium was exchanged three to four times every week. When reaching 70–90% confluence, the fibroblasts were passaged or cryopreserved. Cells at passage three to four were used in the subsequent experiments. And the supernatant was collected.
Enzyme-Linked Immunosorbent Assay (ELISA)
Collected 4 mL cubital venous blood from all subjects on an empty stomach for more than 8 h, then placed in anticoagulation tubes, centrifuge at 1000 × g for 20 min after standing still, took the supernatant. The plasma concentrations of adiponectin, TGF-β1, CTGF, IL-6, and TNF-a, or cell culture supernatant concentrations of FN, and MMP-1 were quantitated by ELISA, using assay kits (Sangon biotech, Shanghai, China) in accordance with the manufacturer’s instructions, collagen I ELISA Kit was from MSK Biotechnology Company (Wuhan, China). The absorbance of colored products was determined by using a microplate reader set to 450 nm (Thermo Scientific, Pittsburgh, PA, USA). The experiments were routinely conducted in duplicate and independently repeated three times.
Quantitative Real-Time RT-PCR (qPCR) Analysis
Specimens were obtained from ten patients (5 males and 5 females, aged 25–48 years) with keloid and the adjacent normal skin tissues, removed from the subcutaneous tissues, and then cut as much as possible. Total RNA was isolated from the keloid tissues, the adjacent normal skin tissues and the collected normal fibroblasts (NFs) or keloid fibroblasts (KFs) by using TRIzol reagent following the manufacturer’s directions. The RNA concentration (OD260/OD280, ratio1.8–2.0) was then measured by Nanodrop 2000 Spectrophotometer. The High Capacity cDNA Reverse Transcription Kits was used to synthesis the cDNA. The expression levels of target genes were assessed using the primer sequences are shown in and performed using the ABI 7500 System (Applied Biosystems). The PCR reaction conditions were as follows: 10 min at 95°C, 40 cycles at 94°C for 15 sec and then 60°C for 1 min. The relative mRNA levels of target genes were determined using the comparative threshold cycle (CT) method using the 2–ΔΔCT equation. GAPDH was used as an internal control gene. The experiments were repeated five times for each experimental condition.
Table 1 Primers Used in qPCR
Statistical Analysis
Measurement data are presented as means ± SE. Statistical significance among multiple groups was used by one-way ANOVA with Dunnett’s tests multiple comparisons. The t test was employed to comparing the differences between the two groups. Pearson’s correlation was used for correlation analysis. All data were analyzed with the statistical software GraphPad Prism 5.0 software (GraphPad Software, SanDiego, CA, USA). And P values <0.05 were considered to indicate a statistical difference.
Results
Comparison of Clinic Data
We compared the differences in clinical features, clinic information of cases and controls. And found that there were no significant differences in terms of gender, ages, body mass index (BMI, kg/m2), systolic blood pressure (SBP, mmHg), diastolic blood pressure (DBP, mmHg), fasting blood glucose (FBG, mmol/L), triglyceride (TG, mmol/L), cholesterol (TC, mmol/L) between the patients with keloid and controls (all P > 0.05). However, noticeable differences were observed in the family history of keloid between the two groups (P < 0.001), as shown in .
Table 2 Comparison of Clinic Data Between Patients with Keloids and Controls
Serum Concentrations of Adiponectin, TGF-β1, CTGF, IL-6 and TNF-α
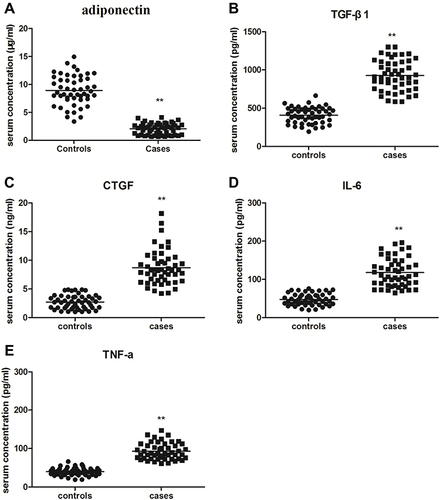
Adiponectin is reported to be widely expressed in various tissues and organs.Citation14 But so far, little is known about the serum concentration of adiponectin, neither the association between adiponectin and inflammatory factors in keloid tissues. Therefore, we measured the expression of adiponectin, TGF-β1, CTGF, IL-6 and TNF-α in peripheral blood between patients with keloid and normal controls by ELISA. We noticed that the serum expression levels of adiponectin in keloid group (2.06 ± 0.99 μg/mL) were lower than the control group (8.95 ± 2.66 μg/mL), and there were statistically significant differences (P < 0.01). However, the higher serum levels of TGF-β1 (928.44 ± 200.02 pg/mL), CTGF (8.71 ± 3.02 ng/mL), IL-6 (117.60 ± 36.82 pg/mL) and TNF-α (92.27 ± 21.49 pg/mL) in peripheral blood were measured in patients with keloid compared with those in normal controls (TGF-β1 was 408.10 ± 104.69 pg/mL, CTGF was 2.72 ± 1.19 ng/mL, IL-6 was 47.46 ± 15.28 pg/mL, and TNF-α was 39.65 ± 9.91 pg/mL), and the all differences were statistically significant (P < 0.01), as shown in and .
Table 3 To Compare the Expression of Adiponectin, TGF-β1, CTGF, IL-6 and TNF-α in Peripheral Blood Between Patients with Keloids and Controls
The mRNAs Expression of the Adiponectin, TGF-β1, CTGF, IL-6 and TNF-α
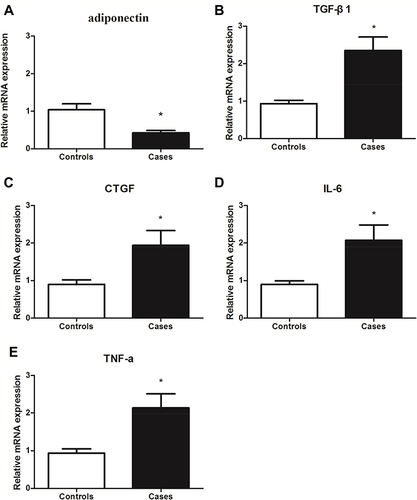
To further confirm the expression of adiponectin and inflammatory factors in patients with keloid. We examined the gene expression levels of adiponectin, TGF-β1, CTGF, IL-6 and TNF-α between the patients with keloid and controls by qPCR. As shown in , adiponectin (2A) genes expression in keloid tissues (0.422±0.064) was decreased than that in normal controls (1.040±0.156), P<0.05. However, compared with the normal controls, the TGF-β1 (2B), CTGF (2C), IL-6 (2D) and TNF-α (2E) mRNA expression showed a marked increase in keloid tissues (TGF-β1 was 2.348±0.361 in keloids and 0.934±0.090 in normal controls, CTGF was 1.938±0.397 in keloids and 0.898±0.120 in normal ones, IL-6 was 2.074±0.411 in keloids and 0.898±0.096 in normal ones, and TNF-α was 2.133±0.374 in keloids and 0.936±0.112 in normal ones), all P<0.05, respectively.
Compared the Serum Concentrations of Adiponectin with Vancouver Scar Scale Scores, Ages and Genders in Keloid Patients
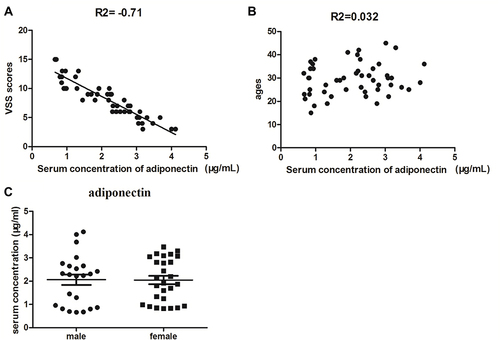
The Vancouver Scar Scale (VSS) was first introduced in 1990,Citation15 which was designed to measure pathological scars, including the parameters pigmentation, vascularity, pliability, and height. VSS has been widely used to evaluate the severity of keloid. In the study, the correlation analysis between serum levels of adiponectin with VSS scores and ages in keloid patients was used by Pearson’s correlation. As shown in , a strong negative correlation was noticed between the serum adiponectin concentration and VSS scores (r = −0.71, P<0.01). Furthermore, no significant association between serum expression of adiponectin and ages was obtained (r = 0.032, P>0.05), as shown in . Next, we measured the serum concentrations of adiponectin in patients with keloid between different genders using t test and found that there was no statistical difference (P>0.05, ).
Adiponectin Attenuated TGF-β1-Mediated Expression of Collagen I and Fibronectin (FN), and Upregulates the Expression of Matrix Metalloproteinase (MMP)-1 in KFs by AMPK Signaling Pathway
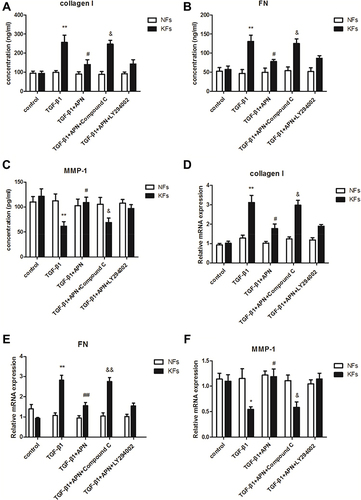
It is known to us that TGF-β1 plays an important role in the inflammation and fibrosis of keloid.Citation16 Our previous research found that adiponectin down-regulated CTGF, a downstream target factor of the TGF-β1/Smad signaling pathway, induced keloid pathogenesis.Citation13 However, whether adiponectin suppressed TGF-β1-induced inflammatory response and fibrosis in keloid is unclear. So, we examined the supernatant concentration and mRNA levels of type I collagen, FN and MMP-1 in KFs and NFs by ELISA and qPCR, respectively. Here, adiponectin was applied at 5µg/mL as described previously,Citation13 and TGF-β1 was 10ng/mL for 24 h in the experiments. Furthermore, the results discovered that adiponectin attenuated TGF-β1 mediated protein and mRNA expression of collagen I (4A, 4D) and FN (4B, 4E), and upregulated the MMP-1 (4C, 4F) expression in KFs, but not in NFs. However, when KFs and NFs were preincubated with Compound C (10 µM as described previously,Citation13 an AMPK inhibitor), or LY294002 (10 µM as described previously,Citation13 a PI3K inhibitor) for 24 h, the inhibitory effect of adiponectin on TGF-β1 in KFs was attenuated by Compound C, but not LY294002, as shown in .
Figure 4 Adiponectin attenuated TGF-β1 mediated expression of collagen I and FN, and upregulates TGF-β1 suppressed MMP-1 expression in KFs by AMPK signaling pathway. KFs and NFs were preincubated with Compound C (10 µM, an AMPK inhibitor), or LY294002 (10 µM, a PI3K inhibitor) or without inhibitors for 24 h. After stimulation with or without adiponectin (APN, 5 µg/mL) in the presence of TGF-β1 (10 ng/mL) for 24 h, the supernatant concentration and mRNA levels of type I collagen (A and D), FN (B and E), and MMP-1 (C and F) in KFs and NFs were examined by ELISA and qPCR, respectively. Data represent at least three independent experiments. *P<0.05 versus the control group, **P<0.01 versus the control group; #P<0.05 versus the TGF-β1 group, ##P<0.01 versus the TGF-β1 group; &P<0.05 versus the TGF-β1+adiponectin group, &&P<0.01 versus the TGF-β1+adiponectin group. The results are shown as means±SE.
Discussion
Keloid is a kind of chronic inflammatory and fibroproliferative disease after injury and inflammation with abnormal accumulation of ECM, particularly collagen, inflammatory cell infiltration, and aggregation of a large number of cytokines and inflammatory factors.Citation17,Citation18 Excessive fibrosis might play a central role in the occurrence of keloid development.Citation19 In addition, inflammatory response is one of the essential characters of keloid,Citation20 but the detailed mechanisms of keloid fibrosis and inflammation are not fully elucidated.
Various cytokines participate in the process of keloid tissue fibrosis and inflammation, among which TGF-β is one of the most critical factors.Citation19 TGF-β belongs to the TGF-β superfamily. It is well known that there are three different isomers, including TGF-β1, TGF-β2 and TGF-β3 in mammalian cells, and TGF-β1 is the most important pathogenic factor of keloid.Citation5 Furthermore, TGF-β1 is also actively participated in the regulation of multiple tissue, organ fibrotic diseases and inflammation.Citation21 Numerous lines of evidence have discovered that CTGF is a downstream effector of the TGF-β1/Smad signaling pathway, and also a critical regulator of tissue remodeling and fibrosis.Citation22 In addition, CTGF could induce the expression TGF-β1, which induces more expression of CTGF.Citation23 Other than that, inhibition of CTGF reversed fibrosis.Citation22 It is reported that keloid expressed more proinflammatory cytokines, such as IL-6, IL-1, and TNF-α, and contained more inflammatory cells than physiological scars and normal skin tissues, revealing that continuous upregulation of proinflammatory factors may be another key factor in the formation of keloid.Citation4 Moreover, IL-6 mediated inflammation is an important player for the development of keloid.Citation24 And TNF-α was reported to induce NF-κB activation in KFs, which disclosed more sensitivity than fibroblasts from normal skin.Citation25
Adiponectin is mostly secreted by adipose tissue and has a negative impact on inflammatory and tissue fibrosis.Citation26 Studies have shown that adiponectin suppressed the production of TNF-α by macrophages, inhibiting its biological activity, moreover, adiponectin decreased the synthesis of IL-6 and IL-18 and exert anti-inflammatory effects.Citation27,Citation28 Recent researches found that adiponectin also has a strong anti-fibrotic effect on various tissues and organs.Citation29 In skin tissues, the serum level of adiponectin in patients with scleroderma was a negative correlation with disease activity, severity, and duration. In addition, adiponectin could antagonize the TGF-β/Smad signaling pathway and down-regulate TGF-β induced expression of type I collagen and α-SMA, therefore inhibiting the occurrence of scleroderma fibrosis.Citation30
In the former study, we discovered that the expression of adiponectin was obviously decreased in keloid tissues compared with those in normal controls, furthermore, adiponectin inhibited CTGF-mediated KFs proliferation, migration and the over-accumulation of ECM.Citation13 But the correlation between adiponectin and inflammatory factors in keloid remains largely unknown. In this research, we noticed that the serum and mRNAs expression of adiponectin were decreased in keloid, but TGF-β1, CTGF, IL-6 and TNF-α was increased compared with that in normal skin tissues, revealing that adiponectin may inhibit the inflammatory response of keloid. Moreover, Pearson’s correlation test discovered that there was a strong inverse relationship between the serum adiponectin concentration and VSS scored in patients with keloid. In addition, adiponectin attenuated TGF-β1 mediated expression of collagen I and FN, but upregulated the MMP-1 expression in KFs by AMPK signaling pathway, but not by PI3K signaling pathway, suggesting that adiponectin may exert an anti-fibrosis effect on the development of keloid.
According to these findings, we speculate that the low expression level of adiponectin in keloid tissues correlates with keloid development and formation. Taken together, adiponectin might play a critical role in inhibiting keloid fibrosis and inflammatory. One of the underlying mechanisms may be the activation of the AMPK signaling pathway. Therefore, adiponectin could be a therapeutic target in keloid pathogenesis. However, the sample size of this research is small. Infurther studies, we will expand the sample size and confirmed the role of adiponectin in vivo with keloid animal models.
Author contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. These authors contributed equally to the research: Limin Luo and Jun Li.
Acknowledgments
The study was supported by the State Key Laboratory of Infectious Diseases, the First Affiliated Hospital of Zhejiang University School of Medicine.
Funding
We gratefully acknowledge financial support from the National Natural Science Foundation of China (no. 82003372), the Natural Science Foundation of Hubei Province (no. 2018CFB747, 2018CFB537), and Educational Commission of Hubei Province (no. B2017112, B20181130).
Disclosure
The authors declared no conflicts of interest for this work.
References
- Kim KK, Sheppard D, Chapman HA. TGF-β1 signaling and tissue fibrosis. Cold Spring Harb Perspect Biol. 2018;10(4):a022293. doi:10.1101/cshperspect.a022293
- Hu HH, Chen DQ, Wang YN, et al. New insights into TGF-β/Smad signaling in tissue fibrosis. Chem Biol Interact. 2018;292:76–83. doi:10.1016/j.cbi.2018.07.008
- Jurzak M, Adamczyk K, Antończak P, et al. Evaluation of genistein ability to modulate CTGF mRNA/protein expression, genes expression of TGFβ isoforms and expression of selected genes regulating cell cycle in keloid fibroblasts in vitro. Acta Pol Pharm. 2014;71(6):972–986.
- Ogawa R. Keloid and hypertrophic scars are the result of chronic inflammation in the reticular dermis. Int J Mol Sci. 2017;18(3):606. doi:10.3390/ijms18030606
- Andrews JP, Marttala J, Macarak E, et al. Keloids: the paradigm of skin fibrosis – pathomechanisms and treatment. Matrix Biol. 2016;51:37–46. doi:10.1016/j.matbio.2016.01.013
- Ghazizadeh M. The existing data suggest that IL-6 mediated inflammation is a key player and may be considered as a common causative factor for development of keloid. J Nippon Med Sch. 2007;74(1):11–22. doi:10.1272/jnms.74.11
- Dong X, Mao S, Wen H. Upregulation of proinflammatory genes in skin lesions may be the cause of keloid formation. Biomed Rep. 2013;1(6):833–836. doi:10.3892/br.2013.169
- Nguyen JK, Austin E, Huang A, et al. The IL-4/IL-13 axis in skin fibrosis and scarring: mechanistic concepts and therapeutic targets. Arch Dermatol Res. 2020;312(2):81–92. doi:10.1007/s00403-019-01972-3
- Lee SY, Kim EK, Seo HB, et al. IL-17 induced stromal cell-derived factor-1 and profibrotic factor in keloid-derived skin fibroblasts via the STAT3 pathway. Inflammation. 2020;43(2):664–672. doi:10.1007/s10753-019-01148-1
- Shi CK, Zhao YP, Ge P, et al. Therapeutic effect of interleukin-10 in keloid fibroblasts by suppression of TGF-β/Smad pathway. Eur Rev Med Pharmacol Sci. 2019;23(20):9085–9092. doi:10.26355/eurrev_201910_19311
- Arita Y, Kihara S, Ouchi N, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. 1999. Biochem Biophys Res Commun. 2012;425(3):560–564. doi:10.1016/j.bbrc.2012.08.024
- Park PH, Sanz-Garcia C, Nagy LE. Adiponectin as an anti-fibrotic and anti-inflammatory adipokine in the liver. Curr Pathobiol Rep. 2015;3(4):243–252. doi:10.1007/s40139-015-0094-y
- Luo L, Li J, Liu H, et al. Adiponectin is involved in connective tissue growth factor-induced proliferation, migration and overproduction of the extracellular matrix in keloid fibroblasts. Int J Mol Sci. 2017;18(5):1044. doi:10.3390/ijms18051044
- Brochu-Gaudreau K, Rehfeldt C, Blouin R, et al. Adiponectin action from head to toe. Endocrine. 2010;37(1):11–32.
- Sullivan T, Smith J, Kermode J, et al. Rating the burn scar. J Burn Care Rehabil. 1990;11(3):256–260. doi:10.1097/00004630-199005000-00014
- Lodyga M, Hinz B. TGF-β1-A truly transforming growth factor in fibrosis and immunity. Semin Cell Dev Biol. 2019. doi:10.1016/j.semcdb.2019.12.010
- Mari W, Alsabri SG, Tabal N, et al. Novel insights on understanding of Keloid scar: article review. J Am Coll Clin Wound Spec. 2015;7(1–3):1–7. doi:10.1016/j.jccw.2016.10.001
- Lee HJ, Jang YJ. Recent understandings of biology, prophylaxis and treatment strategies for hypertrophic scars and Keloids. Int J Mol Sci. 2018;19(3).
- Distler JHW, Györfi AH, Ramanujam M, et al. Shared and distinct mechanisms of fibrosis. Nat Rev Rheumatol. 2019;15(12):705–730.
- Lee WJ, Song SY, Roh H, et al. Profibrogenic effect of high-mobility group box protein-1 in human dermal fibroblasts and its excess in keloid tissues. Sci Rep. 2018;8(1):8434. doi:10.1038/s41598-018-26501-6
- Stewart AG, Thomas B, Koff J. TGF-β: master regulator of inflammation and fibrosis. Respirology. 2018;23(12):1096–1097. doi:10.1111/resp.13415
- Lipson KE, Wong C, Teng Y, et al. CTGF is a central mediator of tissue remodeling and fibrosis and its inhibition can reverse the process of fibrosis. Fibrogenesis Tissue Repair. 2012;5(1):S24. doi:10.1186/1755-1536-5-S1-S24
- Yang H, Huang Y, Chen X, et al. The role of CTGF in the diabetic rat retina and its relationship with VEGF and TGF-β(2), elucidated by treatment with CTGFsiRNA. Acta Ophthalmol. 2010;88(6):652–659. doi:10.1111/j.1755-3768.2009.01641.x
- Ghazizadeh M, Tosa M, Shimizu H, et al. Functional implications of the IL-6 signaling pathway in keloid pathogenesis. J Invest Dermatol. 2007;127(1):98–105. doi:10.1038/sj.jid.5700564
- Zhu G, Cai J, Zhang J, et al. Abnormal nuclear factor (NF)-kappaB signal pathway and aspirin inhibits tumor necrosis factor alpha-induced NF-kappaB activation in keloid fibroblasts. Dermatol Surg. 2007;33(6):697–708. doi:10.1111/j.1524-4725.2007.33146.x
- Wang ZV, Scherer PE. Adiponectin, the past two decades. J Mol Cell Biol. 2016;8(2):93–100. doi:10.1093/jmcb/mjw011
- Liu C, Feng X, Li Q, et al. Adiponectin, TNF-α and inflammatory cytokines and risk of type 2 diabetes: a systematic review and meta-analysis. Cytokine. 2016;86:100–109. doi:10.1016/j.cyto.2016.06.028
- Yuan B, Huang L, Yan M, et al. Adiponectin downregulates TNF-α expression in degenerated intervertebral discs. Spine. 2018;43(7):E381–E389. doi:10.1097/BRS.0000000000002364
- Marangoni RG, Masui Y, Fang F, et al. Adiponectin is an endogenous anti-fibrotic mediator and therapeutic target. Sci Rep. 2017;7(1):4397. doi:10.1038/s41598-017-04162-1
- Fang F, Liu L, Yang Y, et al. The adipokine adiponectin has potent anti-fibrotic effects mediated via adenosine monophosphate-activated protein kinase: novel target for fibrosis therapy. Arthritis Res Ther. 2012;14(5):R229. doi:10.1186/ar4070