Abstract
Objective
This study reports a preclinical evaluation of an alginate/chitosan nanoparticle formulation containing NovaBupi®, a racemic bupivacaine (BVC) containing 25% dextrobupivacaine and 75% levobupivacaine.
Methods
New Zealand White rabbits (n=6) received intraoral or intrathecal injections of BVC 0.5% or BVC 0.5%-loaded alginate–chitosan nanoparticles (BVCALG). BVC plasma levels and pharmacokinetic parameters were determined in blood samples of these rabbits. An infraorbital nerve blockade was performed in male Wistar rats (n=7) with the same formulations and the vehicle (NPALG). Histological evaluation of local toxicity after 6 hours and 24 hours of the treatments was performed in rats’ (n=6) oral tissues.
Results
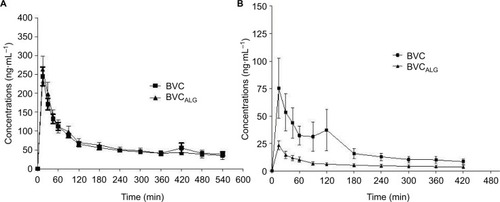
No statistically significant difference was observed between plasma concentrations and pharmacokinetic parameters (p>0.05) after intraoral injections. However, after intrathecal injection BVCALG changed approximately three times the values of volume of distribution and area under the curve (AUC0–t; p<0.05). The total analgesic effect of BVC after infraorbital nerve blockade was improved by 1.4-fold (p<0.001) with BVCALG. BVC and BVCALG did not induce significant local inflammatory reaction.
Conclusion
The encapsulation of BVC prolongs the local anesthetic effect after infraorbital nerve blockade and altered the pharmacokinetics after intrathecal injection.
Introduction
Local anesthetics (LAs) are used for anesthesia and analgesia during trans- and postoperative periods or for management of acute and chronic pain conditions.Citation1 In contact with the nerve fiber trunk, these agents bind to specific sites of sodium channels of the nerve membrane and promote a reversible interruption of the nerve impulses due to decreased permeability to sodium ions.Citation2,Citation3
Bupivacaine (BVC) is a long-acting LA that belongs to the amino-amide class and is widely used during surgical procedures and for postoperative pain. Due to its chemical structure, BVC presents a stereocenter and has two isomers, dextrobupivacaine (R(+)BVC) and levobupivacaine (LBVC; S(−)BVC).Citation4 The physicochemical properties of the two isomers molecules are the same, but these molecules can present different behaviors in their affinity for either the site of action or the sites involved in the generation of side effects. The biologic effects of enantiomers differ, both quantitatively and qualitatively, due to receptor configuration.Citation5
R(+)BVC produces greater tonic and phasic block of sodium channels than S(−) BVC, two and three times, respectively.Citation6 The affinity of R- and S-isomers of BVC is different for ion channels of sodium, potassium, and calcium, and this results in a significant reduction of toxicity in central nervous and cardiac systems of the S(−)BVC.Citation5–Citation9
Therefore, S(−)BVC is a long-acting anesthetic with less risk of cardiotoxicity and neurotoxicity compared with BVC.Citation6 Also, LBVC has a similar clinical profile compared to BVC but presented lower potency for motor blockade, which is a major advantage in regional anesthesia and analgesia.Citation9
Thus, LBVC has become a good option for prolonged regional anesthesia and investigations on stereoselectivity have allowed for changes in BVC stereoisomers concentrations in LA formulations. In this context, an LA formulation with 25% R(+)BVC and 75% S(−)BVC was released in Brazil. This formulation, called NovaBupi® (S75–R25), improved the anesthetic profile compared to LBVC and increased its safety margin.Citation4,Citation10,Citation11
Despite these advantages, all LA molecules present low molecular weight and consequently rapid systemic absorption. As an outcome, their anesthetic effect presents short duration and the risk of systemic toxicity precludes the use of high bolus doses.Citation2 High LA plasma concentration may lead to a progressive range of neurological and cardiac complications with potentially devastating effects.Citation12
The use of LA drug delivery systems, such as liposomes, polymeric nanoparticles, or cyclodextrins as carriers, has improved the therapeutic effects of these agents. These systems are able to prolong LA action, decrease plasma levels, or allow the use of lower LA doses to achieve equivalent analgesia to commercially available formulations.Citation2,Citation4,Citation13–Citation15
A carrier system for BVC (enantiomeric mixture S75–R25) was developed by Grillo et al.Citation4 This system used alginate/chitosan nanoparticles with BVC (0.5%; BVCALG). The amount of BVC associated in the nanoparticles was ~76%. In vitro release kinetics showed that the complete release (100%) of BVC in solution occurred after 350 minutes, while complete release of BVC present in the nanoparticles required >900 minutes. The formulation was tested in 3T3-fibroblasts culture cells and presented low cytotoxicity. BVCALG formulation significantly reduced the cytotoxicity when compared with plain BVC at 20 μM. BVCALG was also tested in vivo after the sciatic nerve blockade model and the formulation promoted an increase in the intensity and in the duration of motor and sensory blockades; also, this formulation enhanced the differential nerve blockade.
Previous data obtained by Grillo et alCitation4 supported the advantages of this new formulation of BVCALG and perspectives for its clinical future use. Nevertheless, before the clinical use of this formulation, it is necessary to determine its pharmacokinetic, efficacy, and local toxicity profile in animal models. This evaluation is important for fundamental information of this new sustained-release pharmaceutical formulation, such as BVCALG. Thus, the aims of this study were to determine plasma levels, efficacy, and local toxicity induced by this new formulation of BVCALG in rats and rabbits, looking forward to its clinical use in dentistry and medicine.
Methods
Chemicals and reagents
The commercial anesthetic solution used in this study was plain 0.5% BVC (NovaBupi®; Cristália Ind Farm Ltda, Itapira, São Paulo, Brazil; batch no 10129262). BVC (S75–R25) salt was donated by Cristália Ind Farm Ltda. Alginate, polyvinyl alcohol, and chitosan were obtained from Sigma-Aldrich Co (St Louis, MO, USA). All other reagents were of analytical grade and deionized water was obtained from an ultrapure water system (Millipore Milli-Q system; Milford, MA, USA).
BVCALG formulation
BVCALG formulation used in this study was identical to that described previously and exhibited the same in vitro characteristics as described by Grillo et al.Citation4 A solution of sodium alginate (0.063% m/v) containing 0.5% BVC (S75–R25) was prepared; 7.5 mL calcium chloride (18 mM) was slowly added in 60 minutes under mechanical agitation. Chitosan solution (0.07%, m/v) was then added over 90 minutes with a peristaltic pump with controlled flow. After preparation, the nanoparticles were stored in an amber flask for later usage.
Animals and in vivo studies: infraorbital nerve blockade, local toxicity, and pharmacokinetics
The animals used in this study were male Wistar rats (Unib: WH) (250–350 g) and New Zealand White rabbits (2.5–3.0 kg). The experimental protocol was approved by the Institutional Committee for Ethics in Animal Research of São Francisco University (protocol #001.09.10) which follows the recommendations of the Guide for the Care and Use of Laboratory Animals. Animals were housed five per cage (rats) or one per cage (rabbits) and received water and food ad libitum with a 12:12 hour light–dark cycle, at 23°C ±2°C.
Pharmacokinetic study
New Zealand White rabbits were randomly divided into four groups (n=6), which received a submucosal intraoral (1 mL) or intrathecal (0.2 mL) injection of the following treatments: BVC plain (BVC) or BVC with nanoparticles (BVCALG). Doses for intraoral and intrathecal injections were 2 and 0.4 mg·kg−1, respectively.
General anesthesia was achieved with α-chloralose (50 mg·kg−1) and urethane (1 g·kg−1) before the injections and an intravascular catheter was inserted in the ear vein of the rabbits. Blood samples (1 mL) were collected via a heparinized cannula pre-dose (0 minutes) and 15, 30, 45, 60, 90, 120, 180, 240, 300, 360, 420, 480, and 540 minutes after the injection of formulations. These intervals were defined to obtain 11 samples between the baseline (0 minutes) and approximately four times the t½ (half-life time) of BVC (~136 minutes).Citation16 Immediately after each blood collection, plasma was separated and stored at −70°C until analysis.
Liquid chromatography-tandem mass spectrometry (LC–MS/MS) assay: apparatus and chromatographic conditions
A Shimadzu LC 20 AD system (Shimadzu Corporation, Kyoto, Japan) coupled with a Micromass Quattro LC® triple stage quadrupole mass spectrometer (LC–MS/MS), equipped with an atmospheric pressure ionization electrospray source, was used to determine the BVC plasma levels. All separations were carried out on a Polaris C18 column (50×2 mm id, 5 μm particle size). The mobile phase was 80% acetonitrile and 20% water with 0.1% formic acid (pH=3.0). The total run time was 2.5 minutes, retention time for BVC was 0.72 minutes. The mass spectrometer was run in the positive mode (ES+) and set for multiple reaction monitoring (MRM). The full-scan single-mass spectrum and the daughter ion-mass spectrum for BVC and ropivacaine (internal standard [IS]) were (m/z) 289.3>140.1 and 275.3>125.9, respectively. Data were integrated using the MassLynx 4.1™ (Waters Corporation, Milford, MA, USA) software.
To validate the method, quality control (QC) samples (90.0, 45.0, and 0.9 ng·mL−1) were prepared by mixing drug-free plasma with appropriate volumes of working solutions.
Sample preparation
The frozen plasma samples (50.0 μL) were thawed at room temperature, followed by the addition of 50 μL of IS work solution (ropivacaine 10.0 μg·mL−1). The samples were previously vortexed for 2 minutes and 1000 μL of hexane/ethyl acetate (1:1; V/V) were added and then vortexed for 5 minutes and centrifuged at 1200× g, for 5 minutes at −4°C. The organic liquid (0.8 μL) layers were transferred to microtubes and the samples were dried under nitrogen flow. After solvent evaporation, samples were reconstituted in 50 μL mobile phase, vortexed for 1 minute, and 50 μL were transferred to LC–MS/MS system vials, for further injection (5.0 μL).
Precision and accuracy of the analytical method were controlled by calculating the intra-batch and inter-batch variation at three concentrations of QC in five replicates (n=5). Three calibration curves were plotted as the peak area ratio versus BVC concentration in the range of 0.3–120.0 ng·mL−1. The limit of quantification (LQ) was defined as the lowest concentration at which precision and accuracy were within 20% of the true value.
Infraorbital nerve blockade
Rat infraorbital nerve blockade was performed as previously described.Citation17,Citation18 The antinociceptive effect was assessed by observation of the aversive response to the rat upper lip pinching, according to the scores: 0 (aversive response) or 1 (no aversive response). The observation was performed by an individual that was blind to the injected formulations. The tested formulations (BVC, BVCALG, and the vehicle alginate and chitosan nanoparticles [NPALG]) were injected into the infraorbital notch (0.1 mL), situated above a gap between the posterior four molars and the anterior incisor, after the animals had been lightly anesthetized with thiopental (25 mg·kg–1) by the intraperitoneal route. NPALG was prepared in the same way as described earlier in the section “BVCALG formulation” without the addition of BVC.
The degree of sedation did not interfere with the generalized aversive response to the upper lip artery forceps pinching. Each formulation was injected unilaterally into the right side, and the intact left side served as a control for each animal. The same investigator performed all experiments. The rats were tested every 5 minutes until the animals presented the first aversive sign in the injected side. The efficacy of the infraorbital nerve block was analyzed by the time for sensory function recovery and the total LA effect. LA effect was estimated by the area under the time curve (AUC) expressed as score/hour.Citation17,Citation18
Local toxicity and histological evaluation
Male Wistar rats (n=3) received slightly general anesthesia induced by an intraperitoneal injection of sodium thiopental solution (40 mg·kg−1), before the administration of the LA formulations. The animals were divided in three groups and received 0.1 mL in the oral mucosa of the upper right first molar of one of the following formulations: 1) BVC; 2) BVCALG; or 3) NPALG. The same amount of saline solution (NaCl 0.9%) was administered in the left side as control.Citation17,Citation18 Animals were sacrificed under anesthesia (urethane 1 g·kg−1 and α-chloralose 50 mg·kg−1) 6 and 24 hours after treatment and the maxilla bones along with soft tissues were removed.
The samples from each animal were prepared to obtain five cross-sections (6 μm thick, 40 μm deep) stained with H&E in the same way as described by de Araujo et al.Citation18 The cross-sections were qualitatively analyzed with a score in order to evaluate the intensity of the leucocitary infiltration and/or any area of necrosis. The score of the local tissue inflammation was defined based on the following descriptions: 1) no infiltrate; 2) minimal infiltrate; 3) mild infiltrate; 4) severe infiltrate; and 5) severe infiltrate with necrosis areas.Citation19,Citation20 The cross-sections were codified by a third subject, and two subjects blindly evaluated all the images according to the qualitative score previously described.
Statistical analysis
The plasma concentrations of BVC were analyzed by an unpaired t-test considering each period of time separately. Pharmacokinetic parameters (maximum plasma concentration, Cmax; time to reach maximum concentration, Tmax; AUC; half-life time, t½; volume of distribution, Vd; clearance, Cl; mean residence time) were calculated using WinNonlin software (WinNonlin version 5.3; Pharsight Corporation, Mountain View, CA, USA). The pharmacokinetic data were also submitted for statistical analysis with unpaired t-test (a=0.05). The results obtained in each time interval (6 and 24 hours) were compared considering each group and considering the control side. Data were analyzed with the Kruskal–Wallis test considering each group (intergroup analysis). The tissue reaction was also analyzed by Wilcoxon paired test considering the treated and control sides (intragroup analysis). Infraorbital nerve blockade data (time for recovery and AUC) were analyzed by the Mann–Whitney test and expressed as medians (minimum and maximum limits). The level of significance was set at 5%.
Results
Analysis using MRM function was highly selective since no interfering compounds and significant ion suppression from endogenous substances were observed at the retention times for BVC and IS. The intra-batch accuracy presented values from 99.25% to 106.00% and precision was in the range of 0.93%–5.77%. The inter-batch accuracy and precision were calculated to be from 99.52% to 102.16% and from 1.29% to 4.46%, respectively. The calibration curve for BVC showed a good response over the range of 0.3–120.0 ng·mL−1. The relative error of the mean of measured concentrations ranged from 0.30% to 1.16%. The determination coefficients (r2) were >0.99 for all curves. In addition, the LQ for BVC was 0.3 ng·mL−1. The described method has proven to be rapid and effective to accurately follow this BVC formulations’ pharmacokinetics.
shows the graph of mean plasma concentrations versus time after the intraoral () and intrathecal () injections of BVC and BVCALG. No statistically significant difference was observed between plasma concentrations of the two formulations for all periods of time (p>0.05) after intraoral injection (). After intrathecal administration (), during 30–180 minutes BVCALG presented lower plasma concentrations when compared with BVC (p<0.05). Two hundred forty minutes after the injections there were no differences between the two BVC formulations after intrathecal administration.
Figure 1 Graph of mean plasma concentration versus time after the intraoral (A) or intrathecal (B) injections of BVC formulations in rabbits. Note: Values are expressed as mean ± SD.
reports the mean (± SD) values of the pharmacokinetic parameters obtained after intraoral and intrathecal injections of the tested formulations. No statistically significant difference was observed between the two formulations (p>0.05) for all pharmacokinetic parameters after intraoral injection. Intrathecal injection of BVCALG changed approximately three times the values of Vd and AUC0–t (p<0.05). Despite the absence of significant differences (p>0.05), Cmax and AUC0–∞ were three times lower for BVCALG and t½ and Cl presented lower values for BVC.
Table 1 Pharmacokinetic parameters after intraoral and intrathecal injections of BVC or BVCALG in rabbits
The BVCALG increased significantly (p<0.001) the duration of sensory blockade, since the intensity of the total analgesic effect was improved (1.4-fold) when compared with BVC plain solution. shows these results expressed as a percentage of animals with analgesia. summarizes the total analgesic effect (expressed as AUC) and the times for recovery, obtained with the tested formulations. NPALG, used as control, presented no effect.
Figure 2 Time-course (minutes) showing the percentage of animals with analgesia after treatment with 0.5% BVC plain solution or encapsulated into alginate–chitosan nanoparticles (BVCALG) as evaluated by the infraorbital nerve blockade test in rats (n=7/group).
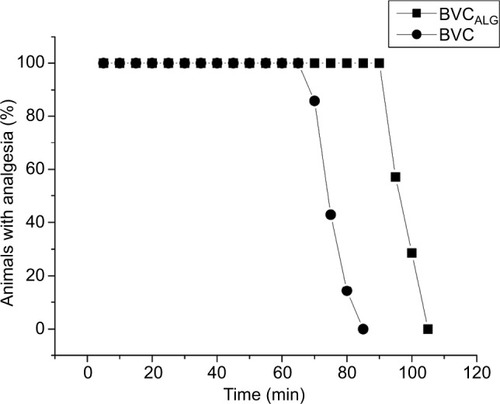
Table 2 Total effect of sensory blockade (AUC) and time for recovery for 0.5% BVC plain and BVCALG in rats
Considering the intragroup analysis (right – treated and left – control sides) after 6 hours, only BVC induced higher inflammatory reaction scores when compared with saline (p<0.05). After 24 hours of treatment, NPALG induced more intense inflammatory reaction when compared with BVC and BVCALG (p<0.05). demonstrates the median values of the scores obtained after 6 and 24 hours of treatments and their controls. and show transverse sections of the maxilla bones and their surrounding soft tissues after 6 and 24 hours of the injections of the tested formulations, respectively.
Figure 3 Histological analysis of the upper right first molar oral mucosa in rats 6 hours after local anesthetic administration: (B) BVC; (D) BVCALG; (F) NPALG. The left side was respectively used as control: (A) control BVC; (C) control BVCALG; (E) control NPALG.
Abbreviations: BVC, bupivacaine; BVCALG, 0.5% bupivacaine-loaded alginate–chitosan nanoparticles; NPALG, alginate/chitosan nanoparticle.
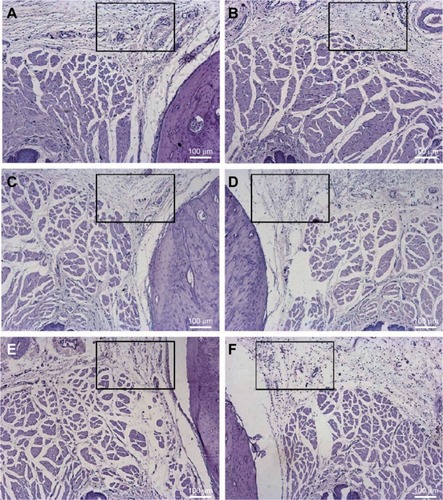
Figure 4 Histological analysis of the upper right first molar oral mucosa in rats 24 hours after local anesthetic administration: (B) BVC; (D) BVCALG; (F) NPALG. The left side was respectively used as control: (A) control BVC; (C) control BVCALG; (E) control NPALG.
Abbreviations: BVC, bupivacaine; BVCALG, 0.5% bupivacaine-loaded alginate–chitosan nanoparticles; NPALG, alginate/chitosan nanoparticle.
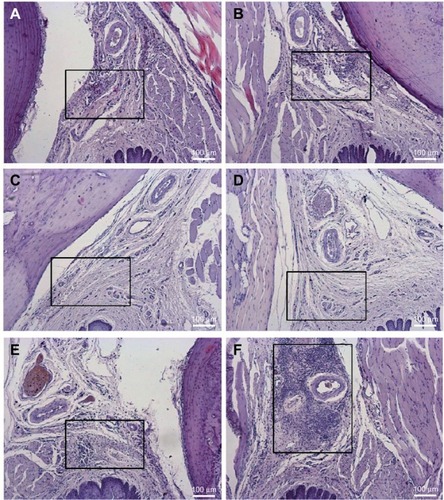
Table 3 Median (minimum–maximum limits) of the inflammatory scores for all treatments and their controls 6 and 24 hours after the treatment (0.1 mL) after intraoral administration in rats
Discussion
Several studies evaluated the effect of drug delivery systems in the pharmacokinetics of BVC used by diverse routes of in systemic LA concentration despite the type of carrier associated with BVC.
In our study, we used rabbits for the pharmacokinetic studies, especially because these animals present a higher volume of blood and easy ways to collect it when compared with rats.Citation24 Their ear vein can be easily cannulated with a simple puncture technique to collect multiple plasma samples. The encapsulation of BVC in NPALG was not capable of altering the pharmacokinetic profile after intraoral injection in rabbits. Also, these results obtained in vivo were not in accordance with the previous in vitro evaluation performed by Grillo et al.Citation4 These authors assessed the release profile of BVC from the nanoparticles in a two-compartment system separated with a cellulose membrane maintained under sink conditions with light agitation. They observed modification of the release profile of BVC when associated with the nanoparticles, the complete transfer of BVC in solution after 350 minutes, while the release of BVC in nanoparticles persisted for about 10 hours. This modification of the release profile in the presence of the nanoparticles is indicative of association, with release being dependent on diffusion through the system, erosion, or other phenomena, before permeation of the pharmaceutical through the membrane separating the donator and receptor compartments. One aspect that must be considered is that the absorption of LA into the circulation depends primarily on the vascularity of the site of injection as well as on the structure composition of the surrounding tissues. Moreover, the vasoactive properties of LA may influence the rate of absorption.Citation25 In general, LA molecules present rapid systemic absorption as a result of their low molecular weight, mainly in the oral mucosa which is an area with high vascularization. In vitro release tests are commonly used to assess the release profiles of drugs from pharmaceutical formulations, enabling comparison between the absence (free drug) and presence of a carrier. Despite the convenience of such tests, the results obtained may not correspond to the in vivo situation, because after intraoral in vivo administration free LA may be absorbed into the adjacent tissues.Citation2
In our study, intrathecal injection of BVCALG promotes slow absorption and Cl of BVC from the cerebrospinal fluid (CSF) demonstrated by the lower BVC concentrations in plasma, higher Vd, and lower AUC. We used healthy animals with similar age and weight, intrathecal injections with the same dosage, volume, and only one trained operator for the injection process. Also, the two formulations presented the same baricity and were isobaric in relation to CSF, which means that this factor was not able to alter the absorption or dispersion of BVC on both formulations.Citation26 Thus, encapsulation in nanoparticles was the factor responsible for the differences in plasma concentrations of BVC and in the pharmacokinetic parameters after intrathecal injection.
Extended or sustained-release formulations are developed to maintain constant or prolonged concentrations of drugs. An ideal LA extended-release formulation would provide consistent pain control and minimization of adverse events associated with peak drug levels. Distinct formulation technologies might produce varied release and pharmacokinetic profiles and it is possible to observe no lag time in drug absorption (no differences in Tmax) but differences in Cmax and AUC values.Citation27 In our study, BVCALG after intrathecal administration presented smaller AUC (AUC0–480). We believe that these results were produced by the immense differences observed in the plasma concentrations of BVC with and without the nanoparticles, mainly in the beginning of the curve. Thus, the encapsulation of BVC in these nanoparticles avoided peak plasma concentrations that are usually related to adverse side effects after LA administration.Citation2,Citation13 We observed the Vd of BVC after only one extravascular administration and we did not measure the tissue concentration of BVC. Nevertheless, it is expected that a drug extensively bound to tissue will generally have an apparent large Vd, which means that probably BVCALG presented a higher affinity for tissue binding and was not available in the plasma (demonstrated by the lower AUC values).
Usually, the efficacy of LA is demonstrated based on their antinociception activity. However, in animal models, like the one used in our study, this can be difficult to achieve. The degree of pain in rabbits can vary importantly between animals and there are no objective criteria for this evaluation. As a prey species, rabbits may hide their pain by remaining motionless. Thus, rabbits appear to respond to pain in a manner contrary to that of mice or rats, and have little activity or behavior to be assessed.Citation28 The lack of pain behavior in rabbits lead to the use of infraorbital nerve blockade to determine the LA formulations efficacy. Also, we selected the infraorbital nerve block to simulate a condition similar to administration of these drugs in the maxillary bone, since our goal was to evaluate a new LA formulation that can be used in both dentistry and medicine. The BVCALG increased the duration of sensory blockade and the intensity of the total analgesic effect was improved by ~1.4-fold. These results corroborate the findings of Grillo et al,Citation4 when the same formulation led to increased analgesia in a mice sciatic nerve blockade. Thus, the new BVC formulations presented a more intense antinociceptive effect after infraorbital nerve blockade.
Evaluation of local toxicity is important to ensure the safeness of new drug delivery systems. The polymeric nanoparticles used in our study are made of natural polymers (alginate and chitosan) that interact to produce a nanoparticle system.Citation4 Despite the natural origin of these polymers, our results showed that NPALG produced an intense inflammatory reaction on the oral mucosa after 24 hours (), and this reaction was more intense when compared with BVC and BVCALG (). Previously results from Grillo et alCitation4 also showed that NPALG reduced the cell viability when compared with the negative control group in tests performed with Balb/c fibroblasts (3T3 cells). On the other hand, BVC significantly reduced the local inflammatory reaction evoked by NPALG in our study as seen in the BVCALG group. Our results could be explained by the anti-inflammatory properties of LA in clinical concentrations.Citation29–Citation31 LAs possess intrinsic anti-inflammatory properties with mechanisms that are not completely elucidated, but diverge from the sodium channels blockade. Among other properties, LA can reduce the synthesis of interleukin (IL)-1α, IL-1β, IL-2, IL-8, tumor necrosis factor-α, and interferon-ϒ.Citation32
Preclinical evaluation is an important (and mandatory) part of new formulations development, since the in vitro results may not be replicable during in vivo studies. This study showed a preclinical evaluation of new polymeric alginate-based nanoparticles with BVC for use in dentistry and medicine. The in vivo intraoral pharmacokinetics did not occur such as the in vitro release test performed earlier by Grillo et al.Citation4 Intrathecal injection of BVCALG promotes slower systemic absorption when compared with BVC. Also, our results showed that this formulation prolonged the LA effect after the infraorbital nerve block and induced tissue reaction comparable to the commercial formulation of BVC. These results encourage the use of this new formulation in dentistry and medicine as a safe and effective option for local anesthesia.
Acknowledgments
The authors thank Cristália Produtos Quim Farm Ltda (São Paulo, Brazil) for the donation of BVC and FAPESP (2014/14457-5) for the financial support. The authors also thank Mr Edvaldo C Coelho for his contribution in the pharmacokinetics analysis.
Disclosure
The authors report no conflicts of interest in this work.
References
- ShiptonEANew formulations of local anaesthetics – part IAnesthesiol Res Pract2012201254640922190922
- de PaulaECeredaCMFracetoLFMicro and nanosystems for delivering local anestheticsExpert Opin Drug Deliv201291505152423140102
- VerlindeMHollmannMWStevensMFHermannsHWerdehausenRLirkPLocal anesthetic-induced neurotoxicityInt J Mol Sci20161733926959012
- GrilloRde MeloNFde AraujoDRde PaulaERosaAHFracetoLFPolymeric alginate nanoparticles containing the local anesthetic bupivacaineJ Drug Target20101868869920196632
- LeoneSDi CianniSCasatiAFanelliGPharmacology, toxicology, and clinical use of new long acting local anesthetics, ropivacaine and levobupivacaineActa Biomed2008799210518788503
- PacellaEPacellaFTroisiFEfficacy and safety of 0.5% levobupivacaine versus 0.5% bupivacaine for peribulbar anesthesiaClin Ophthalmol2013792793223723684
- GulecDKarsliBErtugrulFBigatZKayacanNIntrathecal bupivacaine or levobupivacaine: which should be used for elderly patients?J Int Med Res20144237638524595149
- Bozdogan OzyilkanNKocumASenerMComparison of intrathecal levobupivacaine combined with sufentanil, fentanyl, or placebo for elective caesarean section: a prospective, randomized, double-blind, controlled studyCurr Ther Res Clin Exp201375647024465046
- MisirliogluKSivrikayaGHanciAYalcinkayaAIntrathecal low-dose levobupivacaine and bupivacaine combined with fentanyl in a randomised controlled study for caesarean section: blockade characteristics, maternal and neonatal effectsHippokratia20131726226724470739
- AraujoDRBragaAFMoraesCMFracetoLFPaulaEMistura com excesso enantiomérico de 50% (S75–R25) de bupivacaína complexada com ciclodextrinas e anestesia por via subaracnóidea em ratos [Complexation of 50% enantiomeric excess (S75–R25) bupivacaine with cyclodextrins and spinal block anesthesia in rats]Rev Bras Anestesiol200656495506 Portuguese19468595
- AraujoDRFracetoLFBraga AdeFPaulaESistemas de liberação controlada com bupivacaína racêmica (S50-R50) e mistura enantiomérica de bupivacaína (S75-R25): efeitos da complexação com ciclodextrinas no bloqueio do nervo ciático em camundongos [Drug-delivery systems for racemic bupivacaine (S50–R50) and bupivacaine enantiomeric mixture (S75–R25): cyclodextrins complexation effects on sciatic nerve blockade in mice]Rev Bras Anestesiol200555316328 Portuguese19471836
- DillaneDFinucaneBTLocal anesthetic systemic toxicityCan J Anaesth20105736838020151342
- TofoliGRCeredaCMAraujoDRPharmacokinetic study of liposome-encapsulated and plain mepivacaine formulations injected intraorally in volunteersJ Pharm Pharmacol20126439740322309271
- SeolDMagnettaMJRamakrishnanPSBiocompatibility and preclinical feasibility tests of a temperature-sensitive hydrogel for the purpose of surgical wound pain control and cartilage repairJ Biomed Mater Res B Appl Biomater20131011508151524591226
- BarringtonJWOlugbodeOLovaldSOngKWatsonHEmersonRHJrLiposomal bupivacaine: a comparative study of more than 1000 total joint arthroplasty casesOrthop Clin North Am20154646947726410636
- Ratajczak-EnselmeMEstebeJPRoseFXEffect of epinephrine on epidural, intrathecal and plasma pharmacokinetics of ropivacaine and bupivacaine in sheepBr J Anaesth20079988189017959589
- CeredaCMBrunettoGBde AraújoDRde PaulaELiposomal formulations of prilocaine, lidocaine and mepivacaine prolong analgesic durationCan J Anaesth20065310921097
- de AraujoDRCeredaCMBrunettoGBPharmacological and local toxicity studies of a liposomal formulation for the novel local anaesthetic ropivacaineJ Pharm Pharmacol2008601449145718957165
- CeredaCMTófoliGRde Brito JuniorRBStability and local toxicity evaluation of a liposomal prilocaine formulationJ Liposome Res20081832933918991066
- TofoliGRCeredaCMde AraujoDRPharmacokinetic and local toxicity studies of liposome-encapsulated and plain mepivacaine solutions in ratsDrug Deliv201017687620070242
- Le CorrePEstèbeJPClémentRSpray-dryed bupivacaine-loaded microspheres: in vitro evaluation and biopharmaceutics of bupivacaine following brachial plexus administration in sheepInt J Pharm200223819120311996823
- DavidsonEMBarenholzYCohenRHaroutiunianSKaganLGinosarYHigh-dose bupivacaine remotely loaded into multivesicular liposomes demonstrates slow drug release without systemic toxic plasma concentrations after subcutaneous administration in humansAnesth Analg20101101018102320357145
- BramlettKOnelEViscusiERJonesKA randomized, double-blind, dose-ranging study comparing wound infiltration of DepoFoam bupivacaine, an extended-release liposomal bupivacaine, to bupivacaine HCl for postsurgical analgesia in total knee arthroplastyKnee20121953053622285545
- Removal of blood from laboratory mammals and birdsFirst report of the BVA/FRAME/RSPCA/UFAW Joint Working Group on RefinementLab Anim1993271228437430
- RosenbergPHVeeringBTUrmeyWFMaximum recommended doses of local anesthetics: a multifactorial conceptReg Anesth Pain Med200429564575 discussion 52415635516
- MalinovskyJMCharlesFBaudrimontMIntrathecal ropivacaine in rabbits: pharmacodynamic and neurotoxicologic studyAnesthesiology20029742943512151934
- PapiniJZBCeredaCMSPedrazzoli JúniorJCalafattiSAde AraújoDRTofoliGRPharmacokinetics and pharmacodynamics evaluation of tramadol in thermoreversible gelsBiomed Res Int20172017595462928819627
- BarterLSRabbit analgesiaVet Clin North Am Exot Anim Pract2011149310421074705
- CassutoJSinclairRBonderovicMAnti-inflammatory properties of local anesthetics and their present and potential clinical implicationsActa Anaesthesiol Scand20065026528216480459
- Votta-VelisEGPiegelerTMinshallRDRegional anaesthesia and cancer metastases: the implication of local anaestheticsActa Anaesthesiol Scand2013571211122924134442
- XuanWHankinJZhaoHYaoSMaDThe potential benefits of the use of regional anesthesia in cancer patientsInt J Cancer20151372774278425359704
- GrosuILavand’hommePContinuous regional anesthesia and inflammation: a new targetMinerva Anestesiol2015811001100925317576
