Abstract
Inflammatory diseases provide substantial worldwide concerns, affecting millions of people and healthcare systems by causing ongoing discomfort, diminished quality of life, and increased expenses. In light of the progress made in treatments, the limited effectiveness and negative side effects of present pharmaceuticals need a more comprehensive comprehension of the underlying processes in order to develop more precise remedies. Exosomes, which are tiny vesicles that play a vital role in cell communication, have been identified as prospective vehicles for effective delivery of anti-inflammatory medicines, immunomodulators, and gene treatments. Vesicles, which are secreted by different cells, have a crucial function in communicating between cells. This makes them valuable in the fields of diagnostics and therapies, particularly for inflammatory conditions. Exosomes have a role in regulating the immune system, transporting cytokines, and influencing cell signaling pathways associated with inflammation. They consist of proteins, lipids, and genetic information that have an impact on immune responses and inflammation. Scientists are now investigating exosomes as biomarkers for inflammatory disease. This review article aims to develop non-invasive diagnostic techniques with improved sensitivity and specificity. Purpose of this review is a thorough examination of exosomes in pharmacology, specifically emphasizing their origin, contents, and functions, with the objective of enhancing diagnostic and therapeutic strategies for inflammatory conditions. Gaining a comprehensive understanding of the intricate mechanisms involved in exosome-mediated interactions and their impact on immune responses is of utmost importance in order to devise novel approaches for tackling inflammatory disease and enhancing patient care.
Introduction
Inflammatory diseases include a variety of conditions marked by unregulated immune reactions, leading to tissue inflammation. These diseases can adversely affect multiple organ systems and significantly impact both health and quality of life.Citation1 There is heterogeneity in the occurrence of inflammatory diseases worldwide. For instance, asthma impacts over 300 million individuals globally, whereas conditions like rheumatoid arthritis and inflammatory bowel disease have a substantial effect on millions of people worldwide.Citation2,Citation3 The chronic nature of inflammatory diseases poses significant challenges for people and healthcare systems. These diseases often result in persistent pain, diminished quality of life, and increased healthcare costs.Citation4 Despite advancements in treatment options, therapy remains challenging due to the limited efficacy of current medications and the long-term adverse effects associated with their use. In order to successfully combat inflammatory disorders, it is crucial to have a more thorough knowledge of the underlying mechanisms and develop medications that particularly target those systems. Current treatments often lack specificity, leading to unexpected effects on non-target regions and potential severe responses. Moreover, several inflammatory diseases display repetitive and alternating patterns, requiring the implementation of prolonged treatment interventions.Citation5 below shows prominent studies on exosomes as indicators for inflammation.
Table 1 Shows the Most Recent Research Conducted on Exosomes as Indicators for Inflammation
Presence of a wide range of inflammatory diseases complicates the development of treatment strategies, as individuals may react differently to the same therapeutic intervention. Personalized medicine solutions, including factors such as genetic composition, environmental conditions, and disease manifestation, have the potential to improve treatment outcomes and reduce the effects of inflammatory diseases.Citation6 The advancements in technology, including high-throughput sequencing, omics profiling and advanced imaging approaches, have facilitated the discovery of novel targets for the therapeutic intervention and biomarkers of inflammatory diseases. Targeted therapies, such as biologics and small molecule inhibitors, provide effective means of regulating specific immune pathways implicated in inflammation. Therefore, there is a reduction in the level of disease activity and a positive enhancement in the outcomes experienced by the patients.Citation7 In addition, the emergence of novel treatment strategies using exosomes has received considerable interest in recent years. Scientists are investigating the possibility of using exosomes as carriers for medicinal substances, such as anti-inflammatory medications, immunomodulators, and gene treatments.Citation8,Citation9
Exosomes are small extracellular vesicles (EVs) that have a size ranging from 30–150 nm and play a vital function in communication between cells.Citation10,Citation11 Vesicles are released by several types of cells, including immune cells, to facilitate the transfer of proteins, lipids, and genetic material between cells.Citation12 The role of exosomes in intercellular communication has sparked great anticipation for their potential use in several fields, particularly in diagnostics and therapeutics, with a special focus on inflammatory diseases.Citation13
Exosomes are generated inside intracellular compartments called multivesicular bodies (MVBs), which encapsulate cytoplasmic constituents intended for extracellular release.Citation14,Citation15 Following fusion with the plasma membrane, MVBs release exosomes into the extracellular milieu. Vesicles facilitate the transportation of a substantial amount of nucleic acids and proteins like messenger RNA (mRNA), microRNA (miRNA). This enables them to effectively control the functions of the recipient cells ().Citation16,Citation17
Figure 1 Inward budding of the cell membrane forms early endosomes. Exosomes are produced from endosomes via inward budding of the multi-vesicular bodies (MVBs) membrane, incorporating nucleic acids, proteins, and lipids. Finally, exosomes are released into the extracellular environment through exocytosis. ➀ Exosomes from serum and saliva as novel biomarkers of IBD; ➁ Exosomes from colon cancer cells, IECs, and platelets maintain TJ barrier function; ➂ Exosomes from DCs influence the intestinal microbiota profile via heat shock proteins; ➃ IEC-derived exosomes fuse with DC membranes to induce immune tolerance; ➄ Colitis serum or treated DC exosomes regulate immune cell proliferation through MAPK, NF-κB, and other inflammatory pathways.
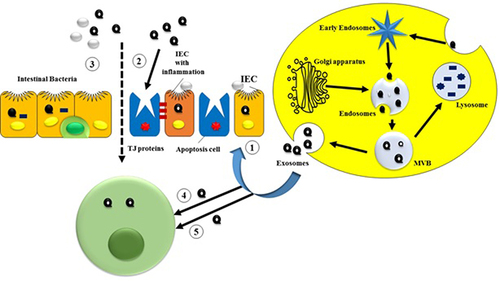
Exosome-mediated communication is essential for several physiological processes and pathological diseases. Exosomes derived from immune cells include immunomodulatory chemicals that regulate immune responses and inflammation.Citation8,Citation18 Exosomes may be recognized and triggered by the immune system because they have the ability to transport antigens.Citation19 Exosomes play a key role in preserving equilibrium and restoring tissues. They participate in the elimination of cellular waste, the conveyance of growth stimuli, and the regulation of stem cell differentiation.Citation20 Moreover, exosomes have been implicated in the pathogenesis of several diseases, including as cancer, neurological disorders, and infectious diseases, highlighting their diverse functions in both promoting health and inducing disease.Citation21,Citation22
Moreover, exosomes are now under scrutiny as biomarkers for inflammatory diseases, presenting the possibility of non-invasive diagnostic procedures with enhanced sensitivity and specificity in comparison to conventional biomarkers.Citation23 Researchers may use exosome cargo analysis, which includes proteins, nucleic acids, and lipids, to discover specific molecular patterns linked to various inflammatory disorders.Citation24 This analysis helps in the early detection, prediction, and monitoring of disease development. Furthermore, the consistent preservation of exosomal cargo in different biofluids, including blood, urine, and cerebrospinal fluid, renders them desirable for liquid biopsy-based methods in clinical settings.Citation24
Rationale of the Review
The motivation for this review arises from the growing recognition of the importance of exosomes in the context of inflammatory diseases, alongside increasing interest in utilizing exosomes as both diagnostic tools and therapeutic targets. Recently, there has been a significant increase in research that emphasizes the various functions of exosomes in communication between cells, maintaining the balance of tissues, and the development of diseases, especially in inflammatory diseases. Gaining a comprehensive understanding of the complex processes that drive exosome-mediated interaction and their role in regulating immune responses and inflammation is essential for the development of innovative diagnostic and therapeutic approaches for inflammatory diseases. This review intends to offer a complete overview of the possible uses of exosomes in pharmacology, specifically focusing on inflammatory disorders. It will elucidate the biogenesis, cargo composition, and activities of exosomes. Furthermore, this review aims to enhance current understanding and incorporate recent developments in exosome research to advance the utilization of exosomes in diagnosing, managing, and treating inflammatory diseases. The ultimate objective is to provide valuable insights that may guide future research paths and clinical applications, resulting in enhanced techniques for addressing inflammatory diseases and improving patient care.
Exosomes have various essential functions in the setting of inflammatory bowel disease (IBD): Initially, when extracted from serum and saliva, they show potential as innovative biomarkers for IBD. In addition, exosomes derived from other sources such as colon cancer cells, intestinal epithelial cells (IECs), and platelets have a role in preserving the integrity of the intestinal barrier. Furthermore, exosomes generated from dendritic cells (DCs) have an impact on the composition of the gut microbiota, and this effect is reliant on heat shock proteins. In addition, exosomes derived from intestinal epithelial cells (IECs) fuse with dendritic cell (DC) membranes in order to enhance immunological tolerance. Finally, exosomes derived from serum of individuals with colitis or from treated dendritic cells modulate the proliferation of immune cells by activating signaling pathways associated with inflammation, such as MAPK and NF-κB.
Exosomes in Inflammation
Exosomes have complex functions in inflammation, affecting immunological responses, transporting cytokines, and modulating cell signaling pathways.Citation25,Citation26 Having an in-depth knowledge of these pathways is essential for comprehending intricate relationship between exosomes and inflammatory processes. This knowledge will provide valuable insights into the possible application of exosomes for diagnosing and treating inflammatory diseases.
Immune Modulation
Exosomes originating from immune cells, namely dendritic cells, macrophages, and T cells, play a significant role in immunological regulation. They have the ability to finely regulate immune responses in both normal and abnormal situations. The nanoscale vesicles, transport a diverse collection of proteins, lipids, and nucleic acids.Citation27 This allows them to coordinate a wide range of immunomodulatory activities.
Exosomes Derived from Dendritic Cell (DEX)
Dendritic cells (DCs) play a pivotal role in the presentation of antigens and the activation of the immune system, which are crucial processes for the initiation and control of adaptive immune responses.Citation28 The exosomes contain a high concentration of major histocompatibility complex (MHC) molecules, including MHC class I and II, along with co-stimulatory molecules like CD80 and CD86.Citation29 DEX enhances antigen presentation to T cells by transferring molecules to recipient cells, resulting in the differentiation of T cells and activation into effector or memory subsets.Citation30 In addition, DEX transport antigens obtained from their parent DCs, enabling the initiation of immune responses that are unique to those antigens. DEX, in addition to their function in presenting antigens, have immunomodulatory characteristics that aid in the control of immunological tolerance and inflammation.Citation31 The exosomes include immunosuppressive elements such as transforming growth factor-beta (TGF-β) and interleukin-10 (IL-10) which may inhibit the activation and functioning of effector T cells and DCs.Citation32 In addition, DEX has been shown to stimulate the production of regulatory T cells (Tregs), a distinct subgroup of T cells that are essential for maintaining immunological balance while avoiding autoimmune reactions.Citation33
MEXs (Macrophage-Derived Exosomes)
The variability of macrophage populations and their involvement in both inflammation and tissue homeostasis enable MEX to demonstrate a wide range of immunomodulatory actions.Citation34 Exosomes transport cytokines, chemokines, and growth factors that have the ability to impact the function of other immune cells and control inflammatory reactions. For example, interleukin-10 (IL-10) produced from MEX has been shown to inhibit the synthesis of pro-inflammatory cytokines in recipient cells, therefore reducing inflammatory responses and enhancing immunological tolerance.Citation35 In addition, MEX has the ability to modify the characteristics of several immune cells, including and natural killer (NK) cells and T cells, via transporting regulatory chemicals. For instance, microRNAs (miRNAs) derived from MEX are associated with regulating T cell activation and differentiation by targeting key signaling pathways involved in immune responses.Citation36
Exosomes Derived from T Cells (TEX)
T cell-derived extracellular vesicles (TEX) serve a crucial function in facilitating communication between T cells and other immune cells, hence contributing to the control of immunological responses and inflammation.Citation37 Exosomes transport a wide range of immunomodulatory chemicals, including as cytokines, chemokines, and cytotoxic proteins that have the ability to affect the activity of certain cells. Regulatory T cells (Tregs) produce TEX, which may block immunological responses and facilitate immune tolerance by releasing inhibitory cytokines such TGF-β and interleukin-35 (IL-35).Citation38 These exosomes hinder the activation and operation of effector T cells and dendritic cells, therefore reducing the intensity of inflammatory reactions and averting the development of autoimmunity.
Cytokine Transport
Exosomes obtained from activated immune cells, such as T cells, macrophages and dendritic cells, contain a high concentration of pro-inflammatory cytokines that have significant functions in immune activation and inflammation.Citation39 These exosomes, containing cytokines, serve as carriers that effectively transport pro-inflammatory substances to specific cells, thereby intensifying inflammatory reactions and promoting immunological activation.Citation24 When recipient cells uptake these exosomes containing cytokines, it can trigger inflammatory signaling pathways, leading to the activation of transcription factors such as nuclear factor-kappa B (NF-κB) and the production of pro-inflammatory chemicals.Citation8 Exosomes generated by active T cells include cytokines, including interleukin-2 (IL-2) and interleukin-17 (IL-17), which stimulate T cell activation and attract immune cells to areas of inflammation.Citation40 T cell-derived exosomes facilitate the transmission of cytokines to specific cells, thereby enhancing and spreading inflammatory reactions, which in turn intensify the immunological cascade.
Transportation of Anti-Inflammatory Cytokines
Unlike pro-inflammatory cytokines, exosomes can carry anti-inflammatory cytokines, which possess immunosuppressive properties and help in resolving inflammation.Citation25 Exosomes derived from regulatory immune cells or stromal cells involved in tissue healing and immunological regulation play a vital role in maintaining immune homeostasis and preventing excessive inflammation.Citation41
Exosomes that contain IL-10 or TGF-β have the ability to strongly suppress the immune system by blocking the activation and activity of immune cells that are engaged in inflammatory reactions.Citation41 These exosomes, which are enriched with anti-inflammatory cytokines, may reduce the activation of inflammatory signaling pathways and modify cellular reactions when ingested by recipient cells. This causes inflammation and increase in tissue healing and regrowth.
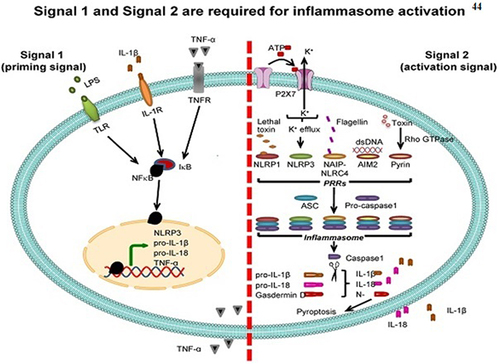
Moreover, it has been shown that exosomes originating from regulatory T cells (Tregs) include immunosuppressive substances such as IL-10, TGF-β, and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4).Citation42 These substances effectively hinder activation, operation of effector T cells and dendritic cells. Treg-derived exosomes facilitate preservation of the immunological tolerance and the avoidance of autoimmune responses by transporting regulatory chemicals to specific cells.Citation43 Exosomes carry cytokines and serve a dual function in inflammation. Exosomes filled with pro-inflammatory cytokines enhance inflammatory responses, whereas exosomes loaded with anti-inflammatory cytokines reduce inflammation ().Citation44
Figure 2 The interaction between exosomes and inflammasomes and their respective functions in inflammatory reactions. Inflammasome activation requires two signals: “signal 1” (priming) and “signal 2” (activation). The priming signal activates NF-κB, upregulating NLRP3, pro-IL-1β, pro-IL-18, and TNF-α. The activation signal induces PRRs, leading to the formation of the inflammasome complex and the conversion of pro-IL-1β and pro-IL-18 to their active forms, IL-1β and IL-18, which are then secreted. Reprinted from Noonin C, Thongboonkerd V. Exosome-inflammasome crosstalk and their roles in inflammatory responses. Theranostics. 2021;11(9):4436. Creative Commons.Citation44
Cellular Communication
Exosomes have important part in helping cell communication pathways related to inflammation.Citation45 They coordinate various cellular activities by transferring bioactive substances. They have the ability to transmit a diverse array of functional proteins to recipient cells, which in turn affects their phenotypic and function in different physiological and pathological situations. The mentioned proteins, which include enzymes, receptors, transcription factors, and signaling molecules, play significant roles in cellular signaling pathways related to inflammation.Citation25
Exosomes obtained from active immune cells, such as macrophages and T cells, have the ability to transport signaling proteins that stimulate inflammatory pathways in target cells.Citation26 These proteins may consist of pro-inflammatory cytokines, like interleukin-1 and tumor necrosis factor-alpha together with chemokines and growth factors that stimulate the recruitment and activation of immune cells.Citation26
Furthermore, the transfer of membrane receptors, including as toll-like receptors (TLRs) and cytokine receptors, via exosomes might impact the sensitivity of recipient cells to inflammatory signals. Exosomes have the ability to augment the responsiveness of target cells to pro-inflammatory signals and intensify inflammatory reactions by delivering these receptors.Citation46 Exosomes not only carry proteins, but also include a wide range of nucleic acids, such as mRNA, miRNA, and long non-coding RNA (lncRNA).Citation47 These nucleic acids are important for controlling gene expression in the cells that receive the exosomes. Exosomal nucleic acids can regulate the expression of specific genes involved in inflammation, immunological responses, and tissue repair processes.Citation46
Exosomal mRNA has the ability to undergo translation in recipient cells, resulting in the production of functional proteins. This process has the potential to impact the phenotypic and function of the recipient cells. Exosomes originating from active immune cells may include mRNA that encodes inflammatory cytokines or chemokines. When these exosomes are taken up by target cells, the mRNA can be translated into proteins, leading to the production of pro-inflammatory mediators.Citation48 Moreover, exosomal miRNA and lncRNA have been identified as powerful controllers of gene expression, with the ability to influence a range of cellular activities such as cell growth, specialization, programmed cell death, and immunological reactions.Citation48 Exosomal miRNAs originating from immune cells have the ability to selectively affect the expression of inflammatory cytokines or transcription factors by targeting their corresponding mRNAs. This process allows for the precise regulation of inflammatory gene expression in recipient cells.Citation48
Significance for Inflammation and Immunity
The transfer of bioactive substances by exosomes between cells is essential for influencing inflammatory responses and maintaining immunological balance. Exosomes have the ability to affect the strength and length of inflammatory reactions, as well as equilibrium between anti-inflammatory and pro-inflammatory signaling pathways, via controlling cell signaling pathways and gene expression patterns in specific cells ().Citation49
Figure 3 Bone marrow mesenchymal stem cells (BMSCs) primarily release exosomes and inflammatory factors. The cytokines generated by BMSCs may be categorized into proinflammatory cytokines and anti-inflammatory cytokines. Both exosomes and cytokines can modulate distant target organs, inducing an inflammatory response.
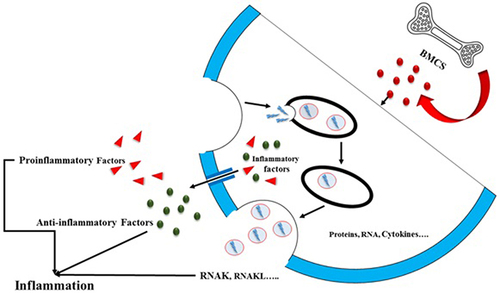
Furthermore, the disruption of exosome-mediated cell signaling has been linked to the development of certain inflammatory disease, such as autoimmune disorders, chronic inflammatory syndromes, and cancer. Understanding the intricate processes of exosome-mediated cell signaling during inflammation is crucial for developing innovative therapeutic approaches. These approaches can target exosome formation, release, and uptake to effectively address inflammatory diseases.Citation8
Exosomes have a diverse function in cell signaling pathways related to inflammation, influencing several cellular processes by transferring bioactive substances. Further investigation is necessary to better understand the processes by which exosomes facilitate cell signaling in inflammation.Citation45 This will help to fully comprehend their potential as a therapeutic approach for managing inflammatory diseases.
Evidence of Exosome Alterations in Various Inflammatory Conditions
Exosomes play a key role in inflammatory diseases by facilitating cellular communication and actively contributing to disease development through intercellular signaling and regulation of immune responses.Citation8
Inflammatory Bowel Disease (IBD)
Inflammatory bowel disease (IBD), encompassing ulcerative colitis and Crohn’s disease can be identified by chronic inflammation of the gastrointestinal tract.Citation50 Research has shown significant changes in the composition of exosomes derived from intestinal epithelial cells, immune cells, and gut microbiota in individuals with IBD.Citation8 These exosomes transport a payload including a high concentration of cytokines, chemokines, microRNAs that contribute to ongoing intestinal inflammation and tissue damage.Citation51
Exosomes originating from inflamed intestinal tissues have a crucial impact on intensifying immune responses and facilitating the attraction of inflammatory cells to the gut mucosa.Citation52 Exosomes deliver pro-inflammatory mediators to target cells, intensifying the inflammatory cascade and exacerbating disease progression and tissue damage in individuals with IBD.Citation53
Rheumatoid Arthritis (RA)
Rheumatoid arthritis (RA) is a persistent inflammatory condition characterized by inflammation of the synovial joints, resulting in the deterioration of cartilage and erosion of bone.Citation54 Research has shown changes in the composition of exosomes obtained from the synovial fluid and serum of individuals with rheumatoid arthritis (RA), with increased amounts of pro-inflammatory cytokines, including different tumor necrosis factors TNF-α, IL-6, and IL-17.Citation55
The presence of cytokine-loaded exosomes in RA contributes to the continuation of synovial inflammation and cartilage damage by facilitating the activation of synovial fibroblasts, triggering the synthesis of matrix metalloproteinases (MMPs), and increasing osteoclastogenesis.Citation56 In addition, exosomes originating from immune cells in the inflamed synovium might intensify local inflammation and tissue harm, hence worsening the course of RA.Citation57
Asthma
Asthma is a long-lasting condition where the airways become inflamed, leading to narrowed air passages, increased sensitivity, and constriction of the bronchial tubes.Citation58 Exosomes obtained from bronchoalveolar lavage fluid and peripheral blood of individuals with asthma show modifications in their payload of inflammatory agents, such as cytokines, chemokines, and growth factors.Citation59 Cytokine-loaded exosomes have a significant impact on regulating airway inflammation, smooth muscle contraction, and mucus production in individuals with asthma. This contributes to the development of the disease and worsens its symptoms. Furthermore, exosomal microRNAs have been linked to the control of immune responses and the alteration of airway structure in asthma.Citation59
Inflammation-Related Skin Conditions
Psoriasis and atopic dermatitis are inflammatory skin diseases characterized by abnormal immune responses and skin inflammation. Exosomes obtained from keratinocytes, fibroblasts, and immune cells present in the skin of patients with these conditions include a load of pro-inflammatory cytokines, chemokines, and antimicrobial peptides. The presence of cytokine-loaded exosomes intensifies cutaneous inflammation, increases epidermal hyperproliferation, and disrupts normal immune responses in inflammatory skin disease, leading to more severe disease and causing damage to the tissues. Furthermore, alterations in the composition of exosomes in the skin’s microenvironment could potentially serve as markers of disease severity and as targets for medical interventions aimed at mitigating skin inflammation and restoring the skin’s natural equilibrium.Citation60 Exosome composition and secretion changes in different inflammatory circumstances, suggesting the complex relation between immune cells, stromal cells, and inflammatory microenvironment.Citation61 An analysis of exosome profiles in various diseases offers useful insights into the formation and progression of diseases and it lead to of new diagnostic biomarkers for inflammatory disorders. Further research is necessary to clarify the intricate function in the disease development and to use these in clinical settings to enhance patient care and treatment.Citation22
Implications for Disease Progression
The alterations in exosome composition and function observed in various inflammatory diseases have important implications for disease progression and the development of therapeutic interventions. By modulating immune responses, inflammation, and tissue remodeling processes, exosomes can either exacerbate or attenuate disease progression, depending on their cargo and target cells.
Diagnostic Biomarkers
Exosome-derived biomarkers, including cytokines, chemokines, microRNAs, and autoantibodies, hold promise for the diagnosis, prognosis, and monitoring of disease activity in inflammatory conditions. The characterization of exosome profiles in biological fluids, such as blood, urine, and synovial fluid, may provide valuable insights into the underlying pathophysiology of inflammatory diseases and help guide therapeutic decision-making.Citation62
Therapeutic Targets
Targeting exosome-mediated pathways represents a novel therapeutic approach for the treatment of inflammatory diseases. Strategies aimed at modulating exosome biogenesis, secretion, and uptake may offer therapeutic benefits by attenuating inflammation, promoting tissue repair, and restoring immune homeostasis. Moreover, modifying exosomes for drug delivery holds promise in developing personalized therapeutic interventions tailored to the specific requirements of patients with inflammatory diseases.Citation63
Prognostic Indicators
Exosome-derived biomarkers have the potential to serve as prognostic indicators of disease progression and treatment response in inflammatory conditions. The longitudinal monitoring of exosome profiles over the course of disease may provide valuable insights into disease trajectory, identify patients at risk of complications, and guide the selection of optimal therapeutic strategies.Citation64
Diagnostic Potential of Exosomes
Exosomes were observed in all body fluids (eg, blood, urine, saliva, milk), and because of their particular protein, RNA, and lipid content, exosomes may be beneficial for early disease detection in contrast to conventional needle or excision biopsies, minimally invasive diagnostics (blood analysis) and non-invasive diagnostics (urinalysis and saliva samples) offer several advantages over the former. These include reduced patient discomfort and inconvenience, expedited and cost-effective analysis, and decreased patient pain and inconvenience.Citation65
Isolation and Characterization Methods
Techniques for Exosome Isolation
Various methodologies are employed for isolating exosomes, each with its own distinct advantages and limitations. The commonly employed techniques comprise ultracentrifugation, size exclusion chromatography, ultrafiltration, and polymer precipitation.Citation66 Ultracentrifugation is widely used and well-regarded technique for isolating exosomes because of its high efficiency in separating different biological components.Citation67 However, because there is no single methodology that meets all criteria for effective exosome isolation, researchers often combine multiple strategies to achieve optimal results. A case in point is by Tayebi et al in 2021, which showcased a method that utilizes electrical and acoustic forces to control exosomes for the purpose of sorting. This approach resulted in exceptionally high levels of purity and recovery rates.Citation68 The intricate nature of biological fluids, the convergence of physical characteristics between exosomes and other entities such as lipoproteins and viruses, and the variability within exosomes themselves provide difficulties in separation methods. Thus, it is generally important to employ a variety of techniques in order to successfully separate exosomes.Citation66 Differential Ultracentrifugation (dUC) is a method that relies on centrifugal forces to separate particles based on size and density over specific periods of time.Citation69 dUC is a highly regarded method for isolating exosomes, allowing for the extraction of reasonably pure populations of these extracellular vesicles. It is widely recognized as the benchmark technique for exosomal isolation.Citation70 Another method used for this is the method of UF ie Ultrafiltration which depends on utilization of membranes that have predetermined pore sizes to separate particles within a specific size range.Citation71 Filters with pore sizes of 0.45 µm and 0.8 µm are used to exclude larger particles, resulting in a filtrate that contains a high concentration of exosomes. Subsequently, membranes having smaller pores are employed to remove smaller vesicles from the filtrate, allowing them to flow into a waste eluate and then they are characterized by a specific size range. This approach can serve as a supplementary method to ultracentrifugation for the separation the exosomes from large microvesicles. However, it can also be utilized independently as a standalone technique. Another technique for isolating exosomes, which utilizes nano-ultrafiltration and involves a series of consecutive filtrations, is referred to as tangential-flow filtration.Citation72
Due to the variations in separation techniques discussed earlier, each with their own merits and drawbacks, researchers in the field of extracellular vehicles (EVs) have not yet reached a consensus on a universally accepted and optimal method. However, in October 2015, a global poll was conducted by ISEV, which collected responses from 196 members using an online questionnaire sent via email.Citation73 According to the survey, dUC was used in 85% of all instances to gather extracellular. In 2019, ISEV performed a follow-up study with 600 respondents. The results showed that although density-gradient ultracentrifugation and dUC remained the widely used methods, utilization of SEC has significantly enhanced. In fact, the percentage of SEC usage has more than doubled since 2015.Citation74
Characterization Methods for Diagnostic Purposes
For diagnostic purposes, characterizing exosomes involves examining their composition and properties using various methods. One of the methods is Nanoparticle Tracking Analysis, Biophysical techniques are employed to determine the size range of exosomes. An example of a biophysical method is optical particle tracking, specifically using NTA, which enables the quantification of exosomes in the size range of 10 nm to 2 µm by measuring their concentration and size distribution. Exosomal migration trajectory has been identified to quantify particle velocity.Citation75 This approach tracks nanoparticle Brownian motion in liquid suspensions on a particle-by-particle basis. NTA uses image analysis to track exosomes, correlating their movement with particle size.Citation76 DLS, or dynamic light scattering, is an alternate method used to determine the size of exosomes. The operational basis of DLS involves the transmission of a single-colored, synchronised laser beam into a solution containing suspended particles.Citation77 Another method is flow cytometry, which is used to analyze and identify the surface proteins of exosomes at a molecular level. Additionally, it enables the quantification of exosome dimensions and composition.Citation78 Atomic force microscopy (AFM) is a distinct and unconventional method for investigating exosomes, offering an alternate solution to electron diffraction and traditional optical methods. The approach reliably identifies and records interactions between a probing point and the sample surface. A key characteristic of this technique is its capacity to assess samples in their original state, requiring no preparation and without causing any damage.Citation79 Some other methods include Resistive Pulse Sensing, Transmission Electron Microscopy, Mass Spectrometry (MS), Tunable Resistive Pulse Sensing (TRPS). These methods are vital for studying the composition, cargo, and traits of exosomes, which are necessary for their diagnostic capacity in diverse ailments such as cancer and autoimmune disorders.Citation80
Biomarkers for Inflammatory Diseases
MicroRNAs, Proteins, Lipids Carried by Exosomes
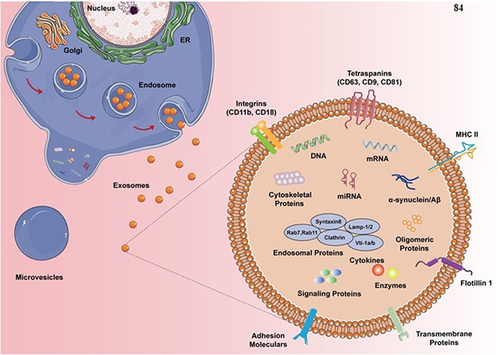
MiRNAs in exosomes regulate gene expression and biological activities. MiRNAs can be transmitted between cells via exosomes, affecting biological functioning and disease processes.Citation81 Proteins from exosomes reflect their cell of origin. Surface markers, enzymes, and signaling molecules present on exosomes can indicate the cell type and its physiological status. Exosome protein analysis can help diagnose disease progression.Citation82 The presence of lipids in exosomes plays an important part in retaining their structural integrity and in facilitating their functioning. The composition ie lipid of exosomes can differ depending on the kind of cell and the environment, providing a distinct pattern that can be used for diagnostic purposes. Exosomes’ lipid patterns have been associated with different disorders, indicating their potential as biomarkers for disease identification and monitoring.Citation83 Exosomes contain a diverse array of molecules, including nucleic acids, proteins, and lipids ().Citation84
Figure 4 Schematic of exosomal molecular composition. Exosomes contain various important biomarkers, such as proteins, lipids, and miRNAs. Reprinted from Guo M, Hao Y, Feng Y, et al. Microglial exosomes in neurodegenerative disease. 2021;14:630808. Creative Commons.Citation84
Clinical Studies Demonstrating Diagnostic Utility
Exosomes, cell-derived vesicles, carry various active biomolecules. Since they may transport different biomarkers, they may be useful for early-stage disease diagnosis. Cancer biomarker studies have demonstrated that exosomes can recognize protein biomarkers. Phase 1 data on non-small cell lung cancer patients showed success with tumor antigen-loaded dendritic cell-derived exosomes as vaccine candidates.Citation85 A different research study used exosomal micro-A as a biomarker to identify early-stage lung cancer, while another study identified ARv7 in the plasma by analyzing blood samples to diagnose metastatic, castrate-resistant prostate cancer.Citation86,Citation87 Another study treated chronic kidney failure with hemodiafiltration (OL-HDF) and serum exosome mRNA analysis. MicroRNA content study using RT-qPCR will revealed inflammatory indicators from chronic renal disease treatment. Obesity is another major healthcare issue. A potential obesity treatment using exosomes has also been researched.Citation88 A number of studies have also been carried out with the purpose of analyzing exosomes extracted from human samples under a variety of situations. According to the findings, a total of 116 studies have been documented, of which 58 (or fifty percent) are associated with biomarker applications. It is necessary for exosomes that are being tested in clinical trials to adhere to good manufacturing practice (GMP). The type of cells, the culture environment, the cultivation equipment, and the culture medium are all components of a GMP-grade exosome production technique.Citation89 Current clinical trials demonstrate the utilisation of exosomes as a means of administering medicinal substances. For instance, in two clinical trials, the scientists sought to enclose curcumin within exosomes derived from plants as a means of treating colon cancer and Irritable Bowel Disease using direct techniques. Furthermore, three more clinical trials were conducted with the objective of genetically altering mesenchymal stem cells (MSCs) to enhance the production of exosomes.None of these trials have been completed and the findings are not yet available. Notably, certain clinical trials included microparticles derived from tumour cells as a means of medication delivery.Citation90
Therapeutic Biomarkers and Targeting Strategies
Exosomes have the potential to be used as biomarkers for diseases and as tools for therapy, providing new approaches for health treatments. These biomarkers can be used for diagnosing and predicting the outcome of different diseases, such as cancer, demonstrating their flexibility in managing diseases.
Identification of Therapeutic Targets
Following the discovery that exosomes contain microRNA, the pharmaceutical industry became highly interested in their potential for delivering specifically engineered small interfering RNA (siRNA) molecules for therapeutic purposes.Citation91 Considerable efforts have been made to load micro RNA into liposomes, which are vesicles enclosed by a membrane. Exosomes, on the other hand, seem to be a more advantageous means of transportation. Van der Meel has provided a summary of the contrasting biochemical and pharmacological characteristics of these two types of corpuscles.Citation92 Emerging research has shown that exosomes can be used both as a therapeutic method and as a target for treating cancer.Citation93 Immunotherapy is a rapidly developing area in treating cancer, and the exosomes can be used for cancer immunotherapy because of their capacity to modulate the immune system.Citation94 Considering the crucial functions that exosomes play in the advancement of cancer, directing efforts towards inhibiting the formation and release of exosomes could have significant consequences for future cancer treatments. Currently, there are no medications available for therapeutic use that effectively removes harmful exosomes in cancer patients. Using a high-throughput pharmacological screening, 4580 samples were tested and five powerful inhibitors of exosome formation and secretion were discovered. The compounds mentioned are triadimenol, ketoconazole, tipifarnib, climbazole, and neticonazole.Citation95
Role of Exosome-Derived Biomarkers
Multiple studies have reported the presence of specific miRNAs (including miRNA-133a, 223, 150, 320b, 143/145, 214, and miRNA-155) in exosomes. These miRNAs have been identified as useful diagnostic biomarkers and predictors of outcomes in atherosclerosis and other vascular inflammatory cases.Citation104 For instance, miR-133a is a diagnostic biomarker for MI (myocardial infarction).Citation105 Krüppel-like factor 2 (KLF2) is a transcription factor that responds to shear stress and have an important role in regulating the expression of genes in endothelial cells, which are activated by flow that protects against atherosclerosis. In addition, atheroprotective stimuli are responsible for initiating a communication by means of microRNA (miRNA) between smooth muscle cells (SMCs) and endothelial cells (ECs). Exosomes released by cells transduced with KLF2 are abundant in miR-143/145 and can regulate the expression of target genes in co-cultured smooth muscle cells.Citation106 CD24 found in exosomes is now known as a diagnostic marker for breast cancer.Citation107 CD44 variant isoform 6 (CD44v6) is a type of protein that is found on the surface of cells and spans across the cell membrane. It is abundantly present in exosomes, which are small vesicles released by pancreatic cancer-initiating cells (PCICs). Exosomes produced from PCICs and expressing CD44v6 have the ability to activate the Wnt/β-catenin signalling pathway and increase the production of plasminogen activator inhibitor 1 (PAI-1) and tissue inhibitor of metalloproteases 1 (TIM-1). Exosomes derived from THP-1 cells have the ability to penetrate and transport miR-150 into human HMEC-1 cells. Exosomes with increased amounts of miR-150 could decrease the expression of c-Myb and promote cell motility in HMEC-1 cells. Elevated exogenous miR-150 effectively mediates the biological communication between vascular endothelial cells (ECs) and macrophages/circulating monocytes in various pathophysiological situations.Citation108
Strategies for Targeted Therapy
Utilization of Exosomes as Drug Delivery Vehicles
Exosomes has numerous characteristics that make them an optimal carrier for drugs delivery. Protein and genetic materials found in exosomes suggest that it is possible to load these biological substances into exosomes. Furthermore, exosomes have a high level of biocompatibility, as demonstrated by their extensive presence in many bodily fluids.Citation109 Consequently, it is expected that drug delivery systems produced from exosomes will be well tolerated, resulting in a longer period of time in which they remain in circulation and improved effectiveness. Furthermore, studies have demonstrated that exosomes are capable of traversing the plasma membrane in order to transport their payload into certain cells. For instance, exosomes generated from dendritic cells have the ability to transport peptide loaded MHC class I and II complexes to other dendritic cells in order to regulate the immune response.Citation110
Extensive research has been conducted on using exosomes for the delivery of therapeutic drugs. Exosomes were used in one study to carry curcumin and treat an inflammatory disease.Citation111 Exosomes have been used to develop the mixture with curcumin in order to augment the efficacy of curcumin. In order to improve the drugs characteristics, exosomes are utilized to transfer small molecular drugs across the blood-brain barrier (BBB). It is true that 98% of potent medications affecting the central nervous system are unable to cross the blood-brain barrier. Additionally, their effectiveness, as demonstrated in laboratory settings, has not translated into success in clinical trials.Citation112 Several Nano-formulations have been used to address the issues related to drug permeability across the BBB. Exosomes, which are naturally produced by the body’s cells, can be modified to pass through the BBB. This modification enhances the delivery of drugs to the brain by reducing the clearance of drugs by the MPS.
Exosomes are utilized not only for transporting small molecules, but also for delivering big molecules like proteins. A study shows that the exosomes containing antioxidant protein catalase were effectively transported across blood-brain barrier, leading to an enhanced disease condition in Parkinson’s disease (PD).Citation113 PD is linked to inflammation in the brain, activation of microglia, and the release of reactive oxygen species (ROS).Citation114 Patients diagnosed with PD exhibit reduced levels of antioxidant enzymes such as catalase and superoxide dismutase. These enzymes play a crucial role in managing oxidative stress and preventing neurodegeneration. Similar to other medications, the transportation of catalase over the BBB presents a challenge. However, the use of exosomes to carry catalase to the brain appears to be a hopeful approach for PD treatment.Citation115 Research has demonstrated that exosomes has the ability to transport nucleic acids, to specific cells, hence causing alterations in both biological and disease-related mechanisms. The characteristics of exosomes have generated significant interest in treatment approaches that involve genetic therapy. Research has been performed to explore the use of exosomes as drug delivery systems for administering therapeutic genetic materials that can modify gene expression in certain disorders and enhance genetic therapy.Citation116
Targeted Therapy Approaches for Inflammatory Disorders
Multiple researches have demonstrated that the exosomes released through various immune cells (such as DCs, macrophages, and neutrophils) as well as non-immune cells (such MSCs and platelets) have a role in the onset and growth of chronic inflammation.Citation51 M2 macrophages secrete exosomes that can generate and discharge anti-inflammatory cytokines (IL-10, IL-1Ra, IL-4) and transforming growth factor TGF-β. These substances have notable therapeutic properties in combating inflammation and promoting tissue repair.Citation117 Furthermore, M2 exosomes demonstrate a pronounced pro-inflammatory inclination, making them a highly efficient bionic carrier for specifically targeting rheumatoid arthritis (RA). Exosomes generated from M2 macrophages contain a diverse range of anti-inflammatory cytokines, which have a crucial function in combating inflammation and facilitating tissue repair, Plasmid exosomes containing betamethasone sodium phosphate and IL-10 are encapsulated within exosomes produced by M2 macrophages to devise a biomimetic drug delivery system. Nano-system achieved M1-to-M2 macrophage polarization by increasing the expression of IL-10. This directed exosomes to inflamed areas, they demonstrated anti-inflammatory effects and reduced joint damage in RA.Citation118 Research has demonstrated that stem cells produced from human urine (HUSCs) can improve the growth and movement of chondrocytes that have been treated with IL-1β.Citation119 In contrast, miR-140–5p transfected exosomes retain these mentioned benefits and target vascular endothelial growth factor to reduce extracellular matrix. MSCs and exosomes regulate immunity, anti-inflammation, homing, tissue repair and differentiation. Therefore, MSC-derived exosome delivery techniques have considerable potential for treating chronic inflammatory disorders.Citation120 Exosomes possess exceptional cellular penetration capabilities and serve as crucial mediators in intercellular communication, either accelerating or mitigating the progression of atherosclerosis. Consequently, they have been extensively used as vehicles for drug delivery to specifically target affected areas, where they suppress the inflammatory response in AS.Citation121 Exosomes generated from M2 macrophages have been chosen as a delivery system for small molecule medications in the treatment of AS due to their anti-inflammatory capabilities and ability to target inflammation. Electroporation-loaded exosomes comprising hexyl 5-aminolevulinate hydrochloride were reportedly generated from M2 macrophages in a recent publication.Citation117
Initially, the exosomes indicated inflammation. After studying their therapeutic and biological potential, they are used for delivery purposes. Engineering design optimization improves chronic inflammatory disease management. Natural exosomes can transport cargo and treat disease due to their stability, minimal immunogenicity, and specific targeting. Various anti-inflammatory drugs, nucleic acids are delivered to the inflammatory milieu in exosomes.Citation9
Challenges and Future Perspectives
Technical Challenges
The primary technological challenge involve the production of adequate amounts of exosomes.Citation122 Additionally, biological fluid exosomes are unpredictable, impure, and low-yield. To reduce EV analysis artefacts, standardized pre-analytical processes are essential. Exosome production and isolation procedures should also address cell culture matrices, polymers, cell confluency and volume, viability, cell passage, microbial contaminants and mycoplasma.Citation123 Exosomes have low zeta potential, a brief half-life, and limited stability, all of which have a detrimental impact on their therapeutic effectiveness and pose challenges in the development of efficacious exosome-based therapeutics.Citation124 It is quite difficult to differentiate exosomes from other extracellular vesicles, like apoptotic bodies and ectosomes, due to the lack of unique biomarkers for exosomes.Citation125
Issues in Exosome Isolation and Characterization
The method of isolation used ultimately determines the degree of purity and the physicochemical properties of exosomes. Conventional procedures such as precipitation and ultracentrifugation are frequently used, however they are not appropriate for therapeutic purposes because they cannot effectively handle the diverse composition, subpopulations, size, aggregations, and protein coronas of exosomes that are obtained by these means.Citation126 Furthermore, the proper preservation of isolated exosomes is essential for maintaining their biological functionality. Exosomes can be preserved by suspending in phosphate-buffered saline and storing at a temperature of −80 °C. Further supplementation of trehalose can provide additional protection to exosomes against cryodamage.Citation127 The utilization of acoustic-based techniques for exosome separation has demonstrated great potential due to its biocompatibility and non-altering effect on exosome morphology post-separation. Nevertheless, the primary drawbacks of this technology are the involvement of intricate fabrication methods.Citation125 Various microfluidics devices use size and density (filtration, on-chip centrifugation). These devices do not need antibodies, but size overlap and clogging are their main difficulties. Exosome specimens that are available for purchase may not correspond to the specific exosome types requested, resulting in erroneous characterization lack of comprehensive analytical characterization of exosomes can impede the comprehension of their features and functions.Citation128
Standardization for Clinical Applications
Considerable efforts have been undertaken to utilize exosomes for the purpose of targeted treatments. However, it is important for future investigations to specifically tackle the primary challenges. The main restriction in the field of exosome isolation and purification is the lack of standardized methodologies.Citation125 The study of extracellular vesicles, which encompasses exosomes, suffers from a lack of uniform norms, standardization, nomenclature, and understanding of kinetics. This hinders the progress towards a standardized approach to exosome research and therapeutic applications.Citation129 Making sure that isolated exosomes are of good quality and consistent for clinical use is important but challenging. It is important to keep quality control and reproducibility in clinical uses by standardizing protocols for isolating, characterizing, and storing exosomes.Citation130 Strictly adhering to Good Manufacturing Practice (GMP) requirements is essential to guarantee the safety and effectiveness of exosome-based therapeutics in clinical environments.Citation129 Furthermore, there exist numerous distinct types of exosomes derived from various human cell types. However, the precise mechanism by which these cell types influence the delivery properties of exosomes remains unknown. This highlights the importance of conducting thorough characterization studies prior to utilizing exosomes for therapeutic purposes. Lastly, while the donor cells employed may be of the same type, the cell cultures that produce exosomes can vary and have unpredictable characteristics.Citation131
Ethical and Regulatory Considerations
Ethical Implications
Ethical implications of the diagnostics based on exosome and therapies cover a variety of factors, including ensuring that individuals who contribute biological samples for exosome analysis have a complete understanding of the procedure’s goal, dangers, and potential benefits, and that they give their informed permission. Another important factor to take into account is the protection of the privacy and safety of patient information collected by exosome-based diagnostics, with the goal of preventing unauthorised access or inappropriate use of the information.Citation132 To guarantee that safety, efficacy, and ethical requirements are followed, it is necessary to put in place comprehensive regulatory frameworks that will oversee testing, and deployment of exosome-based diagnostics and treatments, Moreover, it is essential to uphold transparency in the research, development, and commercialization of technologies based on exosomes in order to establish confidence with patients, healthcare professionals, and the general public.Citation133
Future Directions
Advancements in Exosome Research
The storage suitability is exceptionally high, it is user-friendly, and it never induces immune rejection. Exosomes derived from stem cells provide growth factors in addition to miRNA to promote cellular processes such as differentiation, angiogenesis, and survival. Exosomes have the ability to modulate the inflammatory response and establish a conducive microenvironment following an infarction.Citation20 Exosomes are considered as promising candidates for innovative targeted medication delivery due to their numerous advantages over regular nanoparticles. Currently, exosomes are primarily utilized as biomarkers in clinical trials. Exosomes now have gained consideration in discipline of medication administration because of their inherent origins and the presence of proteins, lipids, and receptors on their surface. In addition, certain research groups have advanced this concept by doing studies on genetic modifications to modify the surface of exosomes.Citation86 Exosomes are now recognized as important contributors to cancer treatment, as more study emphasizes their ability to transport therapeutic substances to specific cancer cells. Due to their safety, biocompatibility, and capacity to transport targeted payload, nanoparticles are highly valuable in the field of cancer treatment.Citation134 Exosome methods for isolation have undergone significant developments, playing a crucial role in biological research. These enhanced techniques improve the quality and quantity of isolated exosomes, making it easier to accurately analyse and use them in subsequent experiments.Citation135 Individuals who have experienced a myocardial infarction often desire immediate intervention. However, this desire poses a challenge for cell-based therapy, as it takes time to identify and cultivate the necessary cells. During intravenous injection, a significant number of cells may become lodged in the lung. Exosomes, due to their small size, are able to travel through the lung and reach the circulation, allowing them to accurately target certain cells. An additional significant advantage of utilizing exosome-based therapy is the ability to modify the exosomal interior content through either genetic engineering or preconditioning.Citation20 Exosomes, specifically containing miRNAs, have demonstrated significant efficacy in the treatment of eye disorders in animal models. Despite experiencing a surge in recent years, research on exosomes in visual systems is still in its early phases of development. Nevertheless, the evidence derived from studies on the typical function of exosomes, as well as their involvement in life-threatening conditions such as neurodegenerative disorders, cardiovascular diseases, and cancer, supports and establishes the original findings, thereby paving the way for future research, Moreover, experimental evidence has shown that exosomes may effectively diagnose and battle important biological processes, indicating their potential use in the treatment of eye diseases.Citation136
Potential for Personalized Medicine and Precision Therapeutics
Ongoing clinical trials, such as those studying exosome-loaded therapeutic compounds for diseases like metastatic pancreatic adenocarcinoma and malignant pleural or ascites fluid, are advancing the use of exosomes in targeted therapeutics. These trials are anticipated to yield crucial knowledge regarding the safety and effectiveness of treatments that are based on the exosomes. Combining nanoparticles (NPs) with hybridization can enhance targeting capability by utilizing the distinct characteristics of NPs for precise release, bioimaging, and adjuvant therapy. This provides versatile delivery systems for precision medicines.Citation137 The utilization of microfluidic technology is expected to have a vital impact on harnessing the potential of exosomes in precision therapies. This encompasses the creation of microfluidic devices for the purpose of isolating, modifying, and loading drugs into exosomes. Additionally, it involves combining microfluidics with on-chip cell culture technologies to create a comprehensive and interconnected system.Citation138 The xylan polysaccharide is commonly employed in the medical field for the production of drug delivery devices. Nanofibrous scaffolds composed of Xylan-PVA were fabricated and the application of glutaraldehyde cross-linkage resulted in improved mechanical characteristics. These scaffolds also exhibited suitable swelling properties, making them suitable for use as a cardiac patch in cases of myocardial infarction. Nanoparticles have the potential to be highly effective in the controlled release of medications and promoting cell growth in cardiac tissue engineering (CTE).Citation139
Conclusion
The increasing interest and expanding research on exosomes suggest that this field of study will continue to flourish. Current knowledge indicates that exosomes have a substantial and complex impact on both human well-being and disease. Exosomes offer remarkable insights into the state of their parent cell and are especially suitable for investigating biomarkers that might be utilized for the diagnosis or prediction of disease progression. Exosomes function as membranous nanocarriers that deliver specific payload to both nearby and distant regions. By engaging in this mechanism of intercellular communication, they have the ability to promote inflammation and modulate the immune response. Exosomes are capable of transporting cytokines, growth factors, and microRNAs. These exosomes are formed from cardiovascular cells such as cardiomyocytes, endothelial cells, and stem cells. Among the functions that exosomes perform, one is to protect the heart, while another is to promote the regeneration of injured tissue in the cardiovascular system. Given that research on exosome biology is still in its early stage, more research in needed to examine the potential effects of exosomes in significant preclinical models. These studies are needed to evaluate safety and toxicity concerns and to guarantee the secure and efficient utilization of exosomes in therapeutic applications.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no competing interest in this work.
Additional information
Funding
References
- Chen L, Deng H, Cui H, et al. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2018;9(6):7204. doi:10.18632/oncotarget.23208
- Shukla SD, Vanka KS, Chavelier A, et al. Chronic respiratory diseases: an introduction and need for novel drug delivery approaches. Targeting chronic inflammatory lung diseases using advanced drug delivery systems. Elsevier. 2020;2020:1–31.
- Halling ML, Kjeldsen J, Knudsen T, Nielsen J, Hansen LK. Patients with inflammatory bowel disease have increased risk of autoimmune and inflammatory diseases. World J Gastroenterol. 2017;23(33):6137. doi:10.3748/wjg.v23.i33.6137
- Lampa J. Pain without inflammation in rheumatic diseases. Best Pract Res. 2019;33(3):101439. doi:10.1016/j.berh.2019.101439
- Bennett JM, Reeves G, Billman GE, Sturmberg JP. Inflammation–nature’s way to efficiently respond to all types of challenges: implications for understanding and managing “the epidemic” of chronic diseases. Front Med. 2018;5:316. doi:10.3389/fmed.2018.00316
- Goyette P, Labbé C, Trinh TT, Xavier RJ, Rioux JD. Molecular pathogenesis of inflammatory bowel disease: genotypes, phenotypes and personalized medicine. Anna Med. 2007;39(3):177–199. doi:10.1080/07853890701197615
- Pedersen J, Coskun M, Soendergaard C, Salem M, Nielsen OH. Inflammatory pathways of importance for management of inflammatory bowel disease. World J Gastroenterol. 2014;20(1):64. doi:10.3748/wjg.v20.i1.64
- Ocansey DK, Zhang L, Wang Y, et al. Exosome‐mediated effects and applications in inflammatory bowel disease. Biol. Rev. 2020;95(5):1287–1307. doi:10.1111/brv.12608
- Wang C, Xu M, Fan Q, Li C, Zhou X. Therapeutic potential of exosome‐based personalized delivery platform in chronic inflammatory diseases. Asian J Pharm Sci. 2023;18(1):100772. doi:10.1016/j.ajps.2022.100772
- Waqas MY, Javid MA, Nazir MM, et al. Extracellular vesicles and exosome: insight from physiological regulatory perspectives. J Physiol Biochem. 2022;78(3):573–580. doi:10.1007/s13105-022-00877-6
- Thakur A, Ke X, Chen Y-W, et al. The mini player with diverse functions: extracellular vesicles in cell biology, disease, and therapeutics. Protein and Cell. 2022;13(9):631–654.
- Gutiérrez‐Vázquez C, Villarroya‐Beltri C, Mittelbrunn M, Sánchez‐Madrid F. Transfer of extracellular vesicles during immune cell‐cell interactions. Immunol Rev. 2013;251(1):125–142. doi:10.1111/imr.12013
- Chung I-M, Rajakumar G, Venkidasamy B, Subramanian U, Thiruvengadam M. Exosomes: current use and future applications. Clin Chim Acta. 2020;500:226–232. doi:10.1016/j.cca.2019.10.022
- Dyball LE, Smales CM. Exosomes: biogenesis, targeting, characterization and their potential as Plug & Play” Vaccine Platforms. Biotechnol J. 2022;17(11):2100646.
- Di Bella MA. Overview and update on extracellular vesicles: considerations on exosomes and their application in modern medicine. Biology. 2022;11(6):804. doi:10.3390/biology11060804
- Rayner KJ, Hennessy EJ. Extracellular communication via microRNA: lipid particles have a new message. J Lipid Res. 2013;54(5):1174–1181. doi:10.1194/jlr.R034991
- Taylor DD, Gercel-Taylor C. The origin, function, and diagnostic potential of RNA within extracellular vesicles present in human biological fluids. Front Genetics. 2013;4:142. doi:10.3389/fgene.2013.00142
- Mahmoudi M, Taghavi Farahabadi M, Hashemi SM. Exosomes: mediators of immune regulation. Immunoregulation. 2019;2(1):3–8.
- Bobrie A, Colombo M, Raposo G, Théry C. Exosome secretion: molecular mechanisms and roles in immune responses. Traffic. 2011;12(12):1659–1668. doi:10.1111/j.1600-0854.2011.01225.x
- Kavya ANL, Subramanian S, Ramakrishna S. Therapeutic applications of exosomes in various diseases: a review. Biomat Advanc. 2022;134:112579. doi:10.1016/j.msec.2021.112579
- Fleming A, Sampey G, Chung MC, et al. The carrying pigeons of the cell: exosomes and their role in infectious diseases caused by human pathogens. Pathog Dis. 2014;71(2):109–120. doi:10.1111/2049-632X.12135
- Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478):eaau6977. doi:10.1126/science.aau6977
- Anastasiu CV, Moga MA, Elena Neculau A, et al. Biomarkers for the noninvasive diagnosis of endometriosis: state of the art and future perspectives. Int J Mol Sci. 2020;21(5):1750. doi:10.3390/ijms21051750
- Gurunathan S, Kang M-H, Kim J-H. A comprehensive review on factors influences biogenesis, functions, therapeutic and clinical implications of exosomes. Int j Nanomed. 2021;1281–1312. doi:10.2147/IJN.S291956
- Chan BD, Wong WY, Lee MML, et al. Exosomes in inflammation and inflammatory disease. Proteomics. 2019;19(8):1800149. doi:10.1002/pmic.201800149
- Shenoda BB, Ajit SK. Modulation of immune responses by exosomes derived from antigen-presenting cells. Clin Med Insigh. 2016;9:S39925. doi:10.4137/CPath.S39925
- Fais S, O’Driscoll L, Borras FE, et al. Evidence-Based Clinical Use of Nanoscale Extracellular Vesicles in Nanomedicine. ACS Publications; 2016.
- Mellman I. Dendritic cells: master regulators of the immune response. Cancer Immunol Res. 2013;1(3):145–149. doi:10.1158/2326-6066.CIR-13-0102
- Benites BD, Alvarez MC, Saad STO. Small particles, big effects: the interplay between exosomes and dendritic cells in antitumor immunity and immunotherapy. Cells. 2019;8(12):1648. doi:10.3390/cells8121648
- Li Q, Wang H, Peng H, Huyan T, Cacalano NA. Exosomes: versatile nano mediators of immune regulation. Cancers. 2019;11(10):1557. doi:10.3390/cancers11101557
- Chaput N, Flament C, Viaud S, et al. Dendritic cell derived-exosomes: biology and clinical implementations. J Leukoc Biol. 2006;80(3):471–478. doi:10.1189/jlb.0206094
- Hosseini R, Asef-Kabiri L, Yousefi H, et al. The roles of tumor-derived exosomes in altered differentiation, maturation and function of dendritic cells. Mol Cancer. 2021;20(1):83. doi:10.1186/s12943-021-01376-w
- Shahbaz SK, Sadeghi M, Koushki K, Penson PE, Sahebkar A. Regulatory T cells: possible mediators for the anti-inflammatory action of statins. Pharmacol Res. 2019;149:104469. doi:10.1016/j.phrs.2019.104469
- Wang Y, Zhao M, Liu S, et al. Macrophage-derived extracellular vesicles: diverse mediators of pathology and therapeutics in multiple diseases. Cell Death Dis. 2020;11(10):924. doi:10.1038/s41419-020-03127-z
- Hyvärinen K, Holopainen M, Skirdenko V, et al. Mesenchymal stromal cells and their extracellular vesicles enhance the anti-inflammatory phenotype of regulatory macrophages by downregulating the production of interleukin (IL)-23 and IL-22. Front Immunol. 2018;9:338017. doi:10.3389/fimmu.2018.00771
- McDonald MK, Tian Y, Qureshi RA, et al. Functional significance of macrophage-derived exosomes in inflammation and pain. Pain®. 2014;155(8):1527–1539. doi:10.1016/j.pain.2014.04.029
- Whiteside TL. Evaluating tumor cell-and T cell-derived extracellular vesicles as potential biomarkers of cancer and immune cell competence. Exp Rev Molec Diagnost. 2023;23(2):109–122. doi:10.1080/14737159.2023.2178902
- Zhao H, Liao X, Kang Y. Tregs: where we are and what comes next? Front Immunol. 2017;8:309575. doi:10.3389/fimmu.2017.01578
- Tavasolian F, Hosseini AZ, Rashidi M, et al. The impact of immune cell-derived exosomes on immune response initiation and immune system function. Curr Pharm Des. 2021;27(2):197–205. doi:10.2174/18734286MTEyiMTQCy
- Gaffen S, Hajishengallis G. A new inflammatory cytokine on the block: re-thinking periodontal disease and the Th1/Th2 paradigm in the context of Th17 cells and IL-17. J Dent Res. 2008;87(9):817–828. doi:10.1177/154405910808700908
- Xiong -Y-Y, Gong Z-T, Tang R-J, Yang Y-J. The pivotal roles of exosomes derived from endogenous immune cells and exogenous stem cells in myocardial repair after acute myocardial infarction. Theranostics. 2021;11(3):1046. doi:10.7150/thno.53326
- Azimi M, Ghabaee M, Moghadasi AN, Noorbakhsh F, Izad M. Immunomodulatory function of Treg-derived exosomes is impaired in patients with relapsing-remitting multiple sclerosis. Immunol Res. 2018;66:513–520. doi:10.1007/s12026-018-9008-5
- Jan AT, Rahman S, Badierah R, et al. Expedition into exosome biology: a perspective of progress from discovery to therapeutic development. Cancers. 2021;13(5):1157. doi:10.3390/cancers13051157
- Noonin C, Thongboonkerd V. Exosome-inflammasome crosstalk and their roles in inflammatory responses. Theranostics. 2021;11(9):4436. doi:10.7150/thno.54004
- Console L, Scalise M, Indiveri C. Exosomes in inflammation and role as biomarkers. Clin Chim Acta. 2019;488:165–171. doi:10.1016/j.cca.2018.11.009
- Madhyastha R, Madhyastha H, Nurrahmah QI, Purbasari B, Maruyama M, Nakajima Y. MicroRNA 21 elicits a pro-inflammatory response in macrophages, with exosomes functioning as delivery vehicles. Inflammation. 2021;44:1274–1287. doi:10.1007/s10753-021-01415-0
- Qiu Y, Li P, Zhang Z, Wu M. Insights into exosomal non-coding RNAs sorting mechanism and clinical application. Front Oncol. 2021;11:664904. doi:10.3389/fonc.2021.664904
- Ti D, Hao H, Fu X, Han W. Mesenchymal stem cells-derived exosomal microRNAs contribute to wound inflammation. Sci China Life Sci. 2016;59:1305–1312. doi:10.1007/s11427-016-0240-4
- Hu Y, Wang Y, Chen T, Hao Z, Cai L, Li J. Exosome: function and application in inflammatory bone diseases. Oxid Med Cell Longev. 2021;2021:1–17. doi:10.1155/2021/6324912
- Fakhoury M, Negrulj R, Mooranian A, Al-Salami H. Inflammatory bowel disease: clinical aspects and treatments. J Inflamm Res. 2014;113–120. doi:10.2147/JIR.S65979
- Zhang H, Wang L, Chen D. Exosome-induced regulation in inflammatory bowel disease. Front Immunol. 2019;10:416673.
- Poggi A, Benelli R, Venè R, et al. Human gut-associated natural killer cells in health and disease. Front Immunol. 2019;10:441580. doi:10.3389/fimmu.2019.00961
- Tang D, Cao F, Fang K, et al. Extracellular vesicle/macrophage axis: potential targets for inflammatory disease intervention. Front Immunol. 2022;13:705472. doi:10.3389/fimmu.2022.705472
- Shrivastava AK, Pandey A. Inflammation and rheumatoid arthritis. J Physiol Biochem. 2013;69:335–347. doi:10.1007/s13105-012-0216-5
- Miao H-B, Wang F, Lin S, Chen Z. Update on the role of extracellular vesicles in rheumatoid arthritis. Expert Rev Molec Med. 2022;24:e12.
- Park S, Bello A, Arai Y, et al. Functional duality of chondrocyte hypertrophy and biomedical application trends in osteoarthritis. Pharmaceutics. 2021;13(8):1139. doi:10.3390/pharmaceutics13081139
- Chang T-H, C-S W, Chiou S-H, Chang C-H, Liao H-J. Adipose-derived stem cell exosomes as a novel anti-inflammatory agent and the current therapeutic targets for rheumatoid arthritis. Biomedicines. 2022;10(7):1725. doi:10.3390/biomedicines10071725
- Saillaja AK. An overall review on chronic asthma. Internat J Pharmaceut Drug Anal. 2014;2014:275–279.
- Sangaphunchai P, Todd I, Fairclough LC. Extracellular vesicles and asthma: a review of the literature. Clin Exp Immunol. 2020;50(3):291–307. doi:10.1111/cea.13562
- Yang G, Waheed S, Wang C, Shekh M, Li Z, Wu J. Exosomes and their bioengineering strategies in the cutaneous wound healing and related complications: current knowledge and future perspectives. Int J Bio Sci. 2023;19(5):1430. doi:10.7150/ijbs.80430
- Skuratovskaia D, Vulf M, Khaziakhmatova O, et al. Exosome limitations in the treatment of inflammatory diseases. Curr Pharm Des. 2021;27(28):3105–3121. doi:10.2174/1381612826666201210120444
- Veziroglu EM, Mias GI. Characterizing extracellular vesicles and their diverse RNA contents. Front Genetics. 2020;11:558262.
- Mishra A, Singh P, Qayoom I, Prasad A, Kumar A. Current strategies in tailoring methods for engineered exosomes and future avenues in biomedical applications. J Mat Chem B. 2021;9(32):6281–6309. doi:10.1039/D1TB01088C
- Lu M, Yuan S, Li S, Li L, Liu M, Wan S. The exosome-derived biomarker in atherosclerosis and its clinical application. J Cardiovasc Translat Res. 2019;12(1):68–74. doi:10.1007/s12265-018-9796-y
- Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochimica et Biophys Acta. 2012;1820(7):940–948. doi:10.1016/j.bbagen.2012.03.017
- Chen J, Li P, Zhang T, et al. Review on strategies and technologies for exosome isolation and purification. Front Bioeng Biotechnol. 2022;9. doi:10.3389/fbioe.2021.811971
- W-z L, Z-j M, X-w K. Current status and outlook of advances in exosome isolation. Anal Bioanal Chem. 2022;414(24):7123–7141. doi:10.1007/s00216-022-04253-7
- Tayebi M, Yang D, Collins DJ, Ai Y. Deterministic sorting of submicrometer particles and extracellular vesicles using a combined electric and acoustic field. Nano Lett. 2021;21(16):6835–6842. doi:10.1021/acs.nanolett.1c01827
- Livshits MA, Khomyakova E, Evtushenko EG, et al. Isolation of exosomes by differential centrifugation: theoretical analysis of a commonly used protocol. Sci Rep. 2015;5(1):1–14. doi:10.1038/srep17319
- Kuo WP, Jia S. Extracellular Vesicles: Methods and Protocols. Springer; 2017.
- Konoshenko MY, Lekchnov EA, Vlassov AV, Laktionov PP. Isolation of extracellular vesicles: general methodologies and latest trends. Biomed Res Int. 2018;2018:1–27. doi:10.1155/2018/8545347
- McNamara RP, Caro-Vegas CP, Costantini LM, et al. Large-scale, cross-flow based isolation of highly pure and endocytosis-competent extracellular vesicles. J Extracell Vesicles. 2018;7(1):1541396. doi:10.1080/20013078.2018.1541396
- Gardiner C, Vizio DD, Sahoo S, et al. Techniques used for the isolation and characterization of extracellular vesicles: results of a worldwide survey. J Extracell Vesicles. 2016;5(1):32945. doi:10.3402/jev.v5.32945
- Royo F, Théry C, Falcón-Pérez JM, Nieuwland R, Witwer KW. Methods for separation and characterization of extracellular vesicles: results of a worldwide survey performed by the ISEV rigor and standardization subcommittee. Cells. 2020;9(9):1955. doi:10.3390/cells9091955
- Dragovic RA, Gardiner C, Brooks AS, et al. Sizing and phenotyping of cellular vesicles using nanoparticle tracking analysis. Nanomed Nanotechnol Biol Med. 2011;7(6):780–788. doi:10.1016/j.nano.2011.04.003
- de Necochea-Campion R, Gonda A, Kabagwira J, et al. A practical approach to extracellular vesicle characterization among similar biological samples. Biomed Phys Eng Express. 2018;4(6):065013. doi:10.1088/2057-1976/aad6d8
- Szatanek R, Baj-Krzyworzeka M, Zimoch J, Lekka M, Siedlar M, Baran J. The methods of choice for extracellular vesicles (EVs) characterization. Int J Mol Sci. 2017;18(6):1153. doi:10.3390/ijms18061153
- Pospichalova V, Svoboda J, Dave Z, et al. Simplified protocol for flow cytometry analysis of fluorescently labeled exosomes and microvesicles using dedicated flow cytometer. J Extracell Vesicles. 2015;4(1):25530. doi:10.3402/jev.v4.25530
- Binnig G, Quate CF, Gerber C. Atomic force microscope. Phys Rev Lett. 1986;56(9):930. doi:10.1103/PhysRevLett.56.930
- Zhu L, Sun H-T, Wang S, et al. Isolation and characterization of exosomes for cancer research. J Hematol Oncol. 2020;13(1):152.
- Jahromi FNA, Dowran R, Jafari R. Recent advances in the roles of exosomal microRNAs (exomiRs) in hematologic neoplasms: pathogenesis, diagnosis, and treatment. Cell Commun Signal. 2023;21(1):88. doi:10.1186/s12964-023-01102-7
- Spada S. Chapter 15 - Study of microRNAs carried by exosomes. In: Kepp O, Galluzzi L, editors. Methods in Cell Biology. Academic Press; 2021:187–197.
- Zhang J, Li S, Li L, et al. Exosome and exosomal MicroRNA: trafficking, sorting, and function. Genom Prot Bioinf. 2015;13(1):17–24. doi:10.1016/j.gpb.2015.02.001
- Guo M, Hao Y, Feng Y, et al. Microglial exosomes in neurodegenerative disease. Front Mol Neurosci. 2021;14:630808. doi:10.3389/fnmol.2021.630808
- Mamdani H, Ahmed S, Armstrong S, Mok T, Jalal SI. Blood-based tumor biomarkers in lung cancer for detection and treatment. Translat Lung Can Res. 2017;6(6):648. doi:10.21037/tlcr.2017.09.03
- Huda MN, Nafiujjaman M, Deaguero IG, et al. Potential use of exosomes as diagnostic biomarkers and in targeted drug delivery: progress in clinical and preclinical applications. ACS Biomater Sci Eng. 2021;7(6):2106–2149. doi:10.1021/acsbiomaterials.1c00217
- Czernek L, Düchler M. Exosomes as messengers between mother and fetus in pregnancy. Int J Mol Sci. 2020;21(12):4264. doi:10.3390/ijms21124264
- Hruby A, Hu FB. The epidemiology of obesity: a big picture. Pharmacoeconomics. 2015;33:673–689. doi:10.1007/s40273-014-0243-x
- Chen Y-S, Lin E-Y, Chiou T-W, Harn H-J. Exosomes in clinical trial and their production in compliance with good manufacturing practice. Tzu Chi Med J. 2020;32(2):113–120. doi:10.4103/tcmj.tcmj_182_19
- Clemmens H, Lambert DW. Extracellular vesicles: translational challenges and opportunities. Biochem Soc Trans. 2018;46(5):1073–1082. doi:10.1042/BST20180112
- Johnsen KB, Gudbergsson JM, Skov MN, Pilgaard L, Moos T, Duroux M. A comprehensive overview of exosomes as drug delivery vehicles—endogenous nanocarriers for targeted cancer therapy. Biochimica et Biophys Acta. 2014;1846(1):75–87. doi:10.1016/j.bbcan.2014.04.005
- van der Meel R, Fens MH, Vader P, Van Solinge WW, Eniola-Adefeso O, Schiffelers RM. Extracellular vesicles as drug delivery systems: lessons from the liposome field. J Control Release. 2014;195:72–85. doi:10.1016/j.jconrel.2014.07.049
- Vader P, Mol EA, Pasterkamp G, Schiffelers RM. Extracellular vesicles for drug delivery. Adv Drug Delivery Rev. 2016;106:148–156. doi:10.1016/j.addr.2016.02.006
- Syn NL, Wang L, Chow EK-H, Lim CT, Goh B-C. Exosomes in cancer nanomedicine and immunotherapy: prospects and challenges. Trends Biotechnol. 2017;35(7):665–676. doi:10.1016/j.tibtech.2017.03.004
- Datta A, Kim H, McGee L, et al. High-throughput screening identified selective inhibitors of exosome biogenesis and secretion: a drug repurposing strategy for advanced cancer. Sci Rep. 2018;8(1):8161. doi:10.1038/s41598-018-26411-7
- Heydari R, Koohi F, Rasouli M, et al. Exosomes as rheumatoid arthritis diagnostic biomarkers and therapeutic agents. Vaccines. 2023;11(3):687. doi:10.3390/vaccines11030687
- Aheget H, Mazini L, Martin F, Belqat B, Marchal JA, Benabdellah K. Exosomes: their role in pathogenesis, diagnosis and treatment of diseases. Cancers. 2020;13(1):84. doi:10.3390/cancers13010084
- Mirzaei R, Zamani F, Hajibaba M, et al. The pathogenic, therapeutic and diagnostic role of exosomal microRNA in the autoimmune diseases. J Neuroimmunol. 2021;358:577640. doi:10.1016/j.jneuroim.2021.577640
- Wu X, Xu X, Xiang Y, et al. Exosome-mediated effects and applications in inflammatory diseases of the digestive system. Eur J Med Res. 2022;27(1):163. doi:10.1186/s40001-022-00792-y
- Yang X, Xia H, Liu C, et al. The novel delivery-exosome application for diagnosis and treatment of rheumatoid arthritis. Pathol Res Pract. 2023;242:154332.
- Ma X, Liu B, Fan L, et al. Native and engineered exosomes for inflammatory disease. Nano Res. 2023;16(5):6991–7006. doi:10.1007/s12274-022-5275-5
- Ozansoy M, Mikati H, Velioglu HA, Yulug B. Exosomes: a missing link between chronic systemic inflammation and Alzheimer’s disease? Biomed. Pharmacother. 2023;159:114161. doi:10.1016/j.biopha.2022.114161
- Han J, Zhang Y, Ge P, et al. Exosome-derived CIRP: an amplifier of inflammatory diseases. Front Immunol. 2023;14:1066721. doi:10.3389/fimmu.2023.1066721
- Cervio E, Barile L, Moccetti T, Vassalli G. Exosomes for intramyocardial intercellular communication. Stem Cells Internat. 2015;2015:1–10. doi:10.1155/2015/482171
- Cheng C, Wang Q, You W, Chen M, Xia J. MiRNAs as biomarkers of myocardial infarction: a meta-analysis. PLoS One. 2014;9(2):e88566. doi:10.1371/journal.pone.0088566
- Hergenreider E, Heydt S, Tréguer K, et al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol. 2012;14(3):249–256. doi:10.1038/ncb2441
- Rupp A-K, Rupp C, Keller S, et al. Loss of EpCAM expression in breast cancer derived serum exosomes: role of proteolytic cleavage. Gynecologic Oncol. 2011;122(2):437–446. doi:10.1016/j.ygyno.2011.04.035
- Zhang Y, Liu D, Chen X, et al. Secreted monocytic miR-150 enhances targeted endothelial cell migration. Molecular Cell. 2010;39(1):133–144. doi:10.1016/j.molcel.2010.06.010
- Caby M-P, Lankar D, Vincendeau-Scherrer C, Raposo G, Bonnerot C. Exosomal-like vesicles are present in human blood plasma. Internat Immunol. 2005;17(7):879–887. doi:10.1093/intimm/dxh267
- André F, Chaput N, Schartz N, et al. Exosomes as potent cell-free peptide-based vaccine. I. Dendritic cell-derived exosomes transfer functional MHC class I/peptide complexes to dendritic cells. J Immunol. 2004;172(4):2126–2136. doi:10.4049/jimmunol.172.4.2126
- Sun D, Zhuang X, Xiang X, et al. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther. 2010;18(9):1606–1614. doi:10.1038/mt.2010.105
- Pardridge WM. Drug transport across the blood–brain barrier. J Cereb Blood Flow Metab. 2012;32(11):1959–1972. doi:10.1038/jcbfm.2012.126
- Haney MJ, Klyachko NL, Zhao Y, et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J Control Release. 2015;207:18–30. doi:10.1016/j.jconrel.2015.03.033
- Ebadi M, Srinivasan SK, Baxi MD. Oxidative stress and antioxidant therapy in Parkinson’s disease. Prog Neurobiol. 1996;48(1):1–19. doi:10.1016/0301-0082(95)00029-1
- Ambani LM, Van Woert MH, Murphy S. Brain peroxidase and catalase in Parkinson disease. Arch Neurol. 1975;32(2):114–118. doi:10.1001/archneur.1975.00490440064010
- Ha D, Yang N, Nadithe V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharmaceutica Sinica B. 2016;6(4):287–296. doi:10.1016/j.apsb.2016.02.001
- Wu G, Zhang J, Zhao Q, et al. Molecularly engineered macrophage‐derived exosomes with inflammation tropism and intrinsic heme biosynthesis for atherosclerosis treatment. Angew Chem. 2020;132(10):4097–4103. doi:10.1002/ange.201913700
- Li H, Feng Y, Zheng X, et al. M2-type exosomes nanoparticles for rheumatoid arthritis therapy via macrophage re-polarization. J Control Release. 2022;341:16–30. doi:10.1016/j.jconrel.2021.11.019
- Liu Y, Zeng Y, Si H-B, Tang L, Xie H-Q, Shen B. Exosomes derived from human urine–derived stem cells overexpressing miR-140-5p alleviate knee osteoarthritis through downregulation of VEGFA in a rat model. Am J Spor Med. 2022;50(4):1088–1105. doi:10.1177/03635465221073991
- Qiu B, Xu X, Yi P, Hao Y. Curcumin reinforces MSC‐derived exosomes in attenuating osteoarthritis via modulating the miR‐124/NF‐kB and miR‐143/ROCK1/TLR9 signalling pathways. J Cell & Mol Med. 2020;24(18):10855–10865. doi:10.1111/jcmm.15714
- Sun L, He X, Zhang T, Han Y, Tao G. Knockdown of mesenchymal stem cell‑derived exosomal LOC100129516 suppresses the symptoms of atherosclerosis via upregulation of the PPARγ/LXRα/ABCA1 signaling pathway Corrigendum in/. IntJ Mol Med. 2021;48(6):1–11. doi:10.3892/ijmm.2021.5041
- Yamashita T, Takahashi Y, Takakura Y. Possibility of exosome-based therapeutics and challenges in production of exosomes eligible for therapeutic application. Biol Pharm Bull. 2018;41(6):835–842. doi:10.1248/bpb.b18-00133
- Ramirez MI, Amorim MG, Gadelha C, et al. Technical challenges of working with extracellular vesicles. Nanoscale. 2018;10(3):881–906. doi:10.1039/C7NR08360B
- Wang Y, Zhang L, Li Y, et al. Exosomes/microvesicles from induced pluripotent stem cells deliver cardioprotective miRNAs and prevent cardiomyocyte apoptosis in the ischemic myocardium. Int J Cardiol. 2015;192:61–69. doi:10.1016/j.ijcard.2015.05.020
- Li X, Corbett AL, Taatizadeh E, et al. Challenges and opportunities in exosome research-Perspectives from biology, engineering, and cancer therapy. APL Bioeng. 2019;3(1):011503. doi:10.1063/1.5087122
- Witwer KW, Buzás EI, Bemis LT, et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles. 2013;2(1):20360. doi:10.3402/jev.v2i0.20360
- Bosch S, de Beaurepaire L, Allard M, et al. Trehalose prevents aggregation of exosomes and cryodamage. Sci Rep. 2016;6(1):36162. doi:10.1038/srep36162
- Lai JJ, Chau ZL, Chen SY, et al. Exosome processing and characterization approaches for research and technology development. Adv Sci. 2022;9(15):e2103222. doi:10.1002/advs.202103222
- Rezaie J, Feghhi M, Etemadi T. A review on exosomes application in clinical trials: perspective, questions, and challenges. Cell Commun Signaling. 2022;20(1):145. doi:10.1186/s12964-022-00959-4
- Cheng K, Kalluri R. Guidelines for clinical translation and commercialization of extracellular vesicles and exosomes based therapeutics. Extracellular Vesicle. 2023;2:100029. doi:10.1016/j.vesic.2023.100029
- Zhuang X, Xiang X, Grizzle W, et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther. 2011;19(10):1769–1779. doi:10.1038/mt.2011.164
- Maqsood Q, Sumrin A, Saleem Y, Wajid A, Mahnoor M. Exosomes in cancer: diagnostic and therapeutic applications. Clin Med Insights Oncol. 2024;18:11795549231215966. doi:10.1177/11795549231215966
- Muthu S, Bapat A, Jain R, Jeyaraman N, Jeyaraman M. Exosomal therapy-a new frontier in regenerative medicine. Stem Cell Investig. 2021;8:7. doi:10.21037/sci-2020-037
- Wang X, Xia J, Yang L, Dai J, He L. Recent progress in exosome research: isolation, characterization and clinical applications. Cancer Genet Ther. 2023;30(8):1051–1065. doi:10.1038/s41417-023-00617-y
- Sharma V, Mukhopadhyay CD. Exosome as drug delivery system: current advancements. Extracellular Vesicle. 2024;3:100032. doi:10.1016/j.vesic.2023.100032
- Klingeborn M, Dismuke WM, Rickman CB, Stamer WD. Roles of exosomes in the normal and diseased eye. Prog Retinal Eye Res. 2017;59:158–177. doi:10.1016/j.preteyeres.2017.04.004
- Zou Z, Li H, Xu G, Hu Y, Zhang W, Tian K. Current knowledge and future perspectives of exosomes as nanocarriers in diagnosis and treatment of diseases. Int J Nanomed. 2023;18:4751–4778. doi:10.2147/IJN.S417422
- Cazzola M, Ferraris S, Boschetto F, et al. Green tea polyphenols coupled with a bioactive titanium alloy surface: in vitro characterization of osteoinductive behavior through a KUSA A1 cell study. Int J Mol Sci. 2018;19(8):2255. doi:10.3390/ijms19082255
- Venugopal J, Rajeswari R, Shayanti M, et al. Xylan polysaccharides fabricated into nanofibrous substrate for myocardial infarction. Mater Sci Eng C. 2013;33(3):1325–1331. doi:10.1016/j.msec.2012.12.032
