Abstract
Chronic low-grade systemic inflammation is frequently observed in patients with chronic obstructive pulmonary disease (COPD), e.g., elevated pentameric CRP (pCRP). However, pCRP can dissociate to form monomeric CRP (mCRP) which exhibits a clear pro-inflammatory behaviour in contrast to the more anti-inflammatory properties of pCRP. Therefore, mCRP may be an informative biomarker to demonstrate chronic low-grade systemic inflammation. This was confirmed by analysing serum samples from 38 patients with COPD and 18 non-COPD control persons (NCCP). mCRP was significantly elevated in patients with COPD vs. NCCP, indicating that mCRP might be considered as a new sensitive marker of chronic low-grade systemic inflammation.
Introduction
Chronic low-grade systemic inflammation is frequently reported in patients with clinically stable chronic obstructive pulmonary disease (COPD).Citation1 High-sensitive C-reactive protein, as measured in its native pentameric conformation (pCRP), is the most commonly measured inflammatory biomarker in research and clinical practice. However, it can irreversibly dissociate to form monomeric CRP (mCRP).Citation2 These two CRP isoforms exhibit different biological functions. The complement pathway activation and low-density lipoprotein (LDL) metabolism are regulated more effective and versatile by mCRP than pCRP.Citation2 Moreover, mCRP exhibits a strong pro-inflammatory behaviour, while pCRP has anti-inflammatory properties.Citation2 Therefore, mCRP may be a potential biomarker to demonstrate the presence of chronic low-grade inflammation. To our knowledge, mCRP has never been determined in patients with COPD. Hence, this study aimed to compare mCRP and pCRP between patients with clinically stable COPD and age/sex matched non-COPD control persons (NCCP). Additionally, clinical correlates of mCRP were studied in the patients. A priori, it was hypothesized that mCRP and pCRP are elevated in patients with COPD.
Methods
Parts of the baseline data of a prospective randomized controlled trial on the efficacy of a nutritional supplement in patients with COPD (NCT02770417) were analysed. Briefly, patients were recruited from June 2016 to November 2018 at the outpatient consultation of Jessa Hospital (Hasselt, Belgium) and were free of exacerbations ≥6 weeks. Age/sex matched NCCP (COPD:NCCP ratio 2:1) were recruited via advertisement. The study was approved by the Ethics Committees of Jessa Hospital (Hasselt, Belgium) and Hasselt University (Diepenbeek, Belgium) (Study registration number: B243201628086), and performed in accordance with the latest revision (2013) of the Declaration of Helsinki. All participants provided written informed consent prior to inclusion.
Age, sex, smoking status, number of respiratory hospitalizations 1 year before study enrolment, Charlson Comorbidity index, body mass index (BMI), fat mass index and lean mass index (fat mass/height2; lean mass/height2; DXA, lunar DPXL, General Electric Company, Boston, USA), spirometry data from NCCP (SpiroUSB, CareFusion, San Diego, USA; according to American Thoracic Society/European Respiratory Society (ATS/ERS) guidelines), pulmonary function testing including post-bronchodilator spirometry, lung volumes and diffusion capacity for carbon monoxide (Master Screen Body and PFT, Jaeger, CareFusion, San Diego, USA; according to ATS/ERS guidelines) and Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage from patients, impact of disease on daily life by COPD Assessment Test (CAT), grade of dyspnea by modified Medical Research Council (mMRC) scale, physical capacity (six-minute walk distance), and physical activity (PA) as steps per day, assessed via a tri-axial accelerometer (wGT3X-BT, Actigraph, Pensacola, USA), were obtained.
Fasted venous blood samples were directly analysed in the Clinical Biology Laboratory of Jessa Hospital (Hasselt, Belgium) for pCRP with a Cobas 8000 modular analyzer (Roche, Basel, Suisse). Serum samples were stored at -80°C (Biobank UBiLim) until analysis of mCRP, which was performed in a blind setup using the in-house developed aptamer-based mCRP competition ELISA (patent pending: EP20212664.5, manuscript under preparation). Briefly, COOH-coated plates (Biomat, TN, Italy) were activated by applying 0.4 M of EDC and 0.1 M of NHS in a 1:1 ratio. After immobilisation of pCRP (2.5 µg/mL) onto the activated plates, the unbound activated COOH surface groups were blocked using 5% PBS-marvel and 1 M ethanolamine, pH 8 overnight and 1 hour respectively. Next, in situ pCRP monomerization was achieved by applying 10 mM NaOH at room temperature (RT) for 30 min. Twenty-five µL of serum sample was incubated with 150 nM biotin labelled aptamer on a rotating mixer for 30 min at RT and then transferred to a mCRP coated plate for 30 min at 37°C on shaker at 400 rpm. After incubation, the wells were washed three times and incubated on a shaker at 400 rpm with SA-HRP (Life Technologies, Belgium). The enzyme-substrate colour reaction was then stopped by adding a stop solution (0.18 M H2SO4) to the wells and the standards and samples were measured at 450 nm wavelength using a MultiskanTM FC Microplate Absorbance Reader (Thermo Scientific, Belgium).
For statistical analysis SPSS version 24.0 (SPSS Inc., Chicago, USA) was used. Results are described as mean ± standard deviation or median (quartile 1–quartile 3), as appropriate. Proportions are expressed in percentages and comparison between groups was performed via Chi Square test for homogeneity or Fisher’s test, as appropriate. All characteristics, pCRP and mCRP were compared between groups by using Independent T-test or Mann–Whitney U-test, as appropriate, and a P-value ≤0.05 was used for significance. Two sub-analyses were performed: i) comparison of mCRP between COPD and NCCP after excluding persons with a pCRP > 3 mg/L; ii) comparison of mCRP and pCRP between COPD ex-smokers and COPD smokers, and between NCCP non-smokers and NCCP ex-smokers. Spearman’s rho correlation coefficients were determined (rho < 0.3 = weak correlation; 0.3–0.7 = moderate correlation; > 0.7 = strong correlation). A P-value of ≤0.01 was used for significance to correct for multiple correlation testing.
Results
Serum samples were available from 38 patients (65±6 years, 74% male, BMI: 25.8±4.7 kg/m2, forced expired volume in 1 sec (FEV1): 55.6±14.0 %predicted), who typically had moderate-to-severe airflow obstruction, static hyperinflation and decreased diffusion capacity, moderate symptom burden, and a median of two comorbidities. Most patients (97%) were (ex)-smoker. Physical capacity was quite well preserved, while patients were generally physically inactive (). Moreover, 18 NCCP (65±6 years, 78% male, BMI: 26.4±3.0 kg/m2, FEV1: 104.8±10.2 %predicted) were analysed. Patients with COPD had a similar body composition compared to NCCP ().
Table 1 Baseline Characteristics of Patients with COPD and Correlation Coefficients with mCRP and pCRP
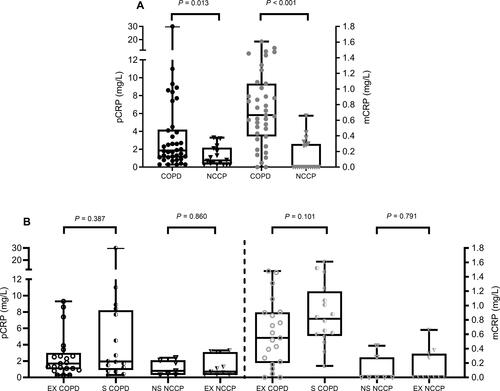
Median pCRP was higher in patients with COPD (1.85 (1.05–4.20) mg/L versus 0.75 (0.30–2.18) mg/L in NCCP; P=0.013; ). Median mCRP levels were higher in patients with COPD (0.66 (0.38–1.03) mg/L) versus NCCP (0.00 (0.00–0.29) mg/L; P<0.001; ). After excluding all participants with a pCRP >3 mg/L (COPD, n=12 and NCCP, n=3), median mCRP was still higher in patients: 0.61 (0.38–0.90) mg/L versus 0.00 (0.00–0.28) mg/L in NCCP (P<0.001). pCRP and mCRP were not different between COPD smokers and COPD ex-smokers, and between NCCP non-smokers and ex-smokers (). pCRP and mCRP did not correlate with each other nor with any of the other patients’ clinical characteristics ().
Figure 1 (A) pCRP (left y-axis; black) and mCRP (right y-axis; grey) levels in 38 patients with COPD (closed circles) and 18 age/gender matched NCCP (closed triangles). (B) pCRP (left y-axis; black) and mCRP (right y-axis; grey) levels in patients with COPD who are ex-smokers (EX COPD; open circles) or current smokers (S COPD; half-open circles) and NCCPs who are non-smokers (NS NCCP; open triangles) or ex-smokers (EX NCCP; half-open triangles).
Discussion and Conclusion
Systemic mCRP levels were increased in patients with clinically stable COPD and did not correlate with pCRP or clinical characteristics. pCRP is a diagnostic biomarker for COPD exacerbationCitation3 and a prognostic biomarker for early mortality.Citation4 However, pCRP may not reveal the complete role of CRP in chronic low-grade systemic inflammation. On binding with liposomes, cell membranes and activated platelets through lysophosphatidylcholine, pCRP dissociates into mCRP subunits revealing new epitopes, which is known to have a more potent pro-inflammatory effect in contrast to pCRP. This mCRP isoform is typically elevated during early low-grade chronic inflammation.Citation2 This explains the current results, as the patients with COPD had a substantial increase in mCRP levels, while mCRP was not detectable in 70% of the NCCP. The difference in mCRP between COPD and NCCP remained after excluding all participants with an increased pCRP level (>3 mg/L). Also, smoking was found to be non-discriminating within patients with COPD or within NCCPs for pCRP and mCRP. Therefore, our results provide a clear indication that mCRP may serve as a potential biomarker to identify early low-grade chronic inflammation in patients with COPD. The current findings need corroboration in larger samples and the investigation of mCRP’s associations with other inflammatory markers is warranted. A limitation of the study is the lack of healthy smoking controls. Though the sub-analysis shows no significant effect of smoking on mCRP level, studies have indicated that smoking increases platelet aggregation, which plays an important role in dissociation of pCRP to mCRP.Citation5 Therefore, it is important to include healthy smoking controls and non-smoking patients with COPD in a larger future study to analyse the precise diagnostic value of mCRP in patients with COPD. Research indicated that CRP levels did not differ significantly according to smoking status or biomass exposure, and whether mCRP levels are influenced by different exposures remains to be elucidated.Citation6
Several studies have been conducted to assess the efficacy of inhaled therapies which is the mainstay of COPD treatment. pCRP levels were found to be reduced following treatment with inhaled corticosteroids.Citation7 However, it remains currently unknown whether and to what extent mCRP transiently increases before, during and/or after a COPD-related exacerbation, and respiratory or other drug therapy can reduce elevated mCRP. To conclude, mCRP is significantly elevated in patients with COPD in comparison to age/sex matched NCCP and may be considered as a potential biomarker of chronic low-grade systemic inflammation.
Data Sharing Statement
Individual participant data that underlie the results reported in this research letter after de-identification (text, figure and table), study protocol and informed consent form can be shared immediately following publication. No end date included. Data can be shared with researchers who provide a methodologically sound proposal to achieve aims in the approved proposal. Proposals should be directed to [email protected]. To gain access, data requestors will need to sign a data access agreement.
Acknowledgments
The authors acknowledge Ms. Bogaers A. of Hasselt University (Diepenbeek, Belgium) for performing blood sampling and DXA scanning. The UBiLim (University Biobank Limburg; Belgium) is acknowledged as they provided storage and release of all human biological material used in this publication. (Linsen L. et al., Raising to the Challenge: Building a Federated Biobank to Accelerate Translational Research-The University Biobank Limburg. Front Med (Lausanne), 2019. 6: p. 224.)
Disclosure
Ms Revathy Munuswamy and Prof. Dr. Luc Michiels report a patent EP20212664 pending to Luc Michiels, UHasselt. The authors report no other conflicts of interest in this work.
Additional information
Funding
References
- Agustí A, Edwards LD, Rennard SI, et al. Persistent systemic inflammation is associated with poor clinical outcomes in COPD: a novel phenotype. PLoS One. 2012;7(5):e37483. doi:10.1371/journal.pone.0037483
- Trial J, Potempa LA, Entman ML. The role of C-reactive protein in innate and acquired inflammation: new perspectives. Inflamm Cell Signal. 2016;3(2).
- Hurst JR, Donaldson GC, Perera WR, et al. Use of plasma biomarkers at exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;174(8):867–874. doi:10.1164/rccm.200604-506OC
- Leuzzi G, Galeone C, Taverna F, et al. C-reactive protein level predicts mortality in COPD: a systematic review and meta-analysis. Eur Respir Rev. 2017;26(143):160070. doi:10.1183/16000617.0070-2016
- de la Torre R, Peña E, Vilahur G, et al. Monomerization of C-reactive protein requires glycoprotein IIb-IIIa activation: pentraxins and platelet deposition. J Thromb Haemost. 2013;11(11):2048–2058. doi:10.1111/jth.12415
- Aksu F, Capan N, Aksu K, et al. C-reactive protein levels are raised in stable chronic obstructive pulmonary disease patients independent of smoking behavior and biomass exposure. J Thorac Dis. 2013;5(4):414–421.
- Perng D-W, Tao C-W, Su K-C, et al. Anti-inflammatory effects of salmeterol/fluticasone, tiotropium/fluticasone or tiotropium in COPD. Eur Respir J. 2009;33(4):778–784. doi:10.1183/09031936.00115308
