Abstract
Helicobacter pylori (HP) infections affect approximately one-third of children worldwide. In China, the incidence of HP infection in children ranges from approximately 30% to 60%. In addition to damaging the gastrointestinal tract mucosa, HP infection in children can negatively affect their growth and development, hematology, respiratory and hepatobiliary system, skin, nutritional metabolism, and autoimmune system. However, the rate of HP eradication also fell considerably from the previous rate due to the presence of drug-resistant HP strains and the limited types of antibiotics that can be used in young patients. Vitamin D3 (VitD3) is a steroid hormone that can reduce inflammation in the stomach mucosa induced by HP and can alleviate and eradicate HP through a variety of pathways and mechanisms, including immune regulation and the stimulation of antimicrobial peptide (AMP) secretion and Ca2+ influx, to reestablish lysosomal acidification; thus, these results provide new strategies and ideas for the eradication of drug-resistant HP strains.
Introduction
Helicobacter pylori (HP) is a helical, microaerobic bacterium that colonizes the epithelium and lamina propria of the gastric mucosa. HP is the only microorganism known to survive in the human stomach, and HP survives by producing ammonia from urea to neutralize gastric acid. As a dependent risk factor for gastric and duodenal erosions, as well as for peptic ulcers,Citation1 HP is thought to induce chronic inflammation, which is characterized by lymphoplasmacytic infiltration, or acute inflammatory responses, which are characterized by neutrophil infiltration.Citation2 The prevalence of HP infection varies between regions of the world, although it affects roughly one-third of children worldwide.Citation3 The incidence of HP infection in children varies significantly by area in China, with infection rates ranging from approximately 30% to 60%.Citation4,Citation5 RenCitation4 and YuanCitation6 discovered that the prevalence of HP infection increased with age while investigating the epidemiology of HP infection in children in China and worldwide. However, the rate of HP eradication also fell considerably from the previous rate due to the presence of drug-resistant HP strains and the limited types of antibiotics available for use in young patients. In addition to causing damage to the gastrointestinal tract mucosa, HP infection in children can negatively affect growth and development, hematology, respiratory and hepatobiliary health, skin, nutritional metabolism, and the autoimmune system.Citation7 Recent research has revealed a link between vitamin D3 (VitD3) deficiency and HP infection.Citation8–10
1.25-dihydroxyvitamin D3 [1,25(OH)2D3] is a steroid hormone derived from vitamin D. 1.25(OH)2D3 is the predominant metabolite used to determine vitamin D levels in the body, and it is derived from both dietary vitamin D (D2) and cholecalciferol (D3) through photochemical interactions with sunshine or ultraviolet light, the latter of which is its principal source. Vitamin D is converted to 25-hydroxyvitamin D3 [25(OH)D3] and 1.25-(OH)2D3 by the enzymes 25-hydroxylase in the microsomes of hepatocytes and 1-a hydroxylase in the mitochondria of proximal renal tubular epithelial cells. In 2006, Kawaura showed that regular usage of an analog of vitamin D3 called 1 alpha-hydroxyvitamin D3 decreased the incidence of Helicobacter pylori infection.Citation10 El ShahawyCitation9 reported that patients with HP infection who were vitamin D deficient had lower rates of successful eradication. Supplemental vitamin intake substantially impacts HP infection, either by directly modifying the host’s inflammatory pathways or by subtly enhancing the host’s immune response.
VitD3 Inhibits HP-Induced Gastric Mucosal Inflammation by Regulating Immunity
It has been reported that HP infection in adults increases the number of CD4+ T cells and induces activation of CD4+ and CD8+ T cells.Citation11 Helper T-cell type 1 (Th1), Th2, Th9, Th17, Th22, and T regulatory (Treg) cells can influence the outcome of HP infection either individually or in combination with each other.Citation12 It has been demonstrated that Th1 cells selectively identify HP antigens. In contrast, the protective response of the immune system to specific HP antigens triggered by Treg and Th2 cells helps reduce tissue damage in the host.Citation13,Citation14 Th1 and Th17 cells promote the expression of interferon-gamma (IFN-γ) and interleukin-17 (IL-17), respectively, the latter of which increases the risk of hyperacidity, gastric atrophy, and gastric adenocarcinomas.Citation15,Citation16
By modulating T-cell proliferation and cytokine production, vitamin D3 may have an impact on the adaptive immune system. By regulating the expression of costimulatory molecules for antigen presentation, such as CD86, CD80, and CD40, as well as major histocompatibility class II (MHCII), VitD3 decreases the antigen-presenting capacity of dendritic cells (DCs)Citation17 and affects cytokine production to control the differentiation of naive CD4+ T cells into various helper subsets, such as Th1 and Th2 cells.Citation18 VitD3 also suppresses IFN-γ expression by binding the VDR/RXR (vitamin D receptor/retinoid X receptor) complex to the silencer promoter region of the gene.Citation19 VitD3 stimulation of human T cells activates VDR signaling, which is connected to the suppression of IFN-γ and IL-10 production, as well as the regulation of the C-C motif chemokine receptor 10 (CCR10) homing receptor.Citation20 However, VitD3 seems to have the opposite effect on IL-10, suggesting that this hormone induces IL-10 in human B cells.Citation18 Furthermore, VitD3 restricts Th17 cell development while promoting Treg cell development,Citation21 and Treg cells both reduce the Th1 cell response and modify the Th2 cell response.Citation12 Furthermore, VitD3 impacts T-cell proliferation, cell cycle progression, and the ability of T cells to produce cytokines, as well as inhibiting the expression of IL-2, a key growth factor for T-lymphocytes. VitD3 inhibited the release of T-cell cytokines and the progression of the cell cycle from G1a to G1b in vitro. VitD3 influences the transition of new T cells toward a Th2-type response by boosting the production of Th2 cytokines (IL-4, IL-5, and IL-10) and decreasing the synthesis of Th1 cytokines (IL-2, IL-12, and IFN-γ).Citation22,Citation23 Vitamin D signaling, on the other hand, suppresses Th17 cytokine productionCitation24 and promotes CD4+CD25+Foxp3+ regulatory T cells.Citation25
More Treg cells were detected in the gastric mucosa of HP-infected children than in that of infected adults, which was accompanied by less severe gastric inflammation; moreover, the decrease in neutrophil aggregation may be due to inhibition of the Th17 and Th1 responses.Citation26 Nevertheless, the activation and expansion of Treg cells in the gastric mucosa, as well as the decreased Th17 cell-associated response in children, may be crucial for reducing inflammation and increasing bacterial density, as well as representing a mechanism of bacterial persistence.Citation27
VitD3 Inhibits HP by Promoting the Secretion of Antimicrobial Peptides (AMPs)
Fleming and Ridley isolated AMPs from human tears in 1922 and categorized them based on their secondary structures, such as cathelicidins (α-defensins), defensins (β-defensins), and bactenecins (θ-defensins); however, no human θ-defensins have been isolated. AMPs are a large class of innate immune system effector molecules with a wide range of biological activities, including immunomodulatory and antimicrobial functions against bacterial, fungal, yeast, and viral pathogens. However, recent studies have identified AMPs as core regulators of autophagy/hemophagy, cytokines, chemokines, and reactive oxygen species (ROS) production, as well as IFN signaling, as they serve as signaling nodes that modulate immune pathways.Citation28,Citation29
VitD3 Inhibits HP by Promoting the Secretion of Cathelicidin
Human cathelicidin antimicrobial peptides (CAMPs), such as LL-37, are alpha-helical peptides with amphipathic properties that are derived from the C-terminal region of the human cationic antimicrobial protein, which is broken down by serine protease and proteinase 3;Citation30 CAMPs can be divided into six categories known as human neutrophil proteins 1 to 4 (HNP1-4) and human defensins 5 and 6 (HD5 and 6). Human CAMPs have antimicrobial activity against both gram-negative and gram-positive bacteria, and their bactericidal activity requires activation by protein hydrolysis of their precursors.Citation31 CAMPs are responsible for modulating the intestinal microbiota and improving bacterial clearance at barrier sites.Citation29 Due to their positive charge, CAMPs preferentially interact with negatively charged bacterial membranes and form pores via detergent-like effects.Citation30 A recent study used superresolution single-particle tracking tools to demonstrate CAMP-mediated penetration and rigidification of the bacterial cytoplasm through the electrostatic linking of chromosomal DNA and a subset of ribosomes.Citation32 CAMPs stimulate mucus synthesis in the gastric mucosa by upregulating the mRNA expression of mucin 1 (MUC1), inhibiting HP growth, disrupting bacterial biofilms, and inducing morphological alterations in the HP membrane.Citation33 Additionally, they alleviate gastric mucosal infections, reduce HP colonization in the gastric mucosa, and decrease the production of gastric mucosal cytokines.Citation34
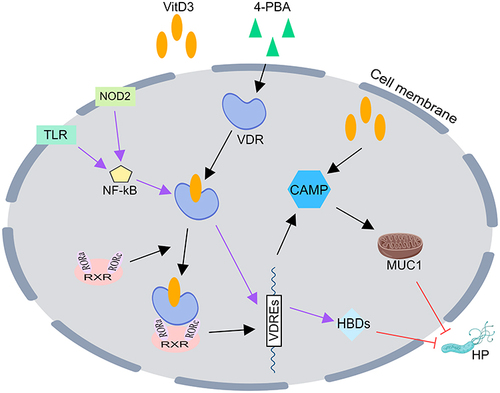
The relationship between CAMPs and VitD3 is not fully known and may be related to the following mechanisms: 1) VitD3 combined with 4-phenylbutyrate results in the activation of functional VDR signaling to trigger the expression of cathelicidin, a secondary messenger for autophagy activation through transcriptional activation of ATGs (autophagy-related) and the enhancement of autophagic flux in human monocytes/macrophages;Citation35 2) VitD3 alone enhances CAMP expression;Citation36 and 3) in mammalian cells, 1.25-dihydroxyvitamin D3 stimulates the production of heterodimers of the vitamin D receptor (VDR) and the retinoid X receptor (RXR), which mostly bind to retinoic acid-related orphan receptors a and γ (RORa and RORγ).Citation37 VDR-RXR coupled with 1a, 25-dihydroxy vitamin D3 binds to vitamin D3 response elements (VDREs) located 59 bp upstream of target genes, acts as a transcription factor, and upregulates the gene expression of various proteins, such as CAMPs ().Citation37
Figure 1 VitD3 inhibits HP by promoting the secretion of antimicrobial peptides (AMPs). Vitamin D3 aloneCitation36 or in combination with retinoic acidCitation37 or 4-PBACitation35 results in the activation of functional VDR signaling to trigger the expression of CAMP, while VitD3 upregulates b-defensins by binding to VDR after TLR2/1 or NOD2 signaling activates NF-kB signaling in human monocytes, which ultimately eradicates HP.Citation38,Citation39 CAMP, cathelicidin antimicrobial peptide; HBDs, human β-defensins; VDR, vitamin D receptor; VDREs, vitamin D3 response elements; 4-PBA, 4-phenylbutyrate; TLR, Toll-like receptor; NOD2, nucleotide-binding oligomerization domain containing 2; NF-kB, nuclear factor kB; RXR, retinoid X receptor; ROR, retinoic acid-related orphan receptor; MUC1, mucin1.
VitD3 Inhibits HP by Promoting the Secretion of β-Defensins
Human β-defensins (HBDs), which are polypeptides with approximately 35 amino acid residues and are primarily expressed in epithelial tissues, act as the first line of defense between people and their surroundings. In the human genome, more than 50 HBD genes have been discovered. Of these, the functions of HBD1, HBD2, HBD3, and HBD4 are the best characterized,Citation40 with HBD-3 having a particularly strong impact on HP.Citation41 However, the specific mechanism by which defensins kill their target cells is still unknown. HBD3 has been shown to have chemo-inductive effects on dendritic cells, memory T cells, and mast cells,Citation40 and the constructive defensin HBD1 and the inducible defensins HBD2 and HBD4 have potent antimicrobial effects against E. coli but have no or minimal effects on HP.Citation42 HBD2 accumulates on the cell surface of HP strains and leads to slight growth inhibition, even though it does not appear to be efficient at reducing the viability of bacteria.Citation42,Citation43 As recently shown by Cullen et al, the modification of lipid A by removing phosphate groups, which results in a comparably less negative surface charge, may increase the resistance of HP to HBD2.Citation44 Pero et al demonstrated that the C-terminal domain (RRKK) of HBD3 and the inner domain of HBD1 (PIFTKIQGT) are essential for antimicrobial activity.Citation45 The removal of six amino acids at the N-terminus of HBD3 did not decrease the activity. Consequently, Pero et al developed an analog that maintained HBD1-mediated killing and was resistant to high NaCl concentrations, similar to HBD3. The activity of this analog (3NI) was not evaluated against HP, and this analog may be a promising tool against HP that can be used to combat infection and reverse DNA methylation.Citation45 By electrostatically interacting with the negative charge of the microbial plasma membrane, the positive residues in HBDs enter the lipid bilayer through the amphipathic region of the membrane, increasing membrane permeability and killing the microbe.Citation46,Citation47 However, altering membrane permeability is not the only mechanism by which HBDs kill pathogens. It is possible that HBDs can inhibit the synthesis of RNA, DNA, and proteins and induce the synthesis of cytokines, such as IFN-γ, in epithelial cells, which contributes to the elimination of bacteria and viruses. It is also possible that several mechanisms cooperate to cause pathogen death.Citation48
The relationship between defensins and VitD3 is more complicated, and it has been reported that after Toll-like receptor 2/1 (TLR2/1) or nucleotide-binding oligomerization domain containing 2 (NOD2) signaling activates nuclear factor kB (NF-kB) signaling in human monocytes, VitD3 increases the abundance of β-defensins by binding to vitamin D response elements in the promoter and enhancer regions of VDR-regulated defensin genes.Citation38,Citation39 However, the details of the pathway are unclear, and further research is needed ().
Antibacterial Activity of Vitamin D3 Decomposition Products Against HP
Vitamin D group has been shown to decompose nonbiologically via exposure to high humidity and high temperature.Citation49 Kouichi et al carried out the nonbiological degradation of vitamin D3 species and investigated the antibacterial activity of the degraded vitamin D3 species against HP.Citation50 Vitamin D3 was dispersed for 1 week into distilled water warmed at 70°C to obtain abundant amounts of vitamin D3 decomposition products (VDPs). Then, the VDPs were divided into four fractions by column chromatography, of which VDP fraction-1 had the most pronounced inhibition of HP. VDP fraction-1 was further divided into nine aliquots by column chromatography, and the two aliquots with the most significant HP inhibition were purified and categorized as VDP1, VDP2 and VDP3. VDP3 seems to be structurally unstable and volatilizes easily, as it disappears during purification.Citation50
VDP1 and VDP2 have antibacterial effects on HP with free cholesterol (FC), but VDP2 has a weaker effect; however, the antibacterial effect of VDP2 against the FC-retaining HP was completely abolished, whereas VDP1 was unaffected.Citation50 Phosphatidylethanolamine (PE) is a myristic acid molecule that is the most common glycerophospholipid in the cell membrane of HP; has a high binding affinity for nonesterified steroidal compounds such as free cholesterol, pregnenolone, and progesterone at the carbon-3 position; and plays an important role in the interaction between bacterial cells and steroidal compounds.Citation51,Citation52 Dimyristoyl PE (DMPE) is one of the most prevalent PE species among HP cell membrane lipids. Among the steroidal compounds, progesterone and its analogs induce the cell lysis of HP by binding to the myristoyl PE of bacterial cells.Citation51,Citation53 VDP1 binds to the DMPE in the HP membrane, induces leakage from the cell membrane, and ultimately lyses bacterial cells; however, VDP2 has a low binding affinity with DMPE.Citation50
The anti-HP effect of VDP1 is extremely selective, and commonplace bacteria can survive even at high concentrations of VDP1.Citation54 Additionally, VDP1, which contains Grundmann’s ketone, is a highly nonpolar ketone.Citation50 Grundmann’s ketone has antimicrobial effects, especially against fungi, gram-positive bacteria, and HP.Citation55 Furthermore, any cytotoxic side effects from VDP1 are likely to be extremely weak in human cells.Citation50
VitD3 Enhances Lysosomal Degradation of HP via Ca2+-Dependent Lysosomal Acidification
Autophagy is characterized by the formation of double-membrane vesicles known as autophagosomes. This process is an evolutionarily conserved self-degradation process that is utilized by host cells to maintain cellular homeostasis and protect them from pathogen invasion.Citation56 Lysosomes are membrane-bound organelles that contain more than 50 different degradative hydrolases. Autophagosomes mature into autolysosomes after merging with lysosomes, and pathogens trapped inside are destroyed by lysosomal proteases after being identified by the innate immune system,Citation57 but this process requires an acidic environment in the lysosomal lumen.Citation58 The autophagy pathway was formerly thought to be a host defense mechanism against infections, but recent research has shown that bacteria are capable of disrupting this process and actively replicating themselves within cells.Citation59 Initially, HP was thought to be a noninvasive pathogen attached to the surface of gastric epithelial cells; however, in the last decade or so, during HP infections, the pathogen was found to invade the cells,Citation60 induce autophagy in several gastric cell lines,Citation61 and cause an abnormal accumulation of Ca2+ in the lysosomes of its infected cells; in these cases, lysosomal acidification is impaired, which prevents HP from being degraded in lysosomes, allowing it to continue to multiply and persist in infections.Citation62
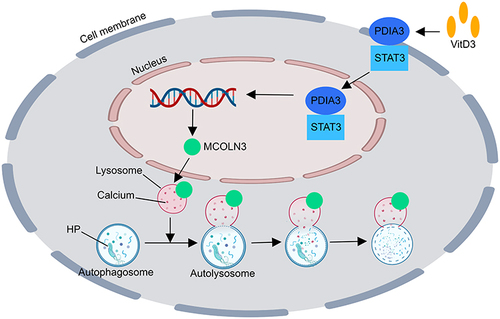
Hu et al demonstrated that vitamin D restores the impaired lysosomal activity of epithelial cells by stimulating the protein disulfide isomerase family A member 3 (PDIA3) receptor, leading to the redistribution of the PDIA3-STAT3 (signal transducer and activator of transcription 3) complex into the nucleus and thereby enhancing the production of the mucolipin 3 (MCOLN3) protein.Citation62 STAT3 is a transcription factor that relays signals from activated cytokine and growth factor receptors in the plasma membrane to the nucleus, where it regulates gene transcription,Citation63 which leads to the upregulation of MCOLN3 protein expression in a promoter-specific manner in HFE145 cells.Citation62 Mucolipins (MCOLNs), a subfamily of transient receptor potential channels, are predicted to encode Ca2+ channels that predominantly function in endolysosomal vesicles.Citation64 The MCOLN3 channel is predominantly expressed on late endosomal and lysosomal membranes (>75%).Citation64 Ca2+ release from endolysosomes via the MCOLN3 channel is necessary for lysosomal acidification and maturation.Citation64,Citation65 Upregulation of the MCOLN3 channel by VitD3 is required for Ca2+ release from lysosomes and consequently normalizes lysosomal acidification. A highly acidic environment (pH < 5.0) in the lumen is extremely important for the lysosome to execute its digestive action and to export recycled cargos. The hydrolytic enzymes within lysosomes are also activated under acidic pH conditions.Citation66 The restoration of the acidic environment of lysosomes damaged by HP infection increased HP degradation by lysosomes ().
Figure 2 The mechanism by which VitD3 enhances the lysosomal degradation of HP via Ca2+-dependent lysosomal acidification.Citation62 The membrane receptor PDIA3 is activated by VitD3 therapy, which also causes the PDIA3-STAT3 complex to relocate into the nucleus and upregulates MCOLN3 protein expression. This process restores Ca2+ release from lysosomes and causes lysosomal acidification. Lysosomes eliminate HP to restore the acidity of the environment. PDIA3, protein disulfide isomerase family A member 3; STAT3, signal transducer and activator of transcription 3; MCOLN3, mucolipin 3.
Conclusions
In summary, the role of VitD3 in the eradication of HP is certain and includes its classical pathway of binding to VDR to regulate the body’s immune defense and increase the secretion of AMPs, while the nonclassical pathway is a novel signaling pathway that initiates activation of the PDIA3-STAT3-MCOLN3-Ca2+ axis to reactivate the acidification and degradation of lysosomes and the collapse of the membrane structure of HP cells via degradation products. VitD3 represents a novel approach and a new option for the eradication of HP, but there are still some pathways or mechanisms that remain unclear and need further in-depth research and development.
Disclosure
The authors report no conflicts of interest in this work.
References
- Burgard M, Kotilea K, Mekhael J, et al. Evolution of Helicobacter pylori associated with gastroduodenal ulcers or erosions in children over the past 23 years: decline or steady state? Helicobacter. 2019;24(5):e12629. doi:10.1111/hel.12629
- Zhang Y, Lai Z, Wang J. 幽门螺杆菌感染患者胃黏膜组织学炎症评价依据及诊断标准的探讨 [Helicobacter pylori associated gastric mucosal inflammation and histopathological assessment]. Zhonghua Yi Xue Za Zhi. 2001;81(13):811–815. Chinese.
- Le LTT, Nguyen TA, Nguyen NA, et al. Antibiotic resistance of helicobacter pylori in children with gastritis and peptic ulcers in Mekong Delta, Vietnam. Healthcare. 2022;10:6.
- Ren S, Cai P, Liu Y, et al. Prevalence of Helicobacter pylori infection in China: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2022;37(3):464–470. doi:10.1111/jgh.15751
- Wen X, Wen D, Yang Y, Chen Y, Wang G, Shan B. Urban-rural disparity in helicobacter pylori infection-related upper gastrointestinal cancer in china and the decreasing trend in parallel with socioeconomic development and urbanization in an endemic area. Ann Glob Health. 2017;83(3–4):444–462. doi:10.1016/j.aogh.2017.09.004
- Yuan C, Adeloye D, Luk TT, et al. The global prevalence of and factors associated with Helicobacter pylori infection in children: a systematic review and meta-analysis. Lancet Child Adolesc Health. 2022;6(3):185–194. doi:10.1016/S2352-4642(21)00400-4
- Xu C, Wu Y, Xu S. Association between Helicobacter pylori infection and growth outcomes in children: a meta‐analysis. Helicobacter. 2022;27(1). doi:10.1111/hel.12861
- Ma PF, Dai Q, Chu J, et al. 25-hydroxyvitamin D levels in children of different ages and with varying degrees of Helicobacter pylori infection and immunological features. Front Pediatr. 2023;11:1157777. doi:10.3389/fped.2023.1157777
- El Shahawy MS, Shady ZM, Gaafar A. Influence of adding vitamin D3 to standard clarithromycin-based triple therapy on the eradication rates of Helicobacter pylori infection. Arab J Gastroenterol. 2021;22(3):209–214. doi:10.1016/j.ajg.2021.08.002
- Mut Surmeli D, Surmeli ZG, Bahsi R, et al. Vitamin D deficiency and risk of Helicobacter pylori infection in older adults: a cross-sectional study. Aging Clin Exp Res. 2019;31(7):985–991. doi:10.1007/s40520-018-1039-1
- Soares TF, Rocha GA, Rocha AM, et al. Phenotypic study of peripheral blood lymphocytes and humoral immune response in Helicobacter pylori infection according to age. Scand J Immunol. 2005;62(1):63–70. doi:10.1111/j.1365-3083.2005.01638.x
- Jafarzadeh A, Larussa T, Nemati M, Jalapour S. T cell subsets play an important role in the determination of the clinical outcome of Helicobacter pylori infection. Microb Pathog. 2018;116:227–236. doi:10.1016/j.micpath.2018.01.040
- Ansari S, Yamaoka Y. Animal models and helicobacter pylori infection. J Clin Med. 2022;11(11):3141. doi:10.3390/jcm11113141
- Maciorkowska E, Kaczmarski M, Stasiak-Barmuta A, et al. Peripheral blood lymphocyte population in children infected with Helicobacter pylori. Rocz Akad Med Bialymst. 2003;48:95–99.
- Sipponen P, Maaroos HI. Chronic gastritis. Scand J Gastroenterol. 2015;50(6):657–667. doi:10.3109/00365521.2015.1019918
- Wilson KT, Crabtree JE. Immunology of Helicobacter pylori: insights into the failure of the immune response and perspectives on vaccine studies. Gastroenterology. 2007;133(1):288–308. doi:10.1053/j.gastro.2007.05.008
- Adorini L, Penna G. Dendritic cell tolerogenicity: a key mechanism in immunomodulation by vitamin D receptor agonists. Hum Immunol. 2009;70(5):345–352. doi:10.1016/j.humimm.2009.01.016
- Bah SY, Dickinson P, Forster T, Kampmann B, Ghazal P. Immune oxysterols: role in mycobacterial infection and inflammation. J Steroid Biochem Mol Biol. 2017;169:152–163. doi:10.1016/j.jsbmb.2016.04.015
- Wu S, Liao AP, Xia Y, et al. Vitamin D receptor negatively regulates bacterial-stimulated NF-kappaB activity in intestine. Am J Pathol. 2010;177(2):686–697. doi:10.2353/ajpath.2010.090998
- Baeke F, Korf H, Overbergh L, et al. Human T lymphocytes are direct targets of 1,25-dihydroxyvitamin D3 in the immune system. J Steroid Biochem Mol Biol. 2010;121(1–2):221–227. doi:10.1016/j.jsbmb.2010.03.037
- Hafkamp FMJ, Taanman-Kueter EWM, van Capel TMM, Kormelink TG, de Jong EC. Vitamin D3 priming of dendritic cells shifts human neutrophil-dependent Th17 cell development to regulatory T cells. Front Immunol. 2022;13:872665. doi:10.3389/fimmu.2022.872665
- Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HF, O’Garra A. 1alpha,25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J Immunol. 2001;167(9):4974–4980. doi:10.4049/jimmunol.167.9.4974
- D’Ambrosio D, Cippitelli M, Cocciolo MG, et al. Inhibition of IL-12 production by 1,25-dihydroxyvitamin D3. Involvement of NF-kappaB downregulation in transcriptional repression of the p40 gene. J Clin Invest. 1998;101(1):252–262. doi:10.1172/JCI1050
- Chang SH, Chung Y, Dong C. Vitamin D suppresses Th17 cytokine production by inducing C/EBP homologous protein (CHOP) expression. J Biol Chem. 2010;285(50):38751–38755. doi:10.1074/jbc.C110.185777
- Hamzaoui A, Berraies A, Hamdi B, Kaabachi W, Ammar J, Hamzaoui K. Vitamin D reduces the differentiation and expansion of Th17 cells in young asthmatic children. Immunobiology. 2014;219(11):873–879. doi:10.1016/j.imbio.2014.07.009
- Serrano C, Wright SW, Bimczok D, et al. Downregulated Th17 responses are associated with reduced gastritis in Helicobacter pylori-infected children. Mucosal Immunol. 2013;6(5):950–959. doi:10.1038/mi.2012.133
- Shi Y, Liu XF, Zhuang Y, et al. Helicobacter pylori-induced Th17 responses modulate Th1 cell responses, benefit bacterial growth, and contribute to pathology in mice. J Immunol. 2010;184(9):5121–5129. doi:10.4049/jimmunol.0901115
- Dimitrov V, White JH. Species-specific regulation of innate immunity by vitamin D signaling. J Steroid Biochem Mol Biol. 2016;164:246–253. doi:10.1016/j.jsbmb.2015.09.016
- Clark A, Mach N. Role of vitamin D in the hygiene hypothesis: the interplay between vitamin D, vitamin D receptors, gut microbiota, and immune response. Front Immunol. 2016;7:627. doi:10.3389/fimmu.2016.00627
- Lee CC, Sun Y, Qian S, Huang HW. Transmembrane pores formed by human antimicrobial peptide LL-37. Biophys J. 2011;100(7):1688–1696. doi:10.1016/j.bpj.2011.02.018
- Wehkamp J, Schauber J, Stange EF. Defensins and cathelicidins in gastrointestinal infections. Curr Opin Gastroenterol. 2007;23(1):32–38. doi:10.1097/MOG.0b013e32801182c2
- Zhu Y, Mohapatra S, Weisshaar JC. Rigidification of the Escherichia coli cytoplasm by the human antimicrobial peptide LL-37 revealed by superresolution fluorescence microscopy. Proc Natl Acad Sci U S A. 2019;116(3):1017–1026. doi:10.1073/pnas.1814924116
- Zhang L, Wu WK, Gallo RL, et al. Critical role of antimicrobial peptide cathelicidin for controlling helicobacter pylori survival and infection. J Immunol. 2016;196(4):1799–1809. doi:10.4049/jimmunol.1500021
- Zhang L, Yu J, Wong CC, et al. Cathelicidin protects against Helicobacter pylori colonization and the associated gastritis in mice. Gene Ther. 2013;20(7):751–760. doi:10.1038/gt.2012.92
- Chung C, Silwal P, Kim I, Modlin RL, Jo EK. Vitamin D-cathelicidin axis: at the crossroads between protective immunity and pathological inflammation during infection. Immune Netw. 2020;20(2):e12. doi:10.4110/in.2020.20.e12
- Steinmann J, Halldorsson S, Agerberth B, Gudmundsson GH. Phenylbutyrate induces antimicrobial peptide expression. Antimicrob Agents Chemother. 2009;53(12):5127–5133. doi:10.1128/AAC.00818-09
- Slominski AT, Kim TK, Takeda Y, et al. RORalpha and ROR gamma are expressed in human skin and serve as receptors for endogenously produced noncalcemic 20-hydroxy- and 20,23-dihydroxyvitamin D. FASEB J. 2014;28(7):2775–2789. doi:10.1096/fj.13-242040
- Wang TT, Dabbas B, Laperriere D, et al. Direct and indirect induction by 1,25-dihydroxyvitamin D3 of the NOD2/CARD15-defensin beta2 innate immune pathway defective in Crohn disease. J Biol Chem. 2010;285(4):2227–2231. doi:10.1074/jbc.C109.071225
- Liu PT, Schenk M, Walker VP, et al. Convergence of IL-1beta and VDR activation pathways in human TLR2/1-induced antimicrobial responses. PLoS One. 2009;4(6):e5810. doi:10.1371/journal.pone.0005810
- Schneider JJ, Unholzer A, Schaller M, Schafer-Korting M, Korting HC. Human defensins. J Mol Med. 2005;83(8):587–595. doi:10.1007/s00109-005-0657-1
- Andresen E, Gunther G, Bullwinkel J, Lange C, Heine H, Idzko M. Increased expression of beta-defensin 1 (DEFB1) in chronic obstructive pulmonary disease. PLoS One. 2011;6(7):e21898. doi:10.1371/journal.pone.0021898
- Pero R, Coretti L, Nigro E, et al. Beta-defensins in the fight against helicobacter pylori. Molecules. 2017;22(3):424. doi:10.3390/molecules22030424
- Nuding S, Gersemann M, Hosaka Y, et al. Gastric antimicrobial peptides fail to eradicate Helicobacter pylori infection due to selective induction and resistance. PLoS One. 2013;8(9):e73867. doi:10.1371/journal.pone.0073867
- Cullen TW, Giles DK, Wolf LN, Ecobichon C, Boneca IG, Trent MS. Helicobacter pylori versus the host: remodeling of the bacterial outer membrane is required for survival in the gastric mucosa. PLoS Pathog. 2011;7(12):e1002454. doi:10.1371/journal.ppat.1002454
- Pero R, Angrisano T, Brancaccio M, et al. Beta-defensins and analogs in Helicobacter pylori infections: mRNA expression levels, DNA methylation, and antibacterial activity. PLoS One. 2019;14(9):e0222295. doi:10.1371/journal.pone.0222295
- Sahl HG, Pag U, Bonness S, Wagner S, Antcheva N, Tossi A. Mammalian defensins: structures and mechanism of antibiotic activity. J Leukoc Biol. 2005;77(4):466–475. doi:10.1189/jlb.0804452
- Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3(9):710–720. doi:10.1038/nri1180
- Sass V, Schneider T, Wilmes M, et al. Human beta-defensin 3 inhibits cell wall biosynthesis in Staphylococci. Infect Immun. 2010;78(6):2793–2800. doi:10.1128/IAI.00688-09
- Grady LT, Thakker KD. Stability of solid drugs: degradation of ergocalciferol (vitamin D2) and cholecalciferol (vitamin D3) at high humidities and elevated temperatures. J Pharm Sci. 1980;69(9):1099–1102. doi:10.1002/jps.2600690932
- Hosoda K, Shimomura H, Wanibuchi K, et al. Identification and characterization of a vitamin D(3) decomposition product bactericidal against Helicobacter pylori. Sci Rep. 2015;5(1):8860. doi:10.1038/srep08860
- Amgalanbaatar A, Shimomura H, Hosoda K, Hayashi S, Yokota K, Hirai Y. Antibacterial activity of a novel synthetic progesterone species carrying a linoleic acid molecule against Helicobacter pylori and the hormonal effect of its steroid on a murine macrophage-like cell line. J Steroid Biochem Mol Biol. 2014;140:17–25. doi:10.1016/j.jsbmb.2013.10.023
- Shimomura H, Hosoda K, Hayashi S, Yokota K, Hirai Y. Phosphatidylethanolamine of Helicobacter pylori functions as a steroid-binding lipid in the assimilation of free cholesterol and 3beta-hydroxl steroids into the bacterial cell membrane. J Bacteriol. 2012;194(10):2658–2667. doi:10.1128/JB.00105-12
- Hosoda K, Shimomura H, Hayashi S, Yokota K, Hirai Y. Steroid hormones as bactericidal agents to Helicobacter pylori. FEMS Microbiol Lett. 2011;318(1):68–75. doi:10.1111/j.1574-6968.2011.02239.x
- Wanibuchi K, Hosoda K, Ihara M, et al. Indene compounds synthetically derived from vitamin d have selective antibacterial action on helicobacter pylori. Lipids. 2018;53(4):393–401. doi:10.1002/lipd.12043
- Krojer M, Keller M, Bracher F. 7-Aza-des-A-steroids with antimicrobial and cytotoxic activity. Sci Pharm. 2013;81(2):329–338. doi:10.3797/scipharm.1303-03
- Klionsky DJ, Abdelmohsen K, Abe A, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy. 2016;12(1):222.
- He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet. 2009;43(1):67–93. doi:10.1146/annurev-genet-102808-114910
- Mindell JA. Lysosomal acidification mechanisms. Annu Rev Physiol. 2012;74(1):69–86. doi:10.1146/annurev-physiol-012110-142317
- Levine B. Eating oneself and uninvited guests: autophagy-related pathways in cellular defense. Cell. 2005;120(2):159–162. doi:10.1016/j.cell.2005.01.005
- Deen NS, Huang SJ, Gong L, Kwok T, Devenish RJ. The impact of autophagic processes on the intracellular fate of Helicobacter pylori: more tricks from an enigmatic pathogen? Autophagy. 2013;9(5):639–652. doi:10.4161/auto.23782
- Terebiznik MR, Raju D, Vazquez CL, et al. Effect of Helicobacter pylori’s vacuolating cytotoxin on the autophagy pathway in gastric epithelial cells. Autophagy. 2009;5(3):370–379. doi:10.4161/auto.5.3.7663
- Hu W, Zhang L, Li MX, et al. Vitamin D3 activates the autolysosomal degradation function against Helicobacter pylori through the PDIA3 receptor in gastric epithelial cells. Autophagy. 2019;15(4):707–725. doi:10.1080/15548627.2018.1557835
- Levy DE, Lee C-K. What does Stat3 do? J Clin Investig. 2002;109(9):1143–1148. doi:10.1172/JCI0215650
- Zeevi DA, Frumkin A, Bach G. TRPML and lysosomal function. Biochim Biophys Acta. 2007;1772(8):851–858. doi:10.1016/j.bbadis.2007.01.004
- Di Palma F, Belyantseva IA, Kim HJ, Vogt TF, Kachar B, Noben-Trauth K. Mutations in Mcoln3 associated with deafness and pigmentation defects in varitint-waddler (Va) mice. Proc Natl Acad Sci U S A. 2002;99(23):14994–14999. doi:10.1073/pnas.222425399
- Xu H, Ren D. Lysosomal physiology. Annu Rev Physiol. 2015;77(1):57–80. doi:10.1146/annurev-physiol-021014-071649
