Abstract
Background
Phorbol 12-myristate 13-acetate (PMA)-induced differentiation of human monocytic THP-1 cells is an experimental model for preparing resting macrophages (M0) for cell polarization toward the different functional specializations of macrophages.
Methods
In this study, we examined the expression of immune checkpoints by using flow cytometry following multicolor staining. The blockade of immune checkpoint by using neutralizing antibodies was performed to assess their role in PMA-induced THP-1-differentiated macrophages.
Results
Upon the inducible macrophage differentiation caused by PMA, increased expression levels of CD11b and CD68 were measured and characterized according to their adherent phenotype accompanied by the generation of cellular complexity. While the cell growth rate was abolished post-differentiation, some cells underwent cell death. Notably, we found increases in the expression of programmed cell death protein 1, also known as PD-1 (CD279), and its ligand PD-L1 (CD274), mainly in differentiated M0 (CD68+CD11b+) macrophages. However, neutralizing PD-L1/PD-1 neither blocked THP-1 cell differentiation toward macrophages nor inhibited macrophage polarization in M1 and M2. In specializing macrophages, a decrease both in CD274 and CD279 was found in M2.
Conclusion
These results revealed the inducible expression of PD-L1/PD-1 in PMA-induced THP-1-differentiated M0 macrophages followed by a decrease in M2 macrophages.
Introduction
Macrophages, a type of phagocyte of innate immunity, are responsible for recognizing, engulfing, and destroying targets such as pathogens and apoptotic cells.Citation1,Citation2 Compared to circulating neutrophils and monocytes, macrophages are professional phagocytes that participate in immune defense. In the human body, macrophages are mainly and notably recruited to the lungs, liver, brain, spleen, and lymph nodes. A relatively low macrophages can be detected in the peripheral blood.Citation3 It is noted that the different types of activated macrophages produce diverse proinflammatory cytokines/chemokines for triggering intercellular inflammation in response to infections, allergens, and cancers.Citation1,Citation2,Citation4 For the regulation in immunity, macrophages can be differentiated toward antigen-presenting cells to modulate cellular T and humoral B cell immunity.Citation5 Therefore, in macrophages, their biological roles and cellular regulation are heterogeneous.
Macrophages are generally and functionally divided into two specialized classes: classically (type I or M1) and alternatively (type II or M2) activated macrophages.Citation2 M1 macrophages are responsible for proinflammation and most antimicrobe and anticancer activities, and M2 macrophages promote anti-inflammation and tumorigenesis, including tumor growth and metastasis.Citation4 To differentiate specialized tissue macrophages, the possible modulators derived from infectious pathogens and cancer cells are essential for macrophage maturation, activation, and polarization.Citation6,Citation7 Notably, blood monocytes are activated and recruited into inflamed tissues and mostly constitute an effective process for further differentiation toward macrophages.Citation1,Citation3,Citation4,Citation8 The processing protocols of monocyte/macrophage differentiation and polarization are currently used for immune cell therapy in treating inflammatory disorders, infectious diseases, and cancers.Citation9,Citation10
For monocyte-derived macrophage differentiation, artificial strategies are processed by exogenously treating monocytes with bioactive growth factors, such as granulocyte-macrophage colony-stimulating factor (GM-CSF) and M-CSF, as well as chemical agents, such as 1,25-dihydroxy vitamin D3 and phorbol-12-myristate-13-acetate (PMA).Citation11 In addition to isolated primary monocytes, human monocytic cell lines such as THP-1 and HL-60 are the most frequently used cell model systems.Citation11,Citation12 Following PMA treatment, THP-1 cells are differentiated into macrophage-like cells, resulting in cells with increased adherence and decreased growth activity.Citation13 At the resting stage of macrophages (M0), the cells can be polarized into M1 and M2 by stimulation with lipopolysaccharide (LPS)/interferon (IFN)-γ and interleukin (IL)-4/IL-10/IL-13/tumor growth factor (TGF)-β, respectively.Citation8 Recent studies have discovered the aberrant expression of immune checkpoints such as programmed cell death protein 1, also known as PD-1 (CD279), and its ligand PD-L1 (CD274) in M2 macrophages, which are involved in tumorigenesis.Citation14–Citation16 However, the regulation of PD-L1/PD-1 expression in monocyte/macrophage differentiation is unknown. In this study, by using PMA-induced THP-1 differentiation toward M0, M1, and M2 macrophages, we examined the regulation of PD-L1/PD-1 expression and its possible role in macrophage differentiation before polarization.
Methods
Cells, Culture Condition, and Reagents
Human THP-1 monocytic cells (ATCC TIB-202) were cultured in RPMI-1640 medium (Invitrogen Life Technologies, Carlsbad, CA, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS, Invitrogen Life Technologies) and maintained at 37°C with 5% CO2. Phorbol 12-myristate 13-acetate (PMA) and LPS were purchased from Sigma-Aldrich (St. Louis, MO, USA) and dissolved in dimethyl sulfoxide (DMSO, Sigma-Aldrich) and double-distilled H2O, respectively. Cytokines, including IFN-γ, IL-4, and IL-13, were purchased from PeproTech (Rocky Hill, NJ). Neutralizing antibodies against CD274 (Cat#10084-R639) and CD279 (Cat# 10377-HN94) was purchased from Sino Biological (Wayne, PA, USA).
Differentiation Protocol
The protocol for THP-1 macrophage differentiation was performed according to previous works with modifications.Citation13,Citation17 THP-1 cells were divided into two groups and seeded in a 6-well culture plate (2×105 cells/mL). Following seeding for 24 h, cells were treated with one microliter per mL of DMSO as a control group and 150 ng/mL of PMA as an M0 group for 48 h. After that, PMA-containing medium was washed away from all cells and replaced with PMA-free culture medium for 24 h then the cells were treated without (for M0) or with LPS (1 μg/mL)/IFN-γ (10 ng/mL) (for M1) and IL-4 (25 ng/mL)/IL-13 (25 ng/mL) (for M2) for an additional 72 h.
Morphological Observation
Cell images were taken for the determination of control and PMA-induced THP-1 macrophage differentiation using an inverted microscope (AxioVert. A1 Inverted Microscope, Carl Zeiss, Germany) under the same magnification of light intensity. Representative images were observed under 10× magnification, and the area of observation was chosen randomly from the whole well.
Immunofluorescence Staining
For flow cytometric analysis, the cells were detached with 0.01% trypsin and then re-suspended in 0.1 mL of Flow Cytometry Staining Buffer (Thermo Fisher Scientific, Carlsbad, CA, USA) in the final cell concentration with 1×107 cells/mL. Cells were stained with fluorescence-conjugated antibodies, including antibodies against human CD11b (Cat# 47-0118-42), CD68 (Cat# 12-0689-42), CD80 (Cat# 67-0809-42), CD209 (Cat# 45-2099-42), CD274 (Cat# 47-5983-42), and CD279 (Cat# 67-2799-42) (Thermo Fisher Scientific). The cells were washed twice in phosphate buffered saline and then analyzed using flow cytometry (Attune Nxt, Invitrogen Life Technologies) with excitation set at 405, 488, and 633 nm, and the data were analyzed using FCS Express 7 Cytometry software (De Novo Software, CA, USA).
MTT Assay and Lactate Dehydrogenase (LDH) Assay
For the detection of cell growth and cytotoxicity, MTT Cell Proliferation Kit (Sigma-Aldrich) and Cytotoxicity Detection kit assays (Roche Diagnostics, Lewes, UK) were performed, respectively, according to the manufacturer’s instructions. A microplate reader (SpectraMax 340PC; Molecular Devices Corporation, Sunnyvale, CA, USA) was used to measure the absorbance at 570 and 490 nm for MTT and LDH, respectively. The data were analyzed using Softmax Pro software (Molecular Devices Corporation).
Statistical Analysis
The values are expressed as the means ± standard deviation (SD). The groups were compared using Student’s two-tailed unpaired t-test or a one-way analysis of variance analysis. A P value of 0.05 was considered significant.
Results
Stimulation of Phorbol 12-Myristate 13-Acetate (PMA) Promotes Macrophage Differentiation in Human Monocytic THP-1 Cells
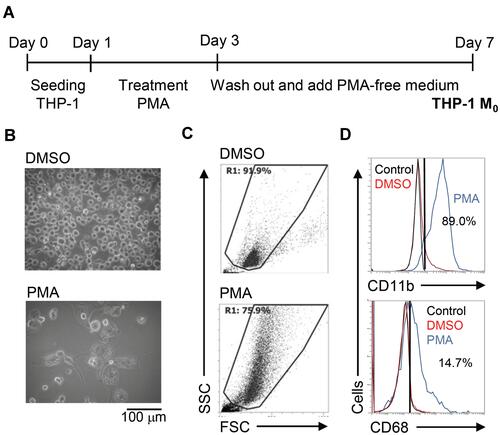
To create an inducible model of macrophage differentiation, human monocytic THP-1 cells were used in this study according to previous works with partial modification.Citation12,Citation13 With PMA stimulation for 2 days, THP-1 cells were cultured only in PMA-free medium for an additional 4 days as a differentiated phenotype of THP-1 M0 (). Image observation showed an adherent phenotype with the enlarged cell size in PMA-stimulated THP-1 cells (). Using flow cytometric analysis, PMA-stimulated THP-1 cells displayed an increase in cell size as well as cellular complexity characterized by high granularity (). Several cell markers could be identified in differentiated macrophages,Citation17 and immunostaining of specific markers followed by flow cytometric analysis showed increased expression of CD11b and CD68 (). As supported by the above experiments, the stimulation of PMA effectively promoted the differentiation of THP-1 cells toward a macrophage-like phenotype.
Figure 1 Phorbol 12-myristate 13-acetate (PMA) treatment triggers macrophage differentiation in human monocytic THP-1 cells. (A) Experimental flowchart of the PMA stimulation performed in this study. (B) Cell morphology evaluation showed cell growth in PMA-treated THP-1 cells. (C) Flow cytometric dot-plot, plotting forward-scattered (FSC) versus side-scattered (SSC) from a population of THP-1 cells, showing the cell size and complexity. (D) Immunostaining followed by flow cytometric histogram analysis showed the expression of CD11b and CD68. Treatment of DMSO was used as control. For all images and flow cytometric analysis, representative staining data of isotype control, DMSO, and PMA were selectively obtained from three individual experiments. For the flow cytometric analysis, the percentage of positive cells in PMA treatment is shown.
Phorbol 12-Myristate 13-Acetate (PMA) Treatment Suppresses Cell Growth Accompanied by Cytotoxicity in THP-1-Differentiated Macrophages
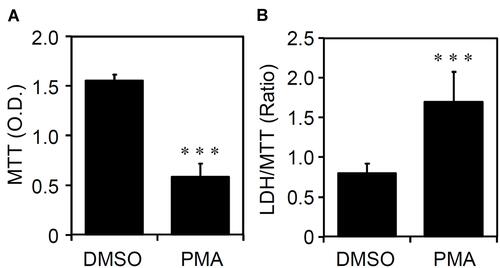
The cell fate of THP-1-differentiated macrophages shows heterogeneity of cell growth and apoptosis.Citation18 Through an MTT cell proliferation assay, which can measure the reduction of tetrazolium into an insoluble formazan product in viable cells,Citation19 PMA-stimulated THP-1 cells significantly (p < 0.05) exhibited a decrease in the cell growth rate (). Compared to the morphological observation, as shown in , the cell density was dramatically reduced in PMA-stimulated THP-1 cells. Among these cells, the induction of cytotoxicity is demonstrated by the formation of detached cells. By using an LDH cytotoxicity assay, which assesses the level of plasma membrane damage in a cell population,Citation20 PMA treatment also caused cellular injury in THP-1-differentiated macrophages (). The results show that PMA induces inducible macrophage differentiation accompanied by cell growth inhibition as well as cytotoxicity.
Figure 2 Phorbol 12-myristate 13-acetate (PMA) treatment causes cell growth inhibition and cytotoxicity in THP-1-differentiated macrophages. According to the experimental design shown in , (A) MTT and (B) lactate dehydrogenase (LDH)/MTT assays showed cell growth and cytotoxicity, respectively, in PMA-treated THP-1 cells. The original optical density (O.D.) of MTT and the ratio of LDH/MTT were shown. All quantitative data are shown as the mean ± SD of three independent experiments. ***p < 0.001.
Expression of PD-L1 (CD274) and PD-1 (CD279) in Phorbol 12-Myristate 13-Acetate (PMA)-Stimulated THP-1-Differentiated Macrophages
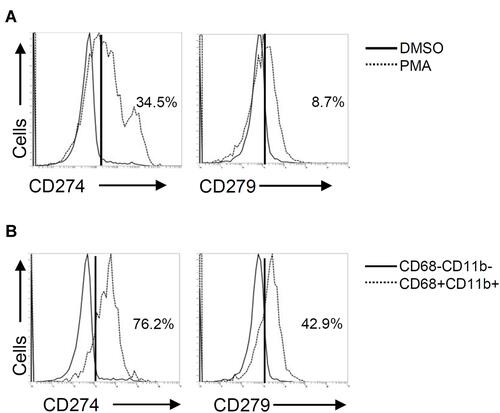
PD-L1 (CD274) and PD-1 (CD279) are expressed not only in cancer cells but also in immune cells, such as tumor-infiltrating lymphocytes and tumor-associated macrophages.Citation21 In PMA-stimulated THP-1 cells, flow cytometric analysis demonstrated considerably increased expression of both PD-L1 (CD274) and PD-1 (CD279) (). Compared to PD-1 (CD279), the expression of PD-L1 (CD274) was more inducible in THP-1-differentiated macrophages. As shown by the gating of CD68+CD11b+ macrophages, the expression levels of PD-L1 (CD274) and PD-1 (CD279) were higher than those in the gating of CD68−CD11b− cells (). These results revealed the inducible expression of PD-L1 (CD274) and PD-1 (CD279) in THP-1-differentiated macrophages.
Figure 3 Phorbol 12-myristate 13-acetate (PMA) treatment induces PD-L1 (CD274) and PD-1 (CD279) expression in THP-1-differentiated macrophages. According to the experimental design shown in , immunostaining followed by flow cytometric histogram and dot-plot analysis showed the expression of PD-L1 (CD274) and PD-1 (CD279) in PMA-treated THP-1 cells (A) without or (B) with the gating of CD68−CD11b− and CD68+CD11b+ cells. Representative data were selectively obtained from three individual experiments, and the percentage of positive cells is shown.
Blockade of PD-L1 (CD274) and PD-1 (CD279) Does Not Disturb Phorbol 12-Myristate 13-Acetate (PMA)-Induced Macrophage Differentiation
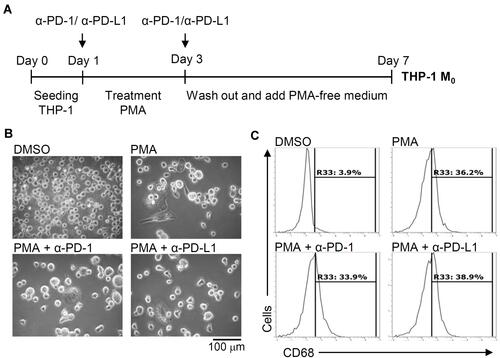
To investigate the role of inducible expression of PD-L1 (CD274) and PD-1 (CD279) in THP-1-differentiated macrophages, we next examined the blockade effects on PD-L1 (CD274) and PD-1 (CD279) using neutralizing antibodies as schematically represented in . Following PMA treatment concurrently incubated with anti-PD-L1 (CD274) and anti-PD-1 (CD279) neutralizing antibodies, no other blockade effects were identified in PMA-induced increased cell adherence and decreased cell density (). As shown by the flow cytometric analysis, the PMA-induced increased expression of CD68 was not affected by the blockade of PD-L1 (CD274) and PD-1 (CD279) (). The results illustrate inducible macrophage differentiation independent of the inducible expression of PD-L1 (CD274) and PD-1 (CD279) in THP-1-differentiated macrophages.
Figure 4 Pharmacologically inhibiting PD-L1 (CD274) and PD-1 (CD279) does not affect phorbol 12-myristate 13-acetate (PMA)-induced macrophage differentiation. (A) In the absence and presence of neutralizing antibodies (5 μg/mL) against CD274 (α-PD-L1) and CD279 (α-PD-1), THP-1 cells were treated with PMA according to the experimental design. (B) Cell morphology showed cell growth. (C) Immunostaining followed by flow cytometric histogram analysis showed the expression of CD68. For all images and flow cytometric analysis, representative data were selectively obtained from three individual experiments, and the percentage of positive cells is shown.
Expression of PD-L1 (CD274) and PD-1 (CD279) and Its Roles in THP-1-Differentia
ted Macrophages M1 and M2
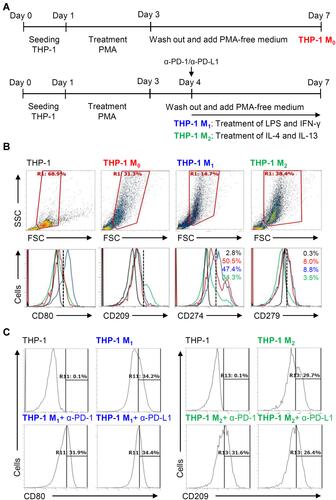
We next investigated the different expression of inducible PD-L1 (CD274) and PD-1 (CD279) in THP-1-differentiated macrophages. Following the inducible THP-1-differentiated macrophages (M0), we used the combination treatment of LPS/IFN-γ and IL-4/IL-13, respectively, to induce the polarization of M0 into M1 and M2 as schematically represented in Figure 5A.Citation8 As shown by the flow cytometric analysis, the LPS/IFN-γ- and IL-4/IL-13-induced increased expression of CD80 (M1 marker) and CD209 (M2 marker) were measured to show the different polarization. In THP-1 M1 and THP-1 M2 macrophages, the different expression of PD-L1 (CD274) and PD-1 (CD279) were shown (). The results elucidate the different expression of inducible PD-L1 (CD274) and PD-1 (CD279) in the different specializing macrophages while a decrease both in these checkpoints was found in THP-1-differentiated M2 macrophages. To further investigate the inhibition of PD-L1/PD-1 on the polarization of M1 and M2 macrophages, anti-PD-L1 (CD274) and anti-PD-1 (CD279) neutralizing antibodies were treated in the processing of THP-1-differentiated M1 and M2 macrophages (). The results showed that PD-1/PD-L1 was also not involved in macrophage polarization.
Figure 5 Different expression of PD-L1 (CD274) and PD-1 (CD279) in THP-1-differentiated macrophages. (A) In PMA-stimulated THP-1-differentiated M0 macrophages, cells were then treated with LPS (1 μg/mL)/IFN-γ (10 ng/mL) and IL-4 (25 ng/mL)/IL-13 (25 ng/mL) for polarization of M1 and M2, respectively, in the absence and presence of neutralizing antibodies (5 μg/mL) against CD274 (α-PD-L1) and CD279 (α-PD-1) according to the experimental design. (B) For immunostaining, cells were stained with CD80 and CD209 for dissecting M1 and M2, respectively. Immunostaining followed by flow cytometric histogram analysis showed the expression of CD274 and CD279 in these cells. (C) Furthermore, the expression of CD80 and CD209 in M1 and M2 without or with the blockade of CD274 and CD279 were shown. For all flow cytometric analysis, representative data were selectively obtained from three individual experiments, and the percentage of positive cells is shown. THP-1 (black); THP-1 M0 (red); THP-1 M1 (blue); THP-1 M2 (green).
Discussion
For anticancer immunotherapy, the blockade of immune checkpoints such as PD-L1 (CD274) and PD-1 (CD279) is currently and widely executed by targeting cancer cells and tumor-infiltrating lymphocytes in principle. However, tumor-associated macrophages also express PD-L1 (CD274) and PD-1 (CD279),Citation14,Citation15,Citation21 increasing alternative therapeutic efficacy by targeting these immune checkpoints for anticancer activity. In this study, by using an inducible model of macrophage differentiation, we demonstrated increased expression of PD-L1 (CD274) and PD-1 (CD279) in PMA-activated human monocytic THP-1 cells. However, pharmacologically neutralizing PD-L1 (CD274) and PD-1 (CD279) did not affect THP-1 macrophage differentiation as well as polarization. Interestingly, a decrease both in these immune checkpoints in M2 macrophages although the previous studies showed the blockade of PD-L1 (CD274) and PD-1 (CD279) increases macrophage phagocytosis as anticancer phenomena.Citation15 However, in this study, the biological effect of down-regulated PD-L1 (CD274) and PD-1 (CD279) in cytokine-driven THP-1 M2 macrophages remains unclear. Future studies are urgently needed to verify the biological roles of PD-L1 (CD274) and PD-1 (CD279) in monocyte/macrophage differentiation.
No further pioneering study has investigated the regulation of PD-L1 (CD274) and PD-1 (CD279) expression in monocyte/macrophage differentiation. Notably, recent studies illustrate that PD-L1 (CD274) is continually expressed on nonclassical monocytes at steady state to promote immunoregulatory function, probably by causing T cell death,Citation22 and the presence of PD-1 (CD279)-expressing intermediate monocytes is upregulated in preterm cases of septic shock or fatal outcomes, showing an immunosuppressive role.Citation23 Classically activated M1 macrophages express more PD-L1 (CD274) due to the presence of type 1 helper T cells and a combination of LPS/IFN-γ.Citation24 In contrast, alternatively activated macrophages, which are polarized by IL-4 stimulation, slightly express PD-L1 (CD274) but effectively express PD-1 (CD279).Citation14,Citation15 In THP-1-differentiated M0 macrophages, both PD-L1 (CD274) and PD-1 (CD279) were evaluated. For macrophage polarization, the different expression in PD-L1 (CD274) and PD-1 (CD279) expression could be critical in polarized M1 and M2, respectively.
Because PD-L1/PD-1 are the main immune checkpoint proteins on the surface of T cells for cancer immune escape,Citation25 the significance of macrophage-associated PD-L1/PD-1 in the relevance to macrophage differentiation and polarization is therefore speculated. However, the role of the evaluated expression of PD-L1 (CD274) and PD-1 (CD279) in M0 macrophages remains unclear. Importantly, patient tumors with a high number of PD-L1 (CD274)-positive macrophages show favorable survival.Citation14,Citation26,Citation27 In contrast, PD-1 (CD279) expressed on macrophages is speculated to be immunosuppressive.Citation14,Citation15,Citation26 According to the current studies, signaling of PD-L1 (CD274) and PD-1 (CD279) may determine the different regulations on macrophage polarization, while PD-L1 (CD274) triggers CD86 and MHC II expression and PD-1 (CD279) promotes phagocytic inhibition.Citation14 The anti- and pro-tumor functions of PD-L1 (CD274) and PD-1 (CD279) have been demonstrated by their aberrant expression and functional regulation in macrophages; however, the mechanisms regulating their expression in macrophages remain unclear.
To control PD-L1 (CD274) and PD-1 (CD279) expression, many immune regulators and signaling pathways are responsible for their transcriptional induction.Citation25,Citation28 For PD-L1 (CD274) expression, proinflammatory cytokines, including type I and type II IFNs, tumor necrosis factor α, vascular endothelial growth factor, protein kinases, and transcription factors, have been reported.Citation25,Citation28 PD-1 (CD279) is induced on activated T cells through mechanisms involving signaling of T-cell antigen receptors and cytokine receptors. In general, the nuclear factor of activated T cells and IFN-regulating factors are required for the transcription of PD-1 in T cells.Citation29 Under PMA stimulation, the activation of mitogen-activated protein kinase signaling networks,Citation30 RhoA/ROCK signaling,Citation31 and several transcription factorsCitation32,Citation33 are essential for macrophage differentiation. Their potential regulation of the expression of PD-L1 (CD274) and PD-1 (CD279) is therefore proposed not only for macrophage differentiation but also for immune modulation.
Limitations of this work need further validation, including the selected differentiation models, the primary responses, and the biological roles of PD-L1/PD-1 in M0 macrophages. Although PMA-differentiated THP-1 is commonly used in macrophage study, other physiological inducible models of macrophage differentiation, such as GM-CSF, M-CSF, and vitamin D3,Citation11 are needed to validate the expression of PD-L1 (CD274) and PD-1 (CD279). In conclusion, these works identified increased expression of PD-L1 (CD274) and PD-1 (CD279) in PMA-activated human monocytic THP-1 cells toward M0 macrophages and a decreased PD-L1/PD-1 in M2 macrophages. Although the blockade of PD-L1/PD-1 did not interfere with macrophage differentiation and polarization, as an inducible model of differentiated macrophages, more investigations on the expression and regulation of PD-L1/PD-1 may advance its physiopathological roles in macrophage intracellular regulation and activation toward M1 and M2.
Disclosure
The authors report no conflicts of interest in this work.
Additional information
Funding
References
- Locati M, Mantovani A, Sica A. Macrophage activation and polarization as an adaptive component of innate immunity. Adv Immunol. 2013;120:163–184. doi:10.1016/B978-0-12-417028-5.00006-5
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8(12):958–969. doi:10.1038/nri2448
- Krikorian G, Marshall WH, Simmons S, Stratton F. Counts and characteristics of macrophage precursors in human peripheral blood. Cell Immunol. 1975;19(1):22–31. doi:10.1016/0008-8749(75)90288-9
- Sica A, Larghi P, Mancino A, et al. Macrophage polarization in tumour progression. Semin Cancer Biol. 2008;18(5):349–355. doi:10.1016/j.semcancer.2008.03.004
- Zou W, Borvak J, Marches F, et al. Macrophage-derived dendritic cells have strong Th1-polarizing potential mediated by beta-chemokines rather than IL-12. J Immunol. 2000;165(8):4388–4396. doi:10.4049/jimmunol.165.8.4388
- Epelman S, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity. 2014;41(1):21–35. doi:10.1016/j.immuni.2014.06.013
- Gordon S, Pluddemann A. Tissue macrophages: heterogeneity and functions. BMC Biol. 2017;15(1):53. doi:10.1186/s12915-017-0392-4
- Italiani P, Boraschi D. From monocytes to M1/M2 macrophages: phenotypical vs functional differentiation. Front Immunol. 2014;5:514. doi:10.3389/fimmu.2014.00514
- Beltraminelli T, De Palma M. Biology and therapeutic targeting of tumour-associated macrophages. J Pathol. 2020;250(5):573–592. doi:10.1002/path.5403
- Hopewell EL, Cox C. Manufacturing dendritic cells for immunotherapy: monocyte enrichment. Mol Ther Methods Clin Dev. 2020;16:155–160. doi:10.1016/j.omtm.2019.12.017
- Murao S, Gemmell MA, Callaham MF, Anderson NL, Huberman E. Control of macrophage cell differentiation in human promyelocytic HL-60 leukemia cells by 1,25-dihydroxyvitamin D3 and phorbol-12-myristate-13-acetate. Cancer Res. 1983;43(10):4989–4996.
- Tsuchiya S, Yamabe M, Yamaguchi Y, Kobayashi Y, Konno T, Tada K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int J Cancer. 1980;26(2):171–176. doi:10.1002/ijc.2910260208
- Schwende H, Fitzke E, Ambs P, Dieter P. Differences in the state of differentiation of THP-1 cells induced by phorbol ester and 1,25-dihydroxyvitamin D3. J Leukoc Biol. 1996;59(4):555–561.
- Lu D, Ni Z, Liu X, et al. Beyond T cells: understanding the role of PD-1/PD-L1 in tumor-associated macrophages. J Immunol Res. 2019;2019:1919082. doi:10.1155/2019/1919082
- Gordon SR, Maute RL, Dulken BW, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545(7655):495–499. doi:10.1038/nature22396
- Hartley GP, Chow L, Ammons DT, Wheat WH, Dow SW. Programmed cell death ligand 1 (PD-L1) signaling regulates macrophage proliferation and activation. Cancer Immunol Res. 2018;6(10):1260–1273. doi:10.1158/2326-6066.CIR-17-0537
- Daigneault M, Preston JA, Marriott HM, Whyte MK, Dockrell DH. The identification of markers of macrophage differentiation in PMA-stimulated THP-1 cells and monocyte-derived macrophages. PLoS One. 2010;5(1):e8668. doi:10.1371/journal.pone.0008668
- Spano A, Barni S, Sciola L. PMA withdrawal in PMA-treated monocytic THP-1 cells and subsequent retinoic acid stimulation, modulate induction of apoptosis and appearance of dendritic cells. Cell Prolif. 2013;46(3):328–347. doi:10.1111/cpr.12030
- Vistica DT, Skehan P, Scudiero D, Monks A, Pittman A, Boyd MR. Tetrazolium-based assays for cellular viability: a critical examination of selected parameters affecting formazan production. Cancer Res. 1991;51(10):2515–2520.
- Decker T, Lohmann-Matthes ML. A quick and simple method for the quantitation of lactate dehydrogenase release in measurements of cellular cytotoxicity and tumor necrosis factor (TNF) activity. J Immunol Methods. 1988;115(1):61–69. doi:10.1016/0022-1759(88)90310-9
- Berghoff AS, Ricken G, Widhalm G, et al. PD1 (CD279) and PD-L1 (CD274, B7H1) expression in primary central nervous system lymphomas (PCNSL). Clin Neuropathol. 2014;33(1):42–49. doi:10.5414/np300698
- Bianchini M, Duchene J, Santovito D, et al. PD-L1 expression on nonclassical monocytes reveals their origin and immunoregulatory function. Sci Immunol. 2019;4:36. doi:10.1126/sciimmunol.aar3054
- Zasada M, Lenart M, Rutkowska-Zapala M, et al. Analysis of PD-1 expression in the monocyte subsets from non-septic and septic preterm neonates. PLoS One. 2017;12(10):e0186819. doi:10.1371/journal.pone.0186819
- Loke P, Allison JP. PD-L1 and PD-L2 are differentially regulated by Th1 and Th2 cells. Proc Natl Acad Sci U S A. 2003;100(9):5336–5341. doi:10.1073/pnas.0931259100
- Sun C, Mezzadra R, Schumacher TN. Regulation and Function of the PD-L1 Checkpoint. Immunity. 2018;48(3):434–452. doi:10.1016/j.immuni.2018.03.014
- Najafi M, Hashemi Goradel N, Farhood B, et al. Macrophage polarity in cancer: a review. J Cell Biochem. 2019;120(3):2756–2765. doi:10.1002/jcb.27646
- Liu CQ, Xu J, Zhou ZG, et al. Expression patterns of programmed death ligand 1 correlate with different microenvironments and patient prognosis in hepatocellular carcinoma. Br J Cancer. 2018;119(1):80–88. doi:10.1038/s41416-018-0144-4
- Boussiotis VA. Molecular and biochemical aspects of the PD-1 checkpoint pathway. N Engl J Med. 2016;375(18):1767–1778. doi:10.1056/NEJMra1514296
- Oestreich KJ, Yoon H, Ahmed R, Boss JM. NFATc1 regulates PD-1 expression upon T cell activation. J Immunol. 2008;181(7):4832–4839. doi:10.4049/jimmunol.181.7.4832
- Richter E, Ventz K, Harms M, Mostertz J, Hochgrafe F. Induction of macrophage function in human THP-1 cells is associated with rewiring of MAPK signaling and activation of MAP3K7 (TAK1) protein kinase. Front Cell Dev Biol. 2016;4:21. doi:10.3389/fcell.2016.00021
- Yang L, Dai F, Tang L, Le Y, Yao W. Macrophage differentiation induced by PMA is mediated by activation of RhoA/ROCK signaling. J Toxicol Sci. 2017;42(6):763–771. doi:10.2131/jts.42.763
- Baek YS, Haas S, Hackstein H, et al. Identification of novel transcriptional regulators involved in macrophage differentiation and activation in U937 cells. BMC Immunol. 2009;10:18. doi:10.1186/1471-2172-10-18
- Lu R, Pitha PM. Monocyte differentiation to macrophage requires interferon regulatory factor 7. J Biol Chem. 2001;276(48):45491–45496. doi:10.1074/jbc.C100421200
