Abstract
Background
Isolated terminal ileitis (ITI) is a phenomenon often observed during colonoscopy, but in most cases, the specific etiology of ITI is unclear. Helicobacter pylori (H. pylori) infection has been reported to be associated with a wide variety of diseases, especially gastrointestinal diseases. Here, we conducted a retrospective study to explore a potential correlation between H. pylori infection and unexplained ITI (UITI).
Materials and Methods
The retrospective study was conducted at Sino-French New City Branch of Tongji Hospital, Wuhan, China, from April 2017 to October 2020. All subjects underwent total colonoscopy, with the endoscope being inserted more than 10cm into the terminal ileum. Subjects also received a 13C-urea breath test (13C-UBT). Data on the age, gender, endoscopic manifestations, and main clinical symptoms of subjects were collected. The presence of H. pylori infection was defined as a positive 13C-UBT result. Logistic regression models were used to analyze the potential correlation between H. pylori infection and UITI.
Results
There were 247 subjects (25.1%) in the H. pylori (+) group and 739 subjects (74.9%) in the H. pylori (-) group. The prevalence of UITI in the H. pylori (+) group was significantly lower than that in the H. pylori (-) group (OR = 0.518; 95% CI 0.281–0.956; P = 0.035), and there was no difference in other clinical features between groups. Stratification analysis results showed that there was an inverse association between H. pylori infection and UITI in subjects with age <60 (P = 0.046).
Conclusion
These data showed that H. pylori infection was negatively correlated with UITI. Additional studies are needed to validate these findings in a larger cohort as well as to explore the underlying mechanisms.
Introduction
Isolated terminal ileitis (ITI) is defined as superficial or deep inflammation of the terminal ileum, without accompanying acute or chronic colitis.Citation1 The occurrence of ITI has been reported to be associated with some identified causes, such as restricted form of Crohn’s disease (CD), nonsteroidal anti-inflammatory drugs (NSAIDs), intestinal infections, rheumatologic diseases, and abdominal lymphoma.Citation2–Citation4 However, in most cases, the etiology of ITI is unclear, and some clinicians often ignore these relevant manifestations.
H. pylori is a common gastrointestinal infection affecting approximately 50% of the world’s population at some point in their lifetime.Citation5,Citation6 H. pylori easily colonizes the gastrointestinal surface due to microaerophilic metabolism, spiral shape, and peculiar motility.Citation7 This microbe has also been reported to be associated with a variety of gastrointestinal pathologies, such as gastritis and peptic ulcers. Chronic H. pylori infection has been considered as the single most important risk factor for the development of gastric adenocarcinoma and mucosa-associated lymphoid tissue (MALT) lymphoma.Citation8,Citation9 Besides, previous studies have also linked H. pylori infection with hematological disease, neural disorders, and autoimmune diseases.Citation10,Citation11 Although H. pylori infection seems to increase the risk of developing both benign and malignant gastric diseases, increasing evidence has also shown that the presence of H. pylori may be negatively associated with several types of intestinal inflammatory diseases. For example, many studies have reported an inverse relationship between H. pylori infection and inflammatory bowel diseases (IBD).Citation12–Citation15 A prior multi-center, cross-sectional study has also reported that in children with celiac disease, a common autoimmune and inflammatory disease of small intestine, the frequency of H. pylori infection was remarkably lower than that of healthy controls.Citation16
There are limited data probing the association between H. pylori infection and ITI, leading us to perform the retrospective study investigating the potential relationship between H. pylori infection and unexplained isolated terminal ileitis (UITI).
Materials and Methods
Study Design and Subjects
The retrospective study was performed at Sino-French New City Branch of Tongji Hospital, Wuhan, China. The subjects who underwent total colonoscopy inserted more than 10cm into the terminal ileum from April 2017 to October 2020 were included. 13C-UBT was performed within 1 week of the procedure. Exclusion criteria were as follows: 1) subjects who underwent colonoscopy without reaching 10 cm from the terminal ileum; 2) subjects who underwent H. pylori eradication therapy previously; 3) subjects who received antibiotics, proton pump inhibitors or glucocorticoids in the 2 weeks before the procedure; 4) subjects who received NSAIDs in the 6 months prior to the procedure; 5) subjects with a history of any underlying chronic intestinal disease, such as inflammatory bowel disease, intestinal tuberculosis, intestinal lymphoma, intestinal malignancy, eosinophilic enterocolitis, allergic enterocolitis, and other identified intestinal infectious diseases; 6) subjects with a history of gastrointestinal surgery; 7) subjects under the age of 14. The data collected include age, gender, endoscopic manifestations, and main clinical manifestations. This study was performed with the approval of the Ethical Committees of Tongji Hospital in accordance with the Declaration of Helsinki, and the written consents were obtained.
Assessment of H. pylori Infection
H. pylori infection was detected by 13C-UBT, which is widely considered as one of the most accurate, non‐invasive tests for H. pylori infection detection.Citation17 Proton pump inhibitors, acid-suppressive drugs, and antibiotics were not permitted for 2 weeks prior to the 13C-UBT. The 13C-UBT was conducted using a urea 13C capsule breath test kit (HEADWAY, Shenzhen, China). After fasting for 2 hours, the subjects were given a tablet labeled with 13C, and breath samples were collected marked as δ‰ (0 min). Then, 30min later, a second breath sample was collected and marked as δ‰ (30 min). A 13C-Breath Test Analyzer (HeliFANplus, Germany) aided in data analysis with a threshold for detected values = δ‰ (30 min) −δ‰ (0 min). Positivity was defined as a value >4.0, indicating the presence of H. pylori infection
Total Colonoscopy Examination
Experienced endoscopists performed all total colonoscopies. All total colonoscopies inserted more than 10cm into the terminal ileum. A clear and detailed description of every segment of colon was recorded, including at least two images demonstrating a stable visualization for at least 30 seconds.Citation18 When subjects had lesions identified at the terminal ileum (eg, congestion, erosion, ulcers), the biopsies were taken and sent for pathological examination under the supervision of nursing staff. The endoscopic equipment used was a gastrointestinal mirror system, CLV-290SL (OLYMPUS, Japan).
Statistical Analysis
Continuous variable (age) was presented as mean ± standard deviation (SD) and compared by Student’s t-test. Fisher’s exact test and Pearson’s chi-square test were applied to explore the differences in categorical variables distribution. Logistic regression models were used to investigate the correlation between H. pylori infection and UITI. The variables of age and gender were adjusted in the above models. Odds ratio (OR) values and 95% confidence interval (CI) were calculated. A two-sided P-value of <0.05 was considered statistically significant. The aforementioned analyses were performed with SPSS 21.0 software (SPSS, Chicago, IL, USA).
Results
Subject Characteristics
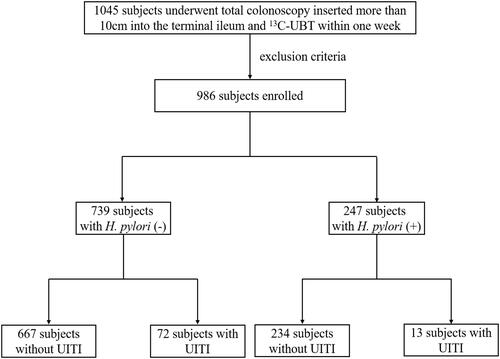
There were 1045 subjects undergoing total colonoscopy and 13C-UBT within 1 week. After excluding some subjects based on the above exclusion factors, a total of 986 subjects were enrolled in this study. The flow diagram of selection of the subjects for the study was shown in , and the characteristics of the subjects are shown in . There were 457 (46.35%) female and 529 (53.65%) male subjects (P=0.268) that were classified as either H. pylori (+) (n=247, 25.1%) or H. pylori (-) (n =739, 74.9%) based on their 13C-UBT result. The mean age of the H. pylori (+) group was 47.47±11.818 years, while the H. pylori (-) group had a mean age of 40.8±12.820 years (P=0.137). Approximately 40% of the subjects did not present any clinically apparent manifestations (n=402). H. pylori (+) patients showed more pronounced clinical symptoms, but did not differ significantly from the H. pylori (-) group.
Table 1 Characteristics of Study Subjects
Correlation Between H. pylori Infection and UITI
There were 85 subjects with UITI, presenting with inflammatory congestion and edema, erosions, and ulcers of mucosa (Supplementary Figure S1). The correlation between H. pylori infection and UITI was shown in . Seventy-two subjects with UITI were in the H. pylori (-) group and 13 subjects with UITI were in the H. pylori (+) group. The prevalence of UITI in the H. pylori (+) group was significantly lower than that in the H. pylori (-) group (OR = 0.518; 95% CI 0.281–0.956; P = 0.035).
Table 2 Correlation Between H. pylori Infection and UITI in the Study
We further analyzed the potential correlation between H. pylori infection and UITI in subgroups stratified by age and gender (). In the stratification analysis by age, an inverse association of H. pylori infection with UITI risk was identified for patients less than 60 years old (OR = 0.521; 95% CI 0.275–0.987; P = 0.046). There were no significant associations between H. pylori infection and UITI in different gender subgroups.
Table 3 Correlation Between H. pylori Infection and UITI in Subgroups of Our Study
Discussion
ITI represents a type of regional ileitis. Patients with ITI may present with abdominal pain, diarrhea, or other digestive system symptoms, with some asymptomatic cases identified during routine screening colonoscopies.Citation3 ITI can further progress to CD in some cases, and a few studies have shown the correlation of infectious pathogens with ITI, such as cytomegalovirus (CMV), Salmonella, Yersinia.Citation1 Nevertheless, the majority of ITI diagnoses have an unknown etiology.
H. pylori infection has been reported to be linked with a wide variety of diseases. Since the intestinal mucosa is continuous with the gastric mucosa, the intestine is another main reservoir for H. pylori infection in human. Previous studies have detected H. pylori in the intestine where thousands of bacteria colonized.Citation19 Therefore, we speculated that H. pylori might have an association with UITI. Here, in this retrospective study, we identified an inverse correlation between H. pylori infection with UITI risk in the entire study sample and in the subjects under the age of 60.
To our knowledge, this is the first reported association between H. pylori infection and UITI in any preclinical or clinical studies. Negative correlations between H. pylori infection and gastrointestinal pathologies are not unheard of however, as an inverse correlation between H. pylori infection and IBD has been shown in many previous epidemiological studies. There is also increasing experimental evidence supporting a protective role of H. pylori to against the chronic intestinal inflammation.Citation9 One explanation for the inverse correlation may be due to the ability of this microbe to downregulate proinflammatory immune responses. Lundgren et al reported that H. pylori colonization might be involved in induction of a regulatory T cells (Treg) driven immune response.Citation20 Another experimental colitis study showed that H. pylori infection resulted in IL-10 upregulation in the mesenteric lymph nodes and Th-17 response suppression, indicating a role of this microbe in extra-gastric immune-modulation.Citation21 Another explanation accounting for the protective role of H. pylori infection may be the production of antibodies against this bacterium, which confer a degree of protective immunity against other infectious bacterial species.Citation12 Some confounding factors, such as inherent genetic or environmental variables, may also contribute to the protective effect of H. pylori infection in intestinal inflammation, including TI. The hygiene hypothesis that IBD is associated with a better hygiene, and the prevalence of H. pylori infection in a cleaner environmental hygiene is relatively lower.Citation22
These data contrast an established role for H. pylori infection as an unfavorable pathogenic factor for multiple diseases. An accepted indication and the detection of H. pylori are prerequisites to eradication treatment.Citation23 However, there is rising clinical abuse of H. pylori eradication treatment, as some clinicians immediately conduct H. pylori eradication treatment as soon as they encounter a positive H. pylori infection result, regardless of whether there is an indication for eradication. Actually, in some cases, patients will not benefit from H. pylori eradication treatment. The overuse of H. pylori eradication drugs also results in the progressive and irreversible increase in the treatment costs for this class of drugs, as well as brings potential harms for some patients.Citation24 Our findings have suggested the protective role of H. pylori, thus providing evidence of the unnecessity of H. pylori eradication treatment under certain circumstances.
This study has several limitations. One is a small sample size, especially in the subgroup analysis, as the data were derived from a single Chinese center with a homogeneous patient population. The retrospective nature of the study also limited the ability to collect other important clinical details such as a detailed family history of digestive system diseases, digestive and dietary habits of the subjects, and other environmental factors. H. pylori status were assessed through 13C-UBT, and some subjects underwent 13C-UBT after bowel cleansing preparation, which might may bias the detection of H. pylori infection. Despite these limitations, it is a novel study that revealed the correlation between H. pylori infection and UITI.
Conclusions
In conclusion, this study is the first to identify an inverse correlation between H. pylori infection and UITI, especially in the people younger than 60. Large-scale, multi-center epidemiologic studies and preclinical mechanistic studies are still required to further explore the relationship of H. pylori infection with UITI.
Abbreviations
UITI, unexplained isolated terminal ileitis; H. pylori, Helicobacter pylori; 13C-UBT, 13C-urea breath test; UC, ulcerative colitis; NSAIDs, nonsteroidal anti-inflammatory drugs; CI, confidence interval; OR, odds ratio; IBD, inflammatory bowel diseases; CD, Crohn’s disease.
Data Sharing Statement
The data analyzed for this study can be accessable from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
This study was performed with the approval of the Ethical Committees of Tongji Hospital in accordance with the Declaration of Helsinki, and the informed consents were obtained.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest.
Additional information
Funding
References
- Kedia S, Kurrey L, Pratap Mouli V, et al. Frequency, natural course and clinical significance of symptomatic terminal ileitis. J Dig Dis. 2016;17(1):36–43. doi:10.1111/1751-2980.12307
- Tse CS, Deepak P, Smyrk TC, Raffals LE. Isolated acute terminal ileitis without preexisting inflammatory bowel disease rarely progresses to crohn’s disease. Dig Dis Sci. 2017;62(12):3557–3562. doi:10.1007/s10620-017-4803-8
- Greaves ML, Pochapin M. Asymptomatic ileitis: past, present, and future. J Clin Gastroenterol. 2006;40(4):281–285. doi:10.1097/01.mcg.0000210104.59370.66
- Zamor R, Emberesh M, Absalon MJ, Koberlein GC, Hariharan S. Abdominal lymphoma presenting as terminal ileitis: a case report. J Emerg Med. 2019;57(1):e13–e16. doi:10.1016/j.jemermed.2019.03.004
- Reshetnyak VI, Burmistrov AI, Maev IV. Helicobacter pylori: commensal, symbiont or pathogen? World J Gastroenterol. 2021;27(7):545–560. doi:10.3748/wjg.v27.i7.545
- Fallone CA, Moss SF, Malfertheiner P. Helicobacter pylori infection. N Engl J Med. 2019;381(6):588. doi:10.1056/NEJMc1905439
- Sonnenberg A. Review article: historic changes of Helicobacter pylori-associated diseases. Aliment Pharmacol Ther. 2013;38(4):329–342. doi:10.1111/apt.12380
- Lehours P, Ferrero RL. Review: helicobacter: inflammation, immunology, and vaccines. Helicobacter. 2019;24(Suppl 1):e12644. doi:10.1111/hel.12644
- Kyburz A, Muller A. Helicobacter pylori and extragastric diseases. Curr Top Microbiol Immunol. 2017;400:325–347. doi:10.1007/978-3-319-50520-6_14
- Youssefi M, Tafaghodi M, Farsiani H, Ghazvini K, Keikha M. Helicobacter pylori infection and autoimmune diseases; Is there an association with systemic lupus erythematosus, rheumatoid arthritis, autoimmune atrophy gastritis and autoimmune pancreatitis? A systematic review and meta-analysis study. J Microbiol Immunol Infect = Wei Mian Yu Gan Ran Za Zhi. 2020.
- Smyk DS, Koutsoumpas AL, Mytilinaiou MG, Rigopoulou EI, Sakkas LI, Bogdanos DP. Helicobacter pylori and autoimmune disease: cause or bystander. World J Gastroenterol. 2014;20(3):613–629. doi:10.3748/wjg.v20.i3.613
- Papamichael K, Konstantopoulos P, Mantzaris GJ. Helicobacter pylori infection and inflammatory bowel disease: is there a link? World J Gastroenterol. 2014;20(21):6374–6385. doi:10.3748/wjg.v20.i21.6374
- Wu XW, Ji HZ, Yang MF, Wu L, Wang FY. Helicobacter pylori infection and inflammatory bowel disease in Asians: a meta-analysis. World J Gastroenterol. 2015;21(15):4750–4756. doi:10.3748/wjg.v21.i15.4750
- Rokkas T, Gisbert JP, Niv Y, O’Morain C. The association between Helicobacter pylori infection and inflammatory bowel disease based on meta-analysis. United European Gastroenterol J. 2015;3(6):539–550. doi:10.1177/2050640615580889
- Luther J, Dave M, Higgins PD, Kao JY. Association between Helicobacter pylori infection and inflammatory bowel disease: a meta-analysis and systematic review of the literature. Inflamm Bowel Dis. 2010;16(6):1077–1084. doi:10.1002/ibd.21116
- Bayrak NA, Tutar E, Volkan B, et al. Helicobacter pylori infection in children with celiac disease: multi-center, cross-sectional study. Helicobacter. 2020;25(3):e12691. doi:10.1111/hel.12691
- Savarino V, Vigneri S, Celle G. The 13C urea breath test in the diagnosis of Helicobacter pylori infection. Gut. 1999;45(Suppl 1):I18–22. doi:10.1136/gut.45.2008.i18
- Borsotti E, Barberio B, D’Inca R, et al. Terminal ileum ileoscopy and histology in patients undergoing high-definition colonoscopy with virtual chromoendoscopy for chronic nonbloody diarrhea: a prospective, multicenter study. United European Gastroenterol J. 2019;7(7):974–981. doi:10.1177/2050640619847417
- Vitetta L, Vitetta G, Hall S. Immunological tolerance and function: associations between intestinal bacteria, probiotics, prebiotics, and phages. Front Immunol. 2018;9:2240. doi:10.3389/fimmu.2018.02240
- Lundgren A, Suri-Payer E, Enarsson K, Svennerholm AM, Lundin BS. Helicobacter pylori-specific CD4+ CD25high regulatory T cells suppress memory T-cell responses to H. pylori in infected individuals. Infect Immun. 2003;71(4):1755–1762. doi:10.1128/IAI.71.4.1755-1762.2003
- Higgins PD, Johnson LA, Luther J, et al. Prior Helicobacter pylori infection ameliorates Salmonella typhimurium-induced colitis: mucosal crosstalk between stomach and distal intestine. Inflamm Bowel Dis. 2011;17(6):1398–1408. doi:10.1002/ibd.21489
- Cholapranee A, Ananthakrishnan AN. Environmental hygiene and risk of inflammatory bowel diseases: a systematic review and meta-analysis. Inflamm Bowel Dis. 2016;22(9):2191–2199. doi:10.1097/MIB.0000000000000852
- Fischbach W, Malfertheiner P. Helicobacter pylori infection. Dtsch Arztebl Int. 2018;115(25):429–436. doi:10.3238/arztebl.2018.0429
- Savarino V, Dulbecco P, de Bortoli N, Ottonello A, Savarino E. The appropriate use of proton pump inhibitors (PPIs): need for a reappraisal. Eur J Intern Med. 2017;37:19–24. doi:10.1016/j.ejim.2016.10.007