Abstract
Background
Sodium bituminosulfonate is derived from naturally occurring sulphur-rich oil shale and is used for the treatment of the inflammatory skin disease rosacea. Major molecular players in the development of rosacea include the release of enzymes that process antimicrobial peptides which, together with reactive oxygen species (ROS) and vascular endothelial growth factor (VEGF), promote pro-inflammatory processes and angiogenesis. The aim of this study was to address the molecular mechanism(s) underlying the therapeutic benefit of the formulation sodium bituminosulfonate dry substance (SBDS), which is indicated for the treatment of skin inflammation, including rosacea.
Methods
We investigated whether SBDS regulates the expression of cytokines, the release of the antimicrobial peptide LL-37, calcium mobilization, proteases (matrix metalloproteinase, elastase, kallikrein (KLK)5), VEGF or ROS in primary human neutrophils. In addition, activity assays with 5-lipoxygenase (5-LO) and recombinant human MMP9 and KLK5 were performed.
Results
We observed that SBDS reduces the release of the antimicrobial peptide LL-37, calcium, elastase, ROS and VEGF from neutrophils. Moreover, KLK5, the enzyme that converts cathelicidin to LL-37, and 5-LO that produces leukotriene (LT)A4, the precursor of LTB4, were both inhibited by SBDS with an IC50 of 7.6 µg/mL and 33 µg/mL, respectively.
Conclusion
Since LTB4 induces LL-37 which, in turn, promotes increased intracellular calcium levels and thereby, ROS/VEGF/elastase release, SBDS possibly regulates the LTB4/LL-37/calcium – ROS/VEGF/elastase axis by inhibiting 5-LO and KLK5. Additional direct effects on other pro-inflammatory pathways such as ROS generation cannot be ruled out. In summary, SBDS reduces the generation of inflammatory mediators from human neutrophils possibly accounting for its anti-inflammatory effects in rosacea.
Introduction
Rosacea is a chronic cutaneous inflammatory disease that affects the face. It can be categorized into erythematotelangiectatic, papulopustular, phymatous and ocular rosacea.Citation1 The overall rosacea manifestations are flushing, transient or persistent erythema, telangiectasia, papules, pustules, phymata, and (micro)edema. Worldwide, the prevalence of rosacea is estimated to reach over 5% in the adult population.Citation2 Rosacea is thought to be triggered by multiple factors such as bacterial proteases, heat, stress/irritant and UVB radiation.Citation3 These factors activate the immune system, induce inflammation, pain, vascular dilatation and angiogenesis in the skin, thus initiating and strengthening the clinical manifestations of rosacea.
The pathophysiology of rosacea remains to be elucidated, but numerous factors are known to contribute. Among these, dysregulation of the innate immune system, specifically macrophages, neutrophils and dendritic cells, is clearly implicated.Citation4 Macrophages and dendritic cells express TLR2 which is activated by external stimuli in rosacea leading to an increased release of interleukin (IL)-8, IL-1β and tumor necrosis factor alpha (TNF-α). Neutrophils are attracted by IL-8 and TNF-α and release proteases (such as kallikrein (KLK)5, matrix metalloprotease (MMP)9, elastase), and the anti-microbial peptide LL-37.Citation5 LL-37 was linked to the generation of reactive oxygen species (ROS), probably through nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activation and intracellular Ca2+ mobilization.Citation6 ROS play a role in the damage seen with photoaging, an etiologic factor in the development of rosacea.Citation7 Moreover, ROS induce production of VEGF,Citation8 which together with IL-1β and TNF-α, contribute to the vascular hyperreactivity seen in rosacea.Citation9 In summary, a variety of factors that activate the immune system (eg cytokines), induce connective tissue damage (eg ROS, proteases) or induce vascularization (eg VEGF) to drive the development of rosacea.
The importance of these factors in the development of rosacea becomes evident from the modes of action of approved drugs for rosacea. Common treatments are doxycycline and topical azelaic acid, among others. Doxycycline inhibits the production and activity of MMP9, inhibits the activity of KLK5, reduces pro-inflammatory cytokine release and the synthesis of ROS.Citation4 It was shown that topical azelaic acid inhibits expression of KLK5 and cathelicidins in keratinocytes and/or in treated rosacea patients.Citation4
Ichthyol (also known as ichthammol, ammonium bituminosulfonate), introduced to dermatology by German dermatologist Paul Gerson Unna in 1882, is an active ingredient derived from sulphur-rich oil shale with anti-inflammatory, antibacterial and antifungal properties.Citation10–Citation16 Different formulations are used worldwide and are employed in clinical practice for the treatment of a variety of skin diseases, including acne, eczema, psoriasis and rosacea.Citation17 According to the “list of preferred Specials” by the British Association of Dermatologists (BAD) Ichthammol can be used in dermatology prescribing to treat acutely inflamed atopic eczema, among others.Citation18 A corresponding recommendation exists for bituminosulfonates in Germany. In a double-blind, placebo-controlled study with 30 papulopustular rosacea patients, sodium bituminosulfonate enteric coated tablets showed a significant improvement in the number of papules/pustules, erythema, and scaling without inducing side effects. The dosage for the treatment of rosacea was two tablets three times per day during the first two weeks followed by one tablet three times per day during the next four weeks.Citation19 In 1952, the formulation of sodium bituminosulfonate dry substance (SBDS) was introduced onto market, and is only approved in Germany and indicated for the treatment of rosacea.
Apart from exerting anti-inflammatory and wound healing effects, Ichthyol has also been shown to inhibit the oxidative burst and migration of macrophages and leukotriene B4 release from neutrophils.Citation12,Citation20,Citation21 However, the detailed mechanisms and potential target cells and target proteins of Ichthyol are mostly unknown. Therefore, in this study, we tried to identify the potential mode(s) of action of SBDS on human neutrophils. For this purpose, we studied the generation of a variety of inflammatory products, including VEGF, ROS, Ca2+, proteases (elastase, MMP9, KLK5), and/or the synthesis of cytokines and chemokines by neutrophils. We also tested whether SBDS interacts with the activity of proteases and proinflammatory enzymes (KLK5, MMP9, 5-lipoxygenase (5-LO)). These products have been proposed to play a role in the etiology of rosacea.Citation3
Materials and Methods
Cells and Reagents
Primary human neutrophils were cultured in RPMI1640 medium supplemented with 10% fetal calf serum (FCS). The media contained 1% penicillin/streptomycin, and the cells were cultured at 37 °C in a 5% CO2 atmosphere. SBDS was dissolved in water and further diluted in media. Oil shale-derived SBDS was provided by the Ichthyol-Gesellschaft. PE Annexin V Apoptosis Detection Kit I was purchased from Becton Dickinson (Heidelberg, Germany). Leuko Spin medium was purchased from pluriSelect (Leipzig, Germany) and GM-CSF was purchased from Miltenyi Biotech GmbH (Bergisch Gladbach, Germany).
Isolation of Human Neutrophils
Human neutrophils were isolated from buffy coats using density double gradient centrifugation. For this, 40 mL buffy coats (German Red Cross, Frankfurt, Germany) or 50 mL of blood from healthy donors who had given their written informed consent for participation, were mixed with the same amount of 2 mM EDTA/PBS and were layered over 15 mL of peripheral blood mononuclear cells (PBMC) Spin Medium (top) and 15 mL Leuko Spin Medium in 50 mL tubes (Greiner Bio-One International GmbH, Frickenhausen, Germany). Tubes were centrifuged (1,000 g, 30 min, 20 °C; without brake), and plasma was removed. Cell layer between peripheral blood mononuclear cells and erythrocytes was collected and transferred to a 50 mL tube and washed with 2 mM EDTA/PBS once and centrifuge (330 g, 10 min, 20 °C). Cell pellet was resuspended in 1 mL 2 mM EDTA/PBS and remaining red blood cells were lysed by adding 25 mL of lysis buffer (155 mM NH4Cl, 10 mM KHCO3, 0.1 mM EDTA) and incubation for 5 min at room temperature (RT), washed again with 2 mM EDTA/PBS and cells were counted using a MACSQuant® Analyser 10 flow cytometer (Miltenyi Biotec, Bergisch Gladbach, Germany).
Cell Cytotoxicity Assay
1 x 105 Neutrophils suspended in RPMI1640-Medium containing 10% FCS and 50 ng/mL GM-CSF were seeded in a 96 well plate. SBDS (10, 50, 100 µg/mL) was added and incubated at 37 °C in an atmosphere containing 5% CO2 for 24h. The annexin staining was achieved as suggested by manufacturer. Neutrophils were washed with cold PBS and resuspended in Annexin V binding buffer supplemented with PE Annexin V and 7-Aminoactinomycin (7-AAD) and incubated for 15 min at RT (25 °C) in the dark. Cells were analyzed by flow cytometry within 1h. For analysis, FlowJo Software (V10) was used. Neutrophils were gated with the FSC and SSC channel. The neutrophil population was further analyzed by 7-AAD and Annexin V staining. Apoptosis was determined by relating the quantity of dead cells (Annexin V positive and 7-ADD positive) to the counts of all cells.
MMP9 Release Assay
1x106 Neutrophils (120 µL) in RPMI1640 medium supplemented with 10% FCS were seeded in a 96 well plate. 0, 10, 50 µg/mL SBDS were added and incubated for 30 min at 37 °C in an atmosphere of 5% CO2. 50 ng/mL GM-CSF was added and incubated for 1h at 37 °C in an atmosphere of 5% CO2. To investigate whether SBDS induces MMP9 release, cells were incubated for further 20 min at 37 °C in an atmosphere of 5% CO2. To investigate whether SBDS inhibits the release of MMP9 1 µM fMLP was added, incubated for 20 min at 37 °C in an atmosphere of 5% CO2, centrifuged at 300 g for 5 min at room temperature and the supernatant was collected. MMP9 concentration was determined with ELISA (Sigma Aldrich, Schnelldorf, Germany) according to the protocol of the supplier. The concentration of MMP9 was extrapolated using the standard curve.
Elastase Assay
The elastase assay was performed as described by Kanashiro et al.Citation22 Briefly, 25,000 neutrophils in 100 µL RPMI medium supplemented with 10% FCS were seeded in a 96 well Plate. 1µM Cytochalasin Band 0, 10, 50 µg/mL SBDS were added and incubated for 30 min at 37 °C in an atmosphere of 5% CO2. 1 mM SAAVNA (N-succinyl-Ala-Ala-Val-p-nitroanilide) (in the half of the wells) was added. To investigate whether SBDS inhibits the elastase release, the cells were stimulated with 50 ng/mL PMA for 30 min 37 °C in an atmosphere of 5% CO2. To investigate whether SBDS induces elastase release, the cells were not stimulated and only vehicle was added and incubated for 30 min at 37 °C in an atmosphere of 5% CO2. The released elastase (induced by PMA or SBDS) converts SAAVNA to p-nitroaniline which can be quantified spectrophotometrically at 410 nm with the EnSpire® (Perkin Elmer, Hamburg, Germany). For analysis, the absorbance values from samples with SAAVNA were corrected with samples without SAAVNA to obtain Δ absorbance.
VEGF Assay
The VEGF assay was adapted from Gaudry et al.Citation23 Briefly, 96-Well plates were precoated with 50 µL 0.01% collagen I (Sigma Aldrich, Schnelldorf, Germany) for at least 6 h at RT. Collagen I was removed and the coated surface was dried overnight. The wells were rinsed with sterile tissue culture grade water before transferring cell suspension. 1×106 Neutrophils in 100 µL RPMI medium supplemented with 10% FCS were seeded in a 96 well plate. SBDS (0, 10, 50 µg/mL) were added and incubated for 30 min at 37 °C in an atmosphere containing 5% CO2. To investigate whether SBDS can prevent VEGF release, neutrophils were stimulated with 50 ng/mL PMA for 2 h. To investigate whether SBDS induces VEGF release, cells were left untreated for 2 h. Cells were centrifuged (300 g, 5 min, RT), supernatant was collected and the concentration of VEGF determined by ELISA (Thermo Fisher Scientific, Germany), as recommended by the supplier. For analysis, the absorbance values from standard and from test samples were corrected with the blank (standard: assay buffer; samples: medium with SBDS), since the values of the controls decreased with increasing concentration of SBDS. The concentration of VEGF was extrapolated using the standard curve.
MMP9 Assay
The MMP9 assay was performed as described by Troeberg et al.Citation24 For the MMP9 assay, recombinant human MMP9 enzyme (R&D Systems, Wiesbaden, Germany), the fluorogenic substrate Mca-PLGL-Dpa-AR-NH2 (R&D Systems, Wiesbaden, Germany) and the activator p-aminophenylmercuric acetate (APMA) (Sigma Aldrich, Schnelldorf, Germany) were used. APMA was diluted in the TCNB-buffer (50 mM Tris HCl, 10 mM CaCl2, 150 mM NaCl, 0.05% Brij-35, pH 7.5). 100 µg/ml rhMMP9 were activated with 1 mM APMA and incubated at 37 °C for 24 h. SBDS and positive control GM6001 (EMD Millipore) were diluted in TCNB buffer and mixed with the activated rhMMP9 to reach the final concentration range of 0.61–10,000 µg/mL and 10 µM, respectively. The enzyme/SBDS mixture was incubated at 37 °C for 1 h, before the substrate 10 µM Mca-PLGL-Dpa-AR-NH2 was added for further 60 min. For background control, SBDS or vehicle were incubated with substrate in TCNB-buffer but without enzyme. The fluorescence was detected with the Multimode Plate Reader (Tecan, Crailsheim, Germany) (Ext 320 nm/Em 405 nm). The inhibition was calculated with the following equation: (1 - (A - B1)/(C - B2)) * 100. A = RFU Test sample; B1 = basal RFU without enzymes with compound; B2 = basal RFU without enzymes with vehicle; C = RFU vehicle control with enzymes. The test samples (A) were corrected for the RFU of samples without enzyme but with SBDS (B1), due to the high background signal of SBDS that was concentration dependent.
KLK5 Assay
The KLK5 assay was performed as described by Matsubara et al.Citation25 For the KLK5 assay, recombinant humane KLK5 enzyme (R&D Systems, Wiesbaden, Germany) and the fluorogenic substrate Boc-V-P-R-AMC substrate (Boc: t-Butyloxycarbonyl; AMC: 7-Amino-4-methylcoumarin) (R&D Systems, Wiesbaden, Germany) were used. The hydrolysis of the R-AMC amide bond releases the highly fluorescent group AMC. rhKLK5 was diluted in 1 M NaH2PO4 pH 8 and SBDS (0.0001 mg/mL – 10 mg/mL) or 42.1 µM Leupeptin or DMSO (background control (C) with enzyme without compound) was added and incubated for 5 min at RT. 100 µM Boc-V-P-R-AMC substrate was added and incubated for 5 min at RT. The fluorescence was detected with the Multimode Plate Reader (Tecan, Crailsheim, Germany) (Ex380 nm/Em460 nm). The inhibition was calculated with the following equation: (1 - (A - B1)/(C - B2)) * 100. A = RFU Test sample; B1 = basal RFU without enzymes with compound; B2 = basal RFU without enzymes with vehicle; C = RFU vehicle control with enzymes. The test samples (A) were corrected for the RFU of samples without enzyme but with SBDS (B1), due to the high background signal of SBDS that was concentration dependent.
LL-37 Detection Assay
The LL-37 assay was adapted from the protocol of Wan et alCitation26 1×106 Neutrophils (120 µL) in RPMI1640 medium supplemented with 10% FCS were seeded in 96 well plates. The neutrophils (isolated from blood) were preincubated with 0, 10, 50 µg/mL of SBDS for 30 min at 37 °C/5% CO2 atmosphere. Leukotriene B4 (LTB4) of 1 µM (Cayman Chemical, Michigan, USA) was added or the cells were left untreated and incubated for 5 min. The supernatant was collected and LL-37 was determined by ELISA, as recommended by the supplier (Hycultec GmbH, Beutelsbach, Germany). For analysis, the absorbance values from standard and from samples were corrected with that of the samples without cells. The concentration of LL-37 was extrapolated using the standard curve.
5-LO Assay
The 5-LO assay was performed as described previously.Citation27 Briefly, human granulocytes were isolated from buffy coats derived from healthy donors, washed once in Dulbecco’s PBS and finally resuspended in 1 mL PBS (with 1 mM Ca2+, 1 mg/mL glucose) and preincubated for 15 min with the test compounds at room temperature. The reaction was started by addition of arachidonic acid and ionophore A23187 in 10 µL of methanol (20 µM and 2.5 µM final concentrations, respectively). After 10 min at 37 °C, the reaction was stopped with 1 mL of methanol and 30 µL of 1N HCl, 200 ng of prostaglandin B1 (internal standard) and 500 µL of PBS were added. After centrifugation (10 min, 800 g, RT), the samples were applied to C-18 solid phase extraction columns (100 mg), which were conditioned with 1 mL methanol and 1 mL water. The columns were washed with 1 mL water and 1 mL 25% methanol. 5-LO metabolites were then extracted with 300 µL methanol. The extract was diluted with 120 µL water and 100 µL of the diluted extract were analyzed by HPLC.
ROS Assay
For the detection of reactive oxygen species (ROS), the dye dihydrorhodamine (DHR) (Sigma Aldrich, Schnelldorf, Germany) and the protocol of Chen et al was used.Citation28 DHR is converted to rhodamine in the presence of ROS which can be detected via flow cytometry. Neutrophils were suspended to a final concentration of 0.5×106 cells/100 µL in 10 mL Hank`s balanced salt solution (HBSS) (w/o Ca2+/Mg2+) and prewarmed in a water bath at 37 °C for 5 min. 200 µl Neutrophil suspension was added to a 96 well plate that already contained SBDS at the final concentration of 0, 10 and 50 µg/mL and incubated for 30 min at 37 °C. 375 ng/mL DHR or HBSS were added and the plate was incubated for 15 min. To investigate whether SBDS can prevent ROS induction, cells were stimulated with 30 ng/mL phorbol-12-myristate-13-acetate (PMA) (Sigma Aldrich, Schnelldorf, Germany). To investigate whether SBDS induces ROS induction, the cells were not stimulated with PMA and instead HBSS was added. The reaction was stopped by cooling the plate on ice for 10 min. The plate was centrifuged (400 × g, 5 min, 4 °C), supernatants were removed and the cells were washed three times with 250 µL HBSS. The cell pellets were resuspended in 300 μL fluorescence-activated cell scanning (FACS) fixing solution (Flow cytometry sheath fluid (Miltenyi Biotec, Bergisch Gladbach, Germany) containing 0.5% formaldehyde) and measured with the MACSQuant10 flow cytometer (Miltenyi Biotec, Bergisch Gladbach, Germany). The fluorescence value of the samples was corrected with that of the unstained sample to obtain the Δmedian value.
Calcium Release Assay
The calcium assay was adapted from Ingelfinger et al.Citation29 Briefly, neutrophils were resuspended in 1 mL HBSS (without Ca2+/Mg2+), centrifuged at 300 g (RT) for 10 min and the supernatant discarded. The wash step with HBSS was repeated. 10×106 neutrophils/mL in HBSS (without Ca/Mg) were mixed with 4 µM Fluo-8 (Abcam, Berlin, Germany) and incubated for 1 h at 37 °C in an atmosphere of 5% CO2. Cells were centrifuged at 300g (RT) for 10 min and the supernatant was removed. Cell pellets were dissolved 1 mL HBSS (without Ca/Mg), centrifuged at 300g (RT) for 10 min and the supernatant was removed. 1×106 Neutrophils (100 µL) in HBSS supplemented with 1.25 mM CaCl2 were seeded in a 96 black well plate (Greiner Bio-One GmbH, Essen, Germany). SBDS (0, 10, 50 µg/mL) was added, the plate was transferred into the Multimode Plate Reader (Tecan, Crailsheim, Germany) and incubated for 15 min at 37 °C and fluorescence (Ext/Em 490/ 520) was detected. 1 µM formyl-Methionyl-Leucyl-Phenylalanine (fMLP) (Sigma Aldrich, Schnelldorf, Germany) was added with the injector of the reader and the fluorescence was followed for 15 min. For analysis, the fluorescence value obtained after addition of fMLP was related to the fluorescence value obtained before the addition of fMLP.
Cytokine Expression
2 x 106 Neutrophils in 2 mL RPMI1640-Medium supplemented containing 10% FCS and 50 ng/mL GM-CSF were seeded in 6 well plates and incubated at 37 °C in an atmosphere of 5% CO2 for 1.5 h. Cells were preincubated with SBDS (0, 10, 50 µg/mL) for 30 min and 50 µg/mL zymosan were added for 1h at 37 °C in an atmosphere of 5% CO2. Cells were harvested and mRNA was extracted with the RNeasy Mini Kit (Qiagen, Hilden, Germany), as recommended by the supplier. mRNA was transcribed to cDNA with RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Germany), as recommended by the supplier. qPCR was achieved with 10 ng cDNA, 0.5 µL 5 mM of each Primer and SYBR® Select Master Mix in the QuantStudio™ 12K Real-Time PCR System. The primers used were partly taken from Hayashi et alCitation30 and are described in . For analysis, the ΔΔCT method was used. The mRNA expression levels were normalized to the reference gene glycerinaldehyde-3-phosphate-dehydrogenase (GAPDH) and related to the control samples.
Table 1 Primer Sequences
Statistical Analyses
Results are presented as means ± standard errors. The data were analysed with one-way ANOVA and with Tukey`s multiple comparisons test or two-way ANOVA and with Dunnett’s or Tukey`s multiple comparisons test. For all calculations and creation of graphs, GraphPad Prism 8 was used and p < 0.05 was considered the threshold for significance.
Results
SBDS Inhibits the Release of Elastase
To assess the cytotoxic effects of SBDS in primary neutrophils, a cytotoxicity assay based on the staining of phosphatidylserine, expressed on the surface of apoptotic cells, was performed.Citation31 The determination of plasma levels of SBDS-treated patients is currently not possible, since sulfonated shale oil consists of more than 10,000 different substances of which only around 100 have been elucidatedCitation17 Therefore, we used a concentration range of sulfonated shale oil that has already shown efficacy in cell culture models.Citation10,Citation13 Neutrophils were treated with SBDS over a concentration range of 10–100 µg/mL for 24 h. Interestingly, 100 µg/mL SBDS showed only a weak cytotoxic effect on neutrophils (). Nevertheless, for subsequent experiments, concentrations of up to 50 µg/mL SBDS were used.
Figure 1 SBDS actions on viability and effector functions of primary neutrophils. (A) Primary neutrophils were treated for 24 h with SBDS at the indicated concentrations. The quantity of dead cells (Annexin V+ and 7-AAD+) was related to that of all cells. The flow cytometry data were analyzed by FlowJo 10.1 Software. (B and C). For MMP9 and VEGF release, primary neutrophils were preincubated for 30 min with SBDS at the indicated concentrations and stimulated with 1 µM fMLP, 50 ng/mL PMA or kept untreated for 20 min or 2h, respectively. For elastase release, neutrophils were preincubated with SBDS at the indicated concentration for 30 min and stimulated with 50 ng/mL PMA for 20h. The experiments were performed in three biological and technical replicates. One way ANOVA with Dunnett`s multiple comparisons test was used to assess statistical significance of differences. *p<0.05, ***p<0.001 indicate significant difference between treated samples and vehicle treated sample (0 µg/mL).
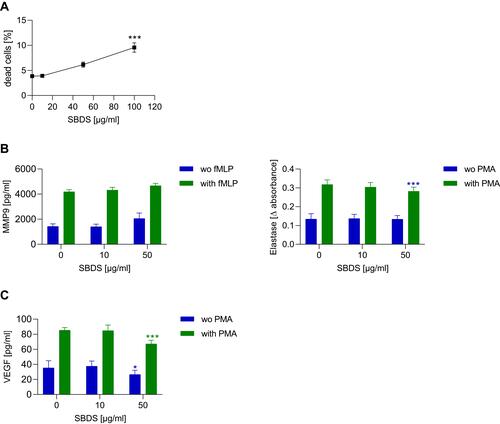
To elucidate how SBDS acts to ameliorate inflammatory processes, we investigated whether it inhibits or induces the release of proteases. To elucidate the inhibitory effects, neutrophils were preincubated with SBDS and then stimulated with PMA for determination of elastase release or with fMLP for MMP9 release. As expected, PMA induced the release of elastase and fMLP the release of MMP9. SBDS did not affect the PMA-induced release of MMP9, but reduced the release of elastase in activated neutrophils (). For the induction assay, neutrophils were only treated with SBDS to investigate whether SBDS is able to induce the release of proteases. SBDS alone did not induce the release of MMP9 or elastase ().
SBDS Inhibited VEGF Release
Besides proteases, neutrophils contain an intracellular pool of VEGF that is secreted under stimulation with PMA.Citation23 Since VEGF plays a major role in rosacea pathology, we also investigated whether SBDS affects the release of VEGF. As expected, PMA increased the release of VEGF about 2.4-fold. Interestingly, SBDS at 50 µg/mL inhibited the release of VEGF in PMA stimulated neutrophils 1.9-fold as well as in unstimulated neutrophils ().
SBDS Inhibits MMP9 and KLK5
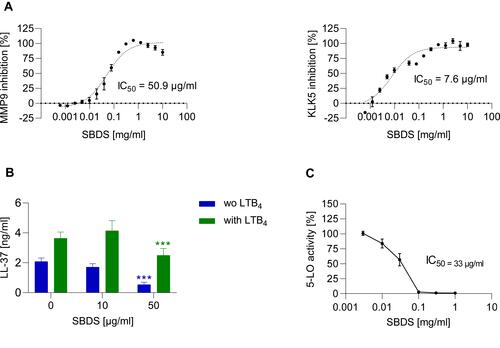
Next, we investigated whether SBDS inhibits the activities of the proteases MMP9 and KLK5. As a positive control, 10 µM GM6001 was used for the MMP9 assay and showed inhibition of ≈ 88%; 42.1 µM Leupeptin was used as a positive control for the KLK5 activity assay, causing inhibition of ≈ 94%. For SBDS, IC50 values of 50.9 µg/mL for MMP9 and of 7.6 µg/mL for KLK5 were observed ().
Figure 2 SBDS inhibits the activity of MMP9, KLK5, 5-LO and the release of LL-37. (A) The inhibition of MMP9 and KLK5 by SBDS was determined using human recombinant enzyme. (B) For the determination of LL-37, primary neutrophils were preincubated for 30 min with SBDS at the indicated concentrations and stimulated with 1 µM LTB4 or left untreated for 5 min. (C) 5-LO inhibition by SBDS was determined in primary human neutrophils. The IC50 values were calculated using GraphPad Prism Software. ***p<0.001 indicate significant difference between treated samples and vehicle treated sample (0 µg/mL).
SBDS Inhibits the Release of LL-37 in Association with Inhibition of 5-LO
Since our data indicate that SBDS inhibits the activity of KLK5, the protease that cleaves cathelicidin to LL-37, we investigated whether SBDS also reduces the release of LL-37. LL-37 release can be stimulated by leukotriene B4 (LTB4) within 5 minutes via the activation of the BLT1 receptor.Citation26 Neutrophils were preincubated with SBDS and subsequently treated with LTB4 or were left unstimulated. LTB4 induced the release of LL-37 which was inhibited by 50 µg/mL SBDS. Interestingly, SBDS significantly reduced the release of LL-37 in unstimulated neutrophils ().
In human neutrophils, a positive feedback circuit exists between LL-37 and LTB4 which mutually stimulates their release.Citation26 LL-37 elicits translocation of 5-LO from the cytosol to the perinuclear membrane in polymorphonuclear cells and promotes the synthesis and release of LTA4 the precursor of LTB4. Since we observed reduced release of LL-37 by SBDS in unstimulated neutrophils, we investigated whether this effect is possibly mediated via inhibition of 5-LO. SBDS inhibited 5-LO with an IC50 value of 33 µg/mL and inhibited LTB4-induced LL-37 release over a comparable concentration range of 10–50 µg/mL (). These data indicate that SBDS inhibits synthesis of LTA4 (product of 5-LO) leading to a reduced level of LTB4. The reduced level of LTB4 in turn, probably lowers the release of LL-37.
SBDS Inhibits ROS Production and Ca2+ Signaling
The generation of ROS, by activation of NADPH oxidase and intracellular Ca2+ mobilization is possibly mediated by LL-37.Citation6 Therefore, we investigated whether SBDS affects ROS production and Ca2+ signaling in neutrophils. ROS production was stimulated by PMA in neutrophils. In unstimulated neutrophils, 50 µg/mL SBDS induced weak ROS release, whereas 50 µg/mL SBDS clearly inhibited PMA-induced ROS release ().
Figure 3 SBDS inhibits ROS release and calcium mobilization. (A) Primary neutrophils were pretreated for 30 min with SBDS at the indicated concentrations and stimulated with 30 ng/mL PMA or were unstimulated and incubated for 1.5 h. ROS were detected via flow cytometry. (B) Primary neutrophils were pretreated with SBDS at the indicated concentrations for 30 min and stimulated with 1 µM fMLP. The experiments were performed in three biological and technical replicates. Two way or One way ANOVA with Dunnett`s multiple comparisons test was used. *p<0.05, ***p<0.001 indicate significant differences between treated samples and vehicle treated sample (0 µg/mL).
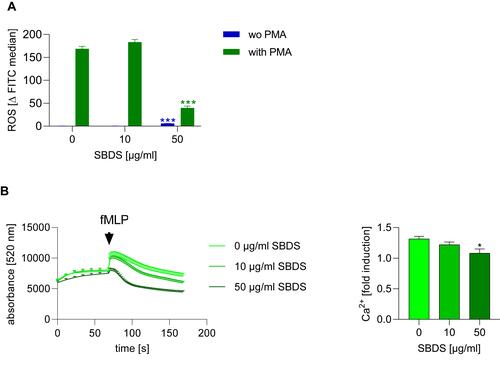
We also investigated whether SBDS influences calcium release. The chemotactic peptide fMLP was used to induce calcium release in neutrophils. As expected, fMLP induced calcium release in HEK293 cells. SBDS was not effective in unstimulated HEK293 cells but inhibited, in a concentration dependent manner, the fMLP induced calcium release ().
SBDS Increased Chemokine mRNA Expression Level
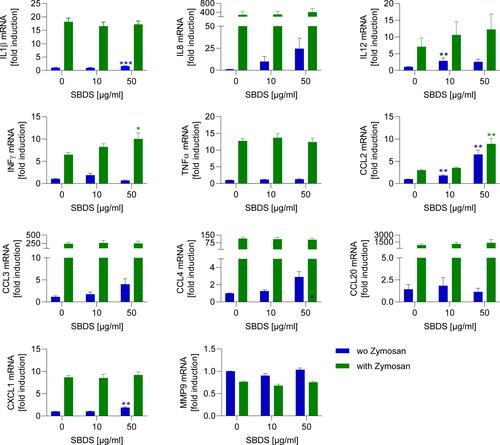
Our results indicate that SBDS inhibits the synthesis of LTA4, the precursor of LTB4, a chemotactic lipid mediator. To investigate whether SBDS also interacts with the generation of chemokines and cytokines, the effect of SBDS on zymosan-induced cytokine and chemokine expression was analyzed. Hayashi et al showed that some cytokines are constitutively expressed (IFNγ, TNFα, IL12, CCL2) and that some are inducible via TLR2 agonists such as zymosan (CCL3, CCL4, CCL20, CXCL1, IL8, IL1β) in neutrophils.Citation30 As expected, the inducible cytokines, apart from CXCL1, were strongly expressed after stimulation with zymosan. In unstimulated neutrophils SBDS upregulated the mRNA expression of IL1β, IL12, CCL2 and CXCL1. However, apart from that of CCL2, the increase was minor (1.5–2.5 fold) in comparison to the zymosan-induced alteration. In zymosan-stimulated neutrophils, SBDS only upregulated constitutively expressed cytokines, IFNγ and CCL2 ().
Figure 4 SBDS action on cytokine release in primary neutrophils. Primary neutrophils were preincubated for 30 min with SBDS at the indicated concentrations and stimulated with 50 µg zymosan or left untreated for 1.5 h and the release of cytokines was determined by quantitative PCR. The mRNA expression of cytokines was normalized to the reference gene GAPDH and related to the vehicle control. Two way ANOVA with Dunnett`s multiple comparisons test was used to assess differences. *p<0.05, **p<0.01, ***p<0.001 indicates significant difference between treated samples and vehicle treated sample (0 µg/mL).
Discussion
Neutrophils are indispensable to defense against microbes, which they effectively counter by releasing toxic enzymes, synthesizing ROS and by producing inflammatory mediators. Recent findings have also highlighted an important role of neutrophils as promoters of the resolution of the inflammatory process, indicating that their biological functions go well beyond simple pathogen killing.Citation32 Moreover, microbes such as demodex mites are thought to induce inflammation, activate neutrophils and contribute thereby to the development of rosacea.Citation33 In this regard, it is highly relevant that our results indicate that SBDS suppresses the inflammatory potential of neutrophils by reducing the release of LL-37, elastase, VEGF, ROS and calcium mobilization, as well as inhibiting 5-LO, KLK-5 and MMP9 activities.
Ichthyol has previously been reported to inhibit 5-LO.Citation10 We also observed that SBDS inhibits 5-LO and over the same concentration range, reduces the release of elastase, VEGF, ROS and LL-37 in neutrophils. In leukocytes, although not having any effect on unstimulated neutrophils, Ichthyol reduced the ionophore-induced synthesis of LTB4.Citation12 We speculate that these effects are linked and that Ichthyol/SBDS interacts with the stimulated LTB4/LL-37/Ca2+/granular release (VEGF, elastase, ROS) axis (). Since Ichthyol did not inhibit leukotriene release in unstimulated neutrophils,Citation12 different mechanisms in stimulated versus unstimulated neutrophils possibly occur.
Figure 5 Scheme of the potential interaction of SBDS with the LTB4/LL-37/Ca2+/ROS-Elastase-VEGF axis. 5-LO synthesizes LTA4, a precursor of LTB4. LTB4 induces the release of LL-37 produced by degradation of cathelicidin by KLK5. LL-37 mediates a positive feedback by activating 5-LO. LL-37 induces mobilization of calcium that in turn, induces KLK5 release and the release of four different granules: primary granules (1 °) loaded with eg elastase, secondary granules (2 °) containing eg cathelicidin, tertiary granules (3 °) with eg MMP9 and fourthly, secretory vesicles (not shown). SBDS directly inhibited the activities of 5-LO, KLK5 and MMP9, as indicated by the IC50 values. In addition, SBDS reduced the release of LL-37, ROS, elastase and VEGF, as indicated by the red arrow, whereas the release of MMP9 was not influenced, as shown by the green arrow.
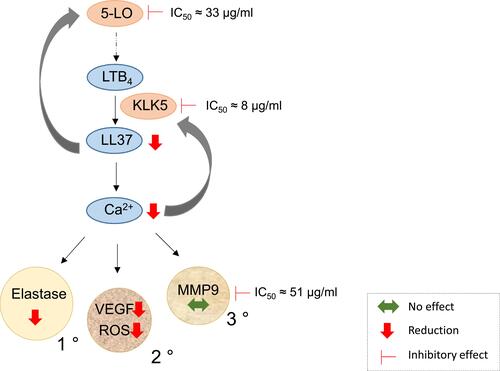
Reduction of the release of LL-37 by SBDS possibly occurs in two ways. First, SBDS inhibits KLK-5, the enzyme that processes the cathelicidin precursor protein (hCAP)18 to LL-37. Second, SBDS inhibits 5-LO, the enzyme that synthesizes LTA4, a precursor of LTB4, a lipid mediator that induces the release of LL-37. Moreover, between LTB4 and LL-37 a positive feedback loop exists, so that an increase in LL-37 further activates the 5-LO. In the presence of SBDS, it is possible that this feedback activation is blocked due to reduced LL-37 generation. The LTB4/LL-37/Ca2+/granular release axis seems to be of relevance in rosacea patients, since LL-37 and KLK-5 are overexpressed in rosacea patients.Citation34 In mice, LL-37 injection led to the exhibition of clinical features of rosacea such as erythema, telangiectasia and inflammation, possibly through the enhanced expression of IL-1, IL6 and MMP9 in mast cells.Citation35,Citation36 In addition, LL-37 affects angiogenesis by enhancing the proliferation of endothelial cells.Citation37
LL-37 induces release of calcium which in turn, is linked to ROS synthesisCitation6 and KLK5 release.Citation38 Moreover, calcium signaling is associated with the mobilization of granules.Citation39 In neutrophils, four type of granules exist: Primary granules contain inflammatory enzymes, such as elastase, proteinase 3 (a further protease that cleaves hCAP18 to LL-37), secondary granules are loaded among others with lactoferrin and hCAP18; in tertiary granules, MMP9 is found; and the secretory vesicle represents the fourth granule type.Citation39–Citation41 These granules differ in content and also their extent of mobilization. Tertiary granules are mobilized more readily than secondary granules, which again are exocytosed more readily than primary granules.Citation42 Components of the NADPH oxidase (gp91 phox, p22phox) that synthesize ROS were found in the secondary, tertiary granules and in the secretory vesicle,Citation42 whereas VEGF was suggested to be located in secondary granules.Citation23 It seems that SBDS prevents specifically the release of primary and secondary granules, because SBDS reduced the release of elastase, VEGF and ROS, but not of MMP9 (). It cannot be ruled out completely that the lack of effect on MMP9 may be related to the high basal MMP9 production in non-stimulated neutrophils (w/o fMLP), possibly due to artifactual effects of isolation of the cells from blood.Citation43 However, the effect of SBDS on ROS release seemed more pronounced than on the other variables, so an additional action of the compound may also occur. The reduction of LL-37 by SBDS could be due to the prevention of the mobilization of the primary and secondary granules, because these granules contain hCAP18. However, the reduced LL-37 level could also be due to the inhibition of 5-LO or KLK5 by SBDS. Whether SBDS inhibits the release of secondary and primary granules and whether this effect is mediated via the inhibition of calcium signaling, remain to be addressed in further studies.
SBDS increased CCL2 mRNA expression in neutrophils and thereby, possibly promotes the recruitment of CCR2 expressing cells such as monocytes. Since monocytes, depending on the inflammatory environment, can differentiate to M1 or M2 macrophages, with pro-inflammatory and pro-resolving roles, respectively, the ultimate effect of SBDS on inflammatory processes is still unclear. Additionally, LL-37 increases cytokine and chemokine liberation from leukocytes and has chemotactic effects on a large number of immune cells.Citation44 Thus, through its LL-37 suppressing effect, SBDS possibly counteracts the outworking of an increased CCL2 expression. Moreover, ROS are responsible for initiation of several proinflammatory effects in the skin, including expression of the leukocyte-attracting chemokines CCL2 and CXCL8.Citation45 Thus, via the reduction of ROS, SBDS possibly counteracts the increased expression of CCL2 and prevents the induction of pro-inflammatory effects. However, the inflammatory response in rosacea appears to be complex and other cells and mechanisms need to be taken into account. The expression of the Toll-like receptor pathway and of several other chemokines than those we measured, generated by lymphocytes or fibroblasts, has very recently been shown to be enhanced in rosacea biopsies.Citation46 Consequently, a more extensive analysis of gene expression of chemokines and other mediators by these other cell types is worth considering in response to SBDS.
Interestingly, doxycycline which is also used for the treatment of rosacea, revealed similar effects to those of SBDS. The following effects have been obtained/published for SBDS and doxycycline: 1) Inhibition of KLK5Citation47 and MMP9 activity,Citation48 reduction of the release of LL-37Citation49 and ROSCitation47 by doxycycline and SBDS. 2) Reduction of the release of VEGF by SBDS and inhibition of angiogenesis by doxycycline shown in HUVECs.Citation48 3) Inhibition of cytokine release (IL6, IL8, TNFα) in LPS-induced HaCaT cells by doxycyclineCitation50 but no effect of SBDS on IL8 and TNFα mRNA expression in neutrophils. These data indicate that doxycycline and SBDS possibly exert their effects via similar pathways. However, further studies are needed which directly compare these treatment strategies in vitro to gain more detailed insight into the mode of action of SBDS.
Conclusions
SBDS appears to exert its anti-inflammatory effects in rosacea via several mechanisms, but a major target could be the LTB4/LL-37/Ca2+/granular release axis. Our data suggest that SBDS possibly regulates anti-inflammatory processes (eg reduction of immune cell recruitment, and cytokine release by other immune cells, proliferation of endothelial cells, and angiogenesis) by suppressing LL-37, ROS, elastase and VEGF release from neutrophils. In summary, SBDS mediates, at least in part, anti-inflammatory effects by reducing some effector functions of neutrophils. Further studies are needed to clarify the biological relevance of the observed effects and to investigate the underlying mechanisms.
Abbreviations
7-AAD, 7-Aminoactinomycin; ANOVA, analysis of variance; APMA, p-aminophenylmercuric acetate; FACS, fluorescence-activated cell sorting; FCS, fetal calf serum; fMLP, Formyl-Methionyl-Leucyl-Phenylalanine; GAPDH, glyceraldehyde-3-phosphate-dehydrogenase; GM-CSF, granulocyte macrophage colony-stimulating factor; HBSS, Hank’s balanced salt solution; IL, interleukin; KLK5, kallikrein 5; 5-LO, 5 Lipoxygenase; LTB4, leukotriene B4; MMP9, matrix metalloproteinase 9; PBS, phosphate-buffered saline; PBMC, peripheral blood mononuclear cells; PMA, phorbol-12-myristate-13-acetate; RFU, relative fluorescence unit; RT, room temperature; SBDS, sodium bituminosulfonate dry substance; SAAVNA, N-succinyl-Ala-Ala-Val-p-nitroanilide; TNFα, tumor necrosis factor alpha.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Acknowledgment
This work was supported by the Landesoffensive zur Entwicklung wissenschaftlich-ökonomischer Exzellenz (LOEWE), Centre Translationale Medizin und Pharmakologie (TMP) and partially funded by ICHTHYOL-GESELLSCHAFT Cordes, Hermanni & Co. (GmbH & Co.) KG.
Disclosure
Current address of M.J.P. is EpiEndo Pharmaceuticals ehf, 170 Seltjarnarnes, Iceland. Prof. M.J.P. also reports contract fee from ICHTHYOL-GESELLSCHAFT Cordes, Hermanni & Co. (GmbH & Co.) KG, grants from Landesoffensive zur Entwicklung wissenschaftlich-ökonomischer Exzellenz (LOEWE), Centre Translationale Medizin und Pharmakologie (TMP) of the State of Hessen, during the conduct of the study. The authors report no other conflicts of interest in this work.
Additional information
Funding
References
- Zhang H, Tang K, Wang Y, Fang R, Sun Q. Rosacea treatment: review and update. Dermatol Ther. 2021;11(1):13–24. doi:10.1007/s13555-020-00461-0
- Gether L, Overgaard LK, Egeberg A, Thyssen JP. Incidence and prevalence of rosacea: a systematic review and meta-analysis. Br J Dermatol. 2018;179(2):282–289. doi:10.1111/bjd.16481
- Buddenkotte J, Steinhoff M. Recent advances in understanding and managing rosacea. F1000Res. 2018;7:1885. doi:10.12688/f1000research.16537.1
- Woo YR, Lim JH, Cho DH, Park HJ. Rosacea: molecular mechanisms and management of a chronic cutaneous inflammatory condition. Int J Mol Sci. 2016;17(9):1562. doi:10.3390/ijms17091562
- Meyer-Hoffert U, Schroder JM. Epidermal proteases in the pathogenesis of rosacea. J Investig Dermatol Symp Proc. 2011;15(1):16–23. doi:10.1038/jidsymp.2011.2
- Zheng Y, Niyonsaba F, Ushio H, et al. Cathelicidin LL-37 induces the generation of reactive oxygen species and release of human alpha-defensins from neutrophils. Br J Dermatol. 2007;157(6):1124–1131. doi:10.1111/j.1365-2133.2007.08196.x
- Millikan L. The proposed inflammatory pathophysiology of rosacea: implications for treatment. Skinmed. 2003;2(1):43–47. doi:10.1111/j.1540-9740.2003.01876.x
- Kuroki M, Voest EE, Amano S, et al. Reactive oxygen intermediates increase vascular endothelial growth factor expression in vitro and in vivo. J Clin Invest. 1996;98(7):1667–1675. doi:10.1172/JCI118962
- Gerber PA, Buhren BA, Steinhoff M, Homey B. Rosacea: the cytokine and chemokine network. J Investig Dermatol Symp Proc. 2011;15(1):40–47. doi:10.1038/jidsymp.2011.9
- Diezel W, Schewe T, Rohde E, Rosenbach T, Czarnetzki BM. [Ammonium bituminosulfonate (Ichthyol). Anti-inflammatory effect and inhibition of the 5-lipoxygenase enzyme]. Hautarzt. 1992;43(12):772–774. [German].
- Unna PG. Aphorismen über Schwefeltherapie und Schwefelpräparate. IV Ichthyol. Monatshefte für Praktische Dermatologie. 1882;1:328–333.
- Czarnetzki BM. Inhibitory effects of shale oils (Ichthyols) on the secretion of chemotactic leukotrienes from human leukocytes and on leukocyte migration. J Investig Dermatol. 1986;87(6):694–697. doi:10.1111/1523-1747.ep12456630
- Schewe C, Schewe T, Rohde E, Diezel W, Czarnetzki BM. Inhibitory effects of sulfonated shale oils (ammonium bituminosulphonates, Ichthyols) on enzymes of polyenoic fatty acid metabolism. Arch Dermatol Res. 1994;286(3–4):137–141. doi:10.1007/BF00374208
- Kownatzki E, Kapp A, Uhrich S. Inhibitory effect of sulfonated shale oils (ammonium bituminosulfonate) on the stimulation of neutrophilic granulocytes by the chemotactic tripeptide f-Met-Leu-Phe. Arch Dermatol Res. 1986;278(3):190–193. doi:10.1007/BF00412922
- Pantke R. [Bacteriological studies of drugs from shale oil]. Arzneimittel-Forschung. 1965;15(5):570–573. [German].
- Listemann H, Scholermann A, Meigel W. [Antifungal activity of sulfonated shale oils]. Arzneimittel-Forschung. 1993;43(7):784–788. [German].
- Boyd AS. Ichthammol revisited. Int J Dermatol. 2010;49(7):757–760. doi:10.1111/j.1365-4632.2010.04551.x
- Buckley DA, Root T, Bath S. Specials recommended by the British association of dermatologists for skin disease. London, UK: Clinical Standards Unit of the British Association of Dermatologists; 2014:9 Available from: www.bad.org.uk/specials. Accessed May 31, 2021.
- Koch R, Wilbrand G. Dark sulfonated shale oil vs placebo in the systemic treatment of rosacea. J Euro Acad Dermatol Venereol. 1999;12(2Suppl):143.
- Rabe KF, Perkins RS, Dent G, Gustmann H, Barnes PJ. Inhibitory effects of sulfonated shale oil fractions on the oxidative burst and Ca++ mobilization in stimulated macrophages. Arzneimittel-Forschung. 1994;44(2):166–170.
- Kownatzki E, Uhrich S, Schopf E. The effect of a sulfonated shale oil extract (Ichthyol) on the migration of human neutrophilic granulocytes in vitro. Arch Dermatol Res. 1984;276(4):235–239. doi:10.1007/BF00414234
- Kanashiro A, Souza JG, Kabeya LM, Azzolini AE, Lucisano-Valim YM. Elastase release by stimulated neutrophils inhibited by flavonoids: importance of the catechol group. Z Naturforsch C J Biosci. 2007;62(5–6):357–361. doi:10.1515/znc-2007-5-607
- Gaudry M, Bregerie O, Andrieu V, El Benna J, Pocidalo MA, Hakim J. Intracellular pool of vascular endothelial growth factor in human neutrophils. Blood. 1997;90(10):4153–4161. doi:10.1182/blood.V90.10.4153
- Troeberg L, Nagase H. Monitoring metalloproteinase activity using synthetic fluorogenic substrates. Curr Protoc Protein Sci. 2004;Unit–21.16.
- Matsubara Y, Matsumoto T, Koseki J, Kaneko A, Aiba S, Yamasaki K. Inhibition of human kallikrein 5 protease by triterpenoids from natural sources. Molecules. 2017;22(11):1829. doi:10.3390/molecules22111829
- Wan M, Sabirsh A, Wetterholm A, Agerberth B, Haeggstrom JZ. Leukotriene B4 triggers release of the cathelicidin LL-37 from human neutrophils: novel lipid-peptide interactions in innate immune responses. FASEB J. 2007;21(11):2897–2905. doi:10.1096/fj.06-7974com
- Wisniewska JM, Rodl CB, Kahnt AS, et al. Molecular characterization of EP6--a novel imidazo[1,2-a]pyridine based direct 5-lipoxygenase inhibitor. Biochem Pharmacol. 2012;83(2):228–240. doi:10.1016/j.bcp.2011.10.012
- Chen Y, Junger WG. Measurement of oxidative burst in neutrophils. Methods Mol Biol. 2012;844:115–124.
- Ingelfinger R, Henke M, Roser L, et al. Unraveling the pharmacological potential of lichen extracts in the context of cancer and inflammation with a broad screening approach. Front Pharmacol. 2020;11:1322. doi:10.3389/fphar.2020.01322
- Hayashi F, Means TK, Luster AD. Toll-like receptors stimulate human neutrophil function. Blood. 2003;102(7):2660–2669. doi:10.1182/blood-2003-04-1078
- Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995;184(1):39–51. doi:10.1016/0022-1759(95)00072-I
- Rosales C. Neutrophil: a cell with many roles in inflammation or several cell types? Front Physiol. 2018;9:113. doi:10.3389/fphys.2018.00113
- McMahon F, Banville N, Bergin DA, et al. Activation of neutrophils via IP3 pathway following exposure to demodex-associated bacterial proteins. Inflammation. 2016;39(1):425–433. doi:10.1007/s10753-015-0264-4
- Yamasaki K, Di Nardo A, Bardan A, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med. 2007;13(8):975–980. doi:10.1038/nm1616
- Kim M, Kim KE, Jung HY, et al. Recombinant erythroid differentiation regulator 1 inhibits both inflammation and angiogenesis in a mouse model of rosacea. Exp Dermatol. 2015;24(9):680–685. doi:10.1111/exd.12745
- Muto Y, Wang Z, Vanderberghe M, Two A, Gallo RL, Di Nardo A. Mast cells are key mediators of cathelicidin-initiated skin inflammation in rosacea. J Investig Dermatol. 2014;134(11):2728–2736. doi:10.1038/jid.2014.222
- Schwab VD, Sulk M, Seeliger S, et al. Neurovascular and neuroimmune aspects in the pathophysiology of rosacea. J Investig Dermatol Symp Proc. 2011;15(1):53–62. doi:10.1038/jidsymp.2011.6
- Yamasaki K, Kanada K, Macleod DT, et al. TLR2 expression is increased in rosacea and stimulates enhanced serine protease production by keratinocytes. J Investig Dermatol. 2011;131(3):688–697. doi:10.1038/jid.2010.351
- Lacy P. Mechanisms of degranulation in neutrophils. Allergy Asthma Clin Immunol. 2006;2(3):98–108. doi:10.1186/1710-1492-2-3-98
- Sorensen OE, Follin P, Johnsen AH, et al. Human cathelicidin, hCAP-18, is processed to the antimicrobial peptide LL-37 by extracellular cleavage with proteinase 3. Blood. 2001;97(12):3951–3959. doi:10.1182/blood.V97.12.3951
- Csernok E, Ludemann J, Gross WL, Bainton DF. Ultrastructural localization of proteinase 3, the target antigen of anti-cytoplasmic antibodies circulating in Wegener’s granulomatosis. Am J Pathol. 1990;137(5):1113–1120.
- Cowland JB, Borregaard N. Molecular characterization and pattern of tissue expression of the gene for neutrophil gelatinase-associated lipocalin from humans. Genomics. 1997;45(1):17–23. doi:10.1006/geno.1997.4896
- Makowski GS, Ramsby ML. Gelatinolytic and fibrinolytic activity in fresh-frozen plasma. J Biomed Sci. 2004;11(4):531–533. doi:10.1007/BF02256103
- Schauber J, Gallo RL. Expanding the roles of antimicrobial peptides in skin: alarming and arming keratinocytes. J Investig Dermatol. 2007;127(3):510–512. doi:10.1038/sj.jid.5700761
- Lee HM, Shin DM, Kim KK, Lee JS, Paik TH, Jo EK. Roles of reactive oxygen species in CXCL8 and CCL2 expression in response to the 30-kDa antigen of Mycobacterium tuberculosis. J Clin Immunol. 2009;29(1):46–56. doi:10.1007/s10875-008-9222-3
- Sun Y, Chen LH, Lu YS, et al. Identification of novel candidate genes in rosacea by bioinformatic methods. Cytokine. 2021;141:155444. doi:10.1016/j.cyto.2021.155444
- Schaller M, Schofer H, Homey B, et al. State of the art: systemic rosacea management. J Dtsch Dermatol Ges. 2016;14(Suppl 6):29–37.
- Su W, Li Z, Li F, Chen X, Wan Q, Liang D. Doxycycline-mediated inhibition of corneal angiogenesis: an MMP-independent mechanism. Invest Ophthalmol Vis Sci. 2013;54(1):783–788. doi:10.1167/iovs.12-10323
- Kanada KN, Nakatsuji T, Gallo RL. Doxycycline indirectly inhibits proteolytic activation of tryptic kallikrein-related peptidases and activation of cathelicidin. J Investig Dermatol. 2012;132(5):1435–1442. doi:10.1038/jid.2012.14
- Di Caprio R, Lembo S, Di Costanzo L, Balato A, Monfrecola G. Anti-inflammatory properties of low and high doxycycline doses: an in vitro study. Mediators Inflamm. 2015;2015:329418. doi:10.1155/2015/329418
