Abstract
Phantom-limb pain (PLP) belongs among difficult-to-treat chronic pain syndromes. Treatment options for PLP are to a large degree implicated by the level of understanding the mechanisms and nature of PLP. Research and clinical findings acknowledge the neuropathic nature of PLP and also suggest that both peripheral as well as central mechanisms, including neuroplastic changes in central nervous system, can contribute to PLP. Neuroimaging studies in PLP have indicated a relation between PLP and the neuroplastic changes. Further, it has been shown that the pathological neuroplastic changes could be reverted, and there is a parallel between an improvement (reversal) of the neuroplastic changes in PLP and pain relief. These findings facilitated explorations of novel neuromodulatory treatment strategies, adding to the variety of treatment approaches in PLP. Overall, available treatment options in PLP include pharmacological treatment, supportive non-pharmacological non-invasive strategies (eg, neuromodulation using transcranial magnetic stimulation, visual feedback therapy, or motor imagery; peripheral transcutaneous electrical nerve stimulation, physical therapy, reflexology, or various psychotherapeutic approaches), and invasive treatment strategies (eg, surgical destructive procedures, nerve blocks, or invasive neuromodulation using deep brain stimulation, motor cortex stimulation, or spinal cord stimulation). Venues of further development in PLP management include a technological and methodological improvement of existing treatment methods, an implementation of new techniques and products, and a development of new treatment approaches.
Introduction
It is estimated that more than 80% of patients with partial or total loss of a limb develop chronic phantom-limb pain (PLP), ie, pain that seems to be located in the missing limb.Citation1 For the purposes of clinical assessment and treatment, it is important to differentiate PLP from other amputation-related phenomena, such as stump-pain, (ie, pain in the remaining part of the limb),Citation2 non-painful phantom-limb sensation, or telescoping, ie, sensation when the distal part of the phantom is gradually felt to approach the residual limb and may even be experienced within the stump.Citation3 Risks factors for PLPCitation4–Citation8 include gender (PLP being more common in women), upper extremity amputation, presence of pre-amputation pain, residual pain in remaining limb, or time after amputation (there are reports of a two-peak period of onset, the first within a month after amputation and the second after 1 year).Citation8 Further, stress, anxiety, depression, and other emotional triggers also highly likely contribute to PLP.Citation7 Recommended preventive measures include using preemptive and postoperative perineural or epidural local anesthetics, intraoperative precautions related to protection of the peripheral nerves, and importance of early rehabilitation.Citation9,Citation10
Treatment options for PLP are, to a large degree, implicated by the level of understanding the mechanisms and nature of PLP. In a historical perspective, PLP had been considered mostly of psychological origin, with the prevalent belief that PLP was generated “in the patient’s head.” However, the development of advanced diagnostic methods, recently including neuroimaging, has facilitated explorations of changes in peripheral and central neural networks after amputation and their putative contribution to the development of PLP.Citation11–Citation13 The findings acknowledged the neuropathic nature of PLP and also suggested that both peripheral, as well as central mechanisms, including neuroplastic changes in central nervous system, can contribute to PLP.Citation14,Citation15 In periphery, axotomized afferent neurons may develop retrograde degeneration and shrinking that primarily involves unmyelinated neurons, and local changes at the site of the remaining nerve endings may contribute to the development of enhanced sensitivity of the afferent fibers.Citation16 Further, a sprouting of injured axon can lead to formation of neuromas in the residual limb. Neuromas show abnormal sensitivity to mechanical and chemical stimuli and can be a source of abnormal ectopic discharges.Citation16,Citation17 Besides neuromas, dorsal root ganglions have also been shown to generate the discharge of ectopic activity,Citation18 substantially increasing the overall barrage of abnormal afferent input to the spinal cord and upper centers, such as the brainstem, thalamus, or cortex. Further, post-amputation changes at the level of the spinal cord include formation of new neural connections between the axonal sprouts from the proximal part of the amputated nerve and spinal cord neurons, which contributes to sensitization of pain-transmission neurons. The sensitization may manifest itself as mechanical hyperalgesia and an expansion of peripheral receptive fields,Citation19 and is linked to an increase of activity on NMDA receptors mediated for example by substance P, neurokinins, and tachykinins at the dorsal horns of the spinal cord.Citation20 There also may be a reduction in the local intersegmental inhibitory mechanisms, resulting in spinal disinhibition and enhanced nociceptive input to the supraspinal centers. Interestingly, a pathological change of the afferent input (whether it is an increase or decrease) represents a significant source of neural plasticity, ie, dynamic changes in the function of neurons and neural networks in the central nervous system, including both spinal and cerebral parts of the Pain Matrix, the pain processing network.Citation18,Citation21 Neuroplastic alterations at the level of the brain include changes in neuronal excitability, changes in the somatotopic organization in the cortical and subcortical areas (eg, in the somatosensory and motor cortices or in thalamus), and structural changes (thickening or thinning) of neuronal layers. Indeed, neuroimaging studies in PLP have shown presence of such neuroplastic changes in amputees and the relation to the presence of PLP.Citation11–Citation13,Citation22–Citation25 Further, it has been shown that the pathological neuroplastic changes can be reverted and that an improvement (reversal) of the neuroplastic changes in PLP patients was paralleled by pain relief.Citation13 These findings facilitated explorations of novel neuromodulatory treatment strategies, such as transcranial magnetic stimulation (TMS) or motor imagery, adding to the variety of treatment approaches in PLP.
Overall, treatment options in PLP include pharmacological treatment, supportive non-pharmacological non-invasive strategies, and invasive treatments.
Pharmacological treatment
The pharmacological armamentarium is vast,Citation26–Citation49 (), but efficacy of most of the drugs utilized to treat PLP have not been determined from PLP controlled trials, but rather extrapolated from positive results obtained on a variety of other neuropathic pain syndromes (eg, painful polyneuropathy [PPN] or post-herpetic neuralgia [PHN]).
Table 1 Level of evidenceTable Footnotea for the drugs utilized to treat phantom-limb pain and other neuropathic-pain conditions
Acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs)
A cross-sectional study found acetaminophen and NSAIDs to be the most common medications used in PLP.Citation4 This widely-used over-the-counter medication has relatively little anti-inflammatory activity as compared to NSAIDs. However, acetaminophen and other NSAIDs all act by the same mechanism (inhibition of prostaglandin synthesis) and all show varying levels of analgesic, anti-inflammatory, antipyretic, and antiplatelet actions, and decrease the nociception at peripheral and central level.Citation28
Antidepressants
Antidepressants have been utilized for the management of neuropathic pain since the eighties after work by Mitchel Max showed analgesic effect by amitriptilyne independent of improvement of depression. Since then many drugs have been added to the list (), with different degrees of success.Citation26,Citation29,Citation30
Tricyclics are on top of the list for their efficacy for the management of neuropathic pain syndromes, but the results for the management of PLP are mixed. Due to the availability of newer agents with similar efficacy and better profile of side effects the tricyclics have been less utilized in the last few years. The anticholinergic effect presented by these drugs is responsible for the major side effects including sedation, constipation, urinary retention, and electrical conduction changes that made them potentially problematic in the elderly. Sometimes the side effects can help to manage comorbidities and a night dose of amitriptyline might be preferable for patients with insomnia. The serotonin-norepinephrine reuptake inhibitors (SNRIs) are antidepressants that block the reuptake of serotonin and norepinephrine (eg, duloxetine) and although they have become popular due to a better profile of side effects and similar good efficacy for neuropathic pain, migraines, and fibromyalgia, the level of evidence for the management of PLP is low (). Milnacipran, in the United States, has been approved for the management of fibromyalgia, but although the level of evidence to treat PLP is low, there are studies that show benefit in neuropathic pain.Citation31 Agents with a preferentially dopaminergic mechanism of action like bupropion have also been shown to be beneficial for the management of neuropathic pain. The selective serotonin reuptake inhibitors (SSRIs) are considered to be less effective but there are positive controlled trials in PPN for escitalopram, fluoxetine, and paroxetine ().Citation26
Anticonvulsants
Some anticonvulsants, like gabapentin and pregabalin, have been shown to be efficacious for the treatment of PLP in controlled trials.Citation26,Citation32,Citation33 The list grows when the evidence of efficacy is looked at on other types of neuropathic pain like painful peripheral neuropathy and post-herpetic neuralgia and includes gabapentin, pregabalin, carbamazepine, lamotrigine, phenytoin, topiramate, and valproate (). Pain with neuralgic characteristics that can occur at the level of the stump, can be successfully treated with the older agents like carbamazepine or the newer oxcarbazepine.
Sodium channel blockers
Sodium channel blockers can be administered orally, intravenously, epidurally, or intrathecally. The evidence that supports its use varies with the agent and the formulation. The only controlled trial that supports its use for PLP is with injectable bupivacaine. A study with lidocaine infusion was negative for PLP and another positive for PPN, while there are no positive trials for the oral formulation mexiletine ().Citation29
NMDA receptor antagonists
Intravenous infusion of ketamine, an NMDA receptor antagonist, has been shown to be beneficial for PLP but other agents of this family have not been consistently shown to be efficacious. More recently it has been utilized to manage pain that does not respond to other agents as a single intravenous infusion or repetitive infusions as outpatient. Many clinicians treat patients that experience at least 50% pain reduction with the infusion, with oral ketamine that can be compounded.Citation26 The long term use is in many occasions not possible due to side effects. There are mixed case reports with memantine, another NMDA antagonist that was developed for the management of dementia in Alzheimer’s disease, for PLP and positive and negative control trials for other forms of neuropathic pain. There are positive control trials with dextromethorphan for PPN and a negative trial for PHN.
Opioids
Morphine has been shown to be beneficial for the management of PLP in a controlled trial and there is case series showing analgesia with methadone and a case report with buprenorphine reporting analgesia.Citation29,Citation38 In addition morphine, oxycodone, levorphanol, and methadone are efficacious for the management of other forms of neuropathic pain including PPN and PHN. The long term use of these drugs has been questioned due to the scarce evidence of long term benefits including function. Concerns about addiction, diversion, and side effects including decrease in testosterone level have also been raised. Despite this, the use of opioids for the management of neuropathic pain has been supported in several guidelines including the American Pain Society (APS) and the American Academy of Pain Medicine (AAPM). Methadone has received a differential treatment due to the additional concern about cardiac toxicity and the Canadian guidelines place it at a lower step than the other opioids.
Tramadol
There are positive clinical trials with tramadol supporting its use for the management of PLP and other forms of neuropathic pain including PPN and PHN.Citation29 Tramadol is an agent with a mixed mechanism of action that includes low affinity for the mu opioid receptors and the ability to blockade the reuptake of norepinerpine and serotonin. It can also produce physical dependency but it is Schedule 3 and this is one of the reasons why it is popular among prescribers.
Others
There are other drugs that have been utilized with variable degree of success, including muscle relaxants like baclofen, the centrally acting benzodiazepines, and the corticosteroids. There is a case report for baclofen in PLP that reports benefit, a positive controlled trial in PHN, a positive controlled trial for diazepam, and an open label trial for alprazolam for the management of PPN. There are case series that report benefit with corticosteroids like prednisone dexamethasone for PPN. Capsaicin has been utilized for neuropathic pain with variable success for many years, which has not been very popular due to the pain that occurs after its application. There is a new formulation in a patch form that seems to be promising, although there is no data available yet for PLP ().
In summary, there is a limited number of control studies done specifically to assess drug efficacy in PLP.Citation26 The ones showing significant beneficial effect include trials with amitriptyline,Citation30 gabapentin,Citation32 tramadol,Citation40 and morphine.Citation33 There are also some case reports/series with mirtazapine,Citation41 duloxetine,Citation33 milnacipran,Citation31 memantine,Citation34,Citation35 baclofen,Citation36 buprenorphine,Citation37 and methadone,Citation38 that suggest beneficial effects on PLP. The selection of the agent should be based on the current understanding of PLP as a neuropathic type of condition. Hence, in addition to drugs that show efficacy in PLP, other drugs with efficacy in other types of neuropathic pain could be prescribed.Citation38 However, the drug selection should take into consideration the clinical presentation of the PLP syndrome. PLP is continuous, intense, sometimes burning and for that reason a drug with efficacy in PPN or PHN may be chosen. Further, as a neuralgic type of pain may be present in PLP (possibly a result of a neuroma formation in the stump), drugs efficacious in neuralgic type of pain, like carbamazepine or oxcarbamazepine, can be added to the regimen. A trial in every patient should include appropriate titration of the drug up to levels where efficacy has been shown in other neuropathic conditions, for at least 4–6 weeks. Thus, completion of an appropriate trial usually takes 1–2 months.Citation39
It has been proposed that the administration of analgesic and anesthetics prior to a surgical interventionCitation9,Citation42 and intraoperative precautions with the handling of peripheral nerves and not to ligate with sutures, could prevent the development of central sensitization due to impulses generated at the level of the amputation, however the results have been mixed. Epidural analgesia, ropivacaine, and patient controlled analgesia during the perioperative period have shown to decrease PLP, but ketamine and ketamine plus bupivacaine showed conflicting results.Citation4,Citation44,Citation45
As PLP is often of severe intensity, causing a long-lasting and debilitating decrease of quality of life in many amputees, Citation48 pharmacological treatment itself in many patients does not provide sufficient pain relief. Therefore, supportive treatments may enhance the treatment regimen.
Supportive non-invasive treatment approaches
Non-invasive neuromodulation
Several approaches are available within non-invasive neuromodulation in PLP, for example repetitive transcranial magnetic stimulation (rTMS), visual feedback (mirror-box therapy), or motor imagery.
Transcranial magnetic stimulation (TMS)
TMS exerts its effects on brain structures via electrical currents induced by a powerful magnetic field delivered with a magnetic coil over the scalp.Citation49 It can be delivered as a single pulse or as sets of pulses (rTMS). Several studies have shown that a single session of rTMS can transiently relieve pain in some patients with chronic neuropathic pain,Citation50–Citation54 and a multiple application on several consecutive days can lead to prolongation of the effects.Citation55,Citation56 However, results from rTMS treatment specifically in patients with PLP are anecdotal, based on case-reports but not sham-controlled trials, and therefore not conclusive.Citation55–Citation57 Although the application of rTMS in PLP patients resulted in pain relief,Citation55–Citation57 short-term durability of the effects is a significant issue limiting clinical use of this treatment.
Visual feedback therapy and motor imagery
Both the visual feedback therapy (mirror-box therapy) and motor imagery are based on behaviorally relevant sensory-motor stimulation of the stump.Citation58–Citation62 A rationale of this approach arises from the existence of maladaptive neuroplastic changes underlying PLP, specifically cortical reorganization in the somatosensory and motor cortices, a relation between the cortical reorganization and the occurrence of PLP, and beneficial effects of cortical normalization on PLP.
The treatment using visual feedback emerged from the observation that looking at the reflection of an intact limb in a mirror box can induce sensations of movement in the phantom limb.Citation59–Citation65 Motor imagery is based on percepts or sensations generated internally by the brain, a mental representation of an actual sensation or movement.Citation58 Physiological studies have shown that both the mirror-box therapy and the motor imagery resulted in increased excitability of the corticospinal pathways.Citation64 This at least partially depends on the so-called mirror-neuron systemCitation61 which includes neurons that are active not only during the execution of the task itself but also during the observation of the task. This finding provides support for the idea that imagined movements share the same cortical pathways as executed motor tasks. Further, it has been shown that visual feedback actually dominates somatosensory feedback and sensory experiences can be evoked by visual information alone, and it has been shown that the visual system enhances tactile sensitivity.Citation61,Citation65
Both the mirror therapy and the motor imagery can be very beneficial in PLP patients.Citation58,Citation60,Citation62,Citation63,Citation66–Citation68 As an example, a controlled neuroimaging study of motor imagery in PLPCitation58 showed evidence of cortical reorganization of motor and somatosensory cortices and its correlation with patients’ pain scores prior to the motor imagery training. The training resulted in a significant decrease of intensity and unpleasantness of pain which correlated with reduction (improvement) of cortical reorganization.
Overall, the mirror-box therapy and motor imagery are safe and non-expensive additions to the PLP treatment options.
Other supportive non-invasive therapies
There is preliminary evidence that PLP patients may benefit from other non-invasive supportive treatments, such as physical therapy including the stump and phantom limb, reflexology or hypnosis,Citation69,Citation70 or peripheral transcutaneous electrical nerve stimulation of the affected limb.Citation71 Although there are multiple reports of transcutaneous electrical nerve stimulation use in PLP, a recent systematic reviewCitation72 revealed lack of evidence from randomized controlled trials on which effectiveness of transcutaneous electrical nerve stimulation for PLP could be judged.
Further, a use of myoelectric prosthesis may improve PLP.Citation73,Citation74 Research of stimulation-induced neural plasticity indicate that extensive behaviorally relevant stimulation of the affected part of the body leads to an expansion of its representation zone, and an intensive use of a myoelectric prosthesis has been shown to be positively linked to decreased PLP as well as decreased degree of cortical reorganization.Citation75 Another venue of non-invasive support to PLP patients includes various psychotherapeutic approaches.Citation76
Invasive treatment strategies
Surgical destructive interventions and nerve blocks
Because of a high rate of refractory chronic pain among PLP patients with failed non-invasive treatments, all kinds of invasive procedures were performed in the history of those cases.Citation77–Citation93 Destructive procedures like thermal nerve root destruction, rhizotomy, spinal ganglionectomy, and also dorsal root entry zone lesion have been performed.Citation83–Citation93 As per their nature, most of these procedures led to unrestorable damage of nervous tissue and functions. Initial results with high rates of pain relief were accompanied with a high rate of complications and followed by a high rate of recurrent pain and therefore yielding disappointing long term results after several months. Nowadays, the indication for destructive procedures is limited only to a few diagnoses for severe, refractory pain patients with short life expectancy. The dorsal root entry zone lesion is the only destructive procedure for selected patients with deafferentation pain and brachial plexus avulsion or cervical nerve root injury. In total, numbers of destructive procedures are decreasing year by year.
Non-destructive interventions such as nerve blocks have also been used in the PLP treatment. For example, interscalene blocks or stellate ganglion blocks for upper extremity PLP, or lumbar sympathetic blocks for lower extremity PLP can lead to decrease of PLP.Citation95 These blocks are often combined with physical therapy.
Invasive neuromodulation
Invasive neuromodulation is considered the last-resort treatment for patients who failed various trials of non-invasive treatments. On contrary to surgical destructive procedures, neuromodulatory techniques are PLP-mechanisms-driven, specifically addressing maladaptive central neuroplastic changes in the Pain Matrix (brain pain-processing networks).
Deep brain stimulation (DBS)
DBS is an electrical stimulation performed after stereotactic implantation of thin stick leads into subcortical areas such as the thalamus or basal ganglia. In the early 1950s, the first clinicians used thalamic stimulation to treat chronic pain patients.Citation78–Citation83 Since these first clinical applications a lot of case series, retrospective patient samples, and reviews were published.Citation81,Citation82,Citation88–Citation91,Citation93,Citation96,Citation97 A recent reviewCitation98 lists a total of 600 patients with DBS due to different chronic pain syndromes. Evidence up to date suggests that the significance of DBS for PLP is controversial. However, some patients clearly benefit from DBS, experiencing long term pain relief >25% and improved quality of life.Citation97
Motor cortex stimulation (MCS)
MCS () is an electrical stimulation of the precentral gyrus using epidural surgical leads and subthreshold stimulation.Citation99–Citation103
Figure 1 Lateral x-ray of the skull documenting the position of the implanted paddle leads. Matching of postoperative ct-scan and preoperative MRI-3D-neuronavigation data with inserted position of the leads over the pre- and postcentral gyrus.
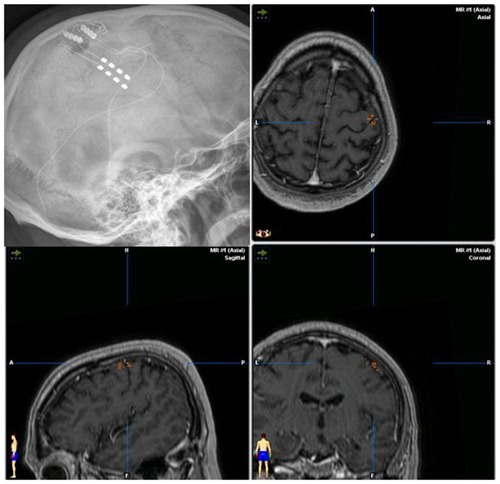
Historically, after the application of MCS in patients with post-stroke-pain or trigeminal neuropathic pain, also patients with PLP became candidates for the MCS treatment.Citation98,Citation104–Citation107 Some centers perform a direct implantation of the neuro-stimulator in the subcutaneous tissue (infraclavicular or abdominal), while others perform also a postoperative testing with externalized extensions of the lead and an external stimulation.Citation98,Citation106,Citation108,Citation109 Such a testing phase allows for a placebo double-blinded stimulation period, so that false positive responders can be identified.Citation106 It has been suggestedCitation106 that the definite implantation of neurostimulator would be performed only in patients with a positive effect of the real MCS and a pain reduction of >30%, and the subjective impression of pain improvement by the patient. Information from different centers and expertsCitation98,Citation105,Citation109,Citation110 show that PLP is an accepted indication for MCS. PLP of the upper limb seems to be favorable due to the large representation on the convex part of the precentral gyrus, but interhemispheral lead implantation for the lower limb has also been reported, and a review of evidenceCitation98 suggests that MCS yields favorable results in about 53% of PLP patients.
Spinal cord stimulation (SCS)
SCS involves the placement of electrodes in the epidural space adjacent to the spinal area presumed to be the source of pain. An electric current is then applied to achieve sympatholytic and other neuromodulatory effects.Citation111 Typically, the first phase of treatment involves the temporary placement of an electrical stimulator. Patients are monitored over a period of time to determine the pain reduction. Only patients positively responding to the stimulation would be considered for a permanent implantation. Clinical results indicate beneficial effects of SCS in PLP patients on immediate as well as long term outcomes,Citation112,Citation113 although the percentage of benefiting patients declined with time. For example, good results have been observed at 2 year follow-up in 52.4% of 64 PLP patients, that decreased to 39% at 5 year follow-up.Citation113 However, given the fact that PLP is a difficult-to-treat pain syndrome and many PLP patients do not respond to other treatment strategies, SCS represents a promising treatment option for selected subpopulation of patients. Similarly to other invasive treatments, such as MCS or DBS, SCS is also reserved for patients that had not benefited from non-invasive treatment strategies.
Conclusions and future directions
Although some of the currently available therapies yield promising results, many PLP patients still remain without satisfactory pain relief. Therefore, continual advances in the PLP treatment are of high importance. It can be anticipated that pain management in PLP patients may in the future benefit from the following venues:
Technological and methodological improvement of existing treatment methods.
Improved formulations of pharmacological agents, such as long-acting formulations or new routes of applications, may improve pain relief and overall quality of life in patients with PLP. Further, as discussed in other sections of this article, efficacy of various neuropathicpain medications has not yet been established for PLP. Establishing the drug efficacy specifically for PLP can certainly facilitate a patient-tailored selection of more-benefiting pharmacological regimens for PLP patients.
Further, it is anticipated that an improvement of invasive DBS and MCS treatments will be facilitated by technological advances of imaging techniques, as higher resolution would provide more accurate information about the relevant brain structures. Furthermore, technological advances in the detection of pain-related electrical signals in the brain or at its surface would enable more specific and target-oriented DBS and MCS.
Implementation of new methods and products within current PLP treatment-strategies.
Besides the fast progress of the pharmaceutical industry and implementation of new agents, also non-pharmacological treatment strategies in PLP may enrich their repertoire of available methods. For example, within the non-invasive neuromodulatory strategy, transcranial direct current stimulationCitation114 that has recently shown promising clinical potential in some neuropathic pain syndromes, may be in the future explored in the treatment of PLP. As transcranial direct current stimulation yielded pain relief in various patient-populations (eg, patients with fibromyalgia, Parkinson’s disease, pelvic pain, central pain due to spinal cord injuries),Citation115–Citation119 it can be anticipated that PLP patients as well may benefit from this method. Further, a wider implementation of the use of myoelectric prosthesis in clinical practice may enrich PLP management in the future. Behaviorally relevant stimulation of the stump delivered via myoelectric prosthesis addresses cortical reorganizationCitation75 has been identified among pathological mechanisms underlying PLP.
Development of new treatment approaches.
Pain research incorporating new perspectives, such as genetics and epi-genetics, provides an exciting opportunity to enhance the insight into the mechanisms and development of chronic pain syndromes, including PLP, and to provide an evidence base for the development of novel treatment approaches and targets. For example, recent studies indicate that gene CACNG2, which is expressed in the peripheral and central nervous system and encodes neuroprotein stargazin, may be implicated in the development neuropathic pain. If confirmed by future studies, the gene may be of high significance for PLP. Understanding genetic and epigenetic factors (ie, functionally relevant modifications to the genome that do not involve a change in the nucleotide sequence) in the development of PLP may lead to exploration of epigenetic pharmaceuticals for this difficult-to-treat condition, as recently witnessed in other areas of medicine, for example in oncology.Citation120
Disclosure
The authors report no conflicts of interest in this work.
References
- KooijmanCMDijkstraPUGeertzenJHElzingaAvan der SchansCPPhantom pain and phantom sensations in upper limb amputees: an epidemiological studyPain2000871334110863043
- JensenTSKrebsBNielsenJRasmussenPNon-painful phantom limb phenomena in amputees: incidence, clinical characteristics and temporal courseActa Neurol Scand19847064074146516789
- NikolajsenLJensenTSPhantom limb painBr J Anaesth200187110711611460799
- SubediBGrossbergGTPhantom limb pain: mechanisms and treatment approachesPain Res Treat20112011861605
- HirshATDillworthTMEhdeDMJensenMPSex differences in pain and psychological functioning in persons with limb lossJ Pain2010111798619734105
- BosmansJCGeertzenJHPostWJvan der SchansCPDijkstraPUFactors associated with phantom limb pain: a 3 1/2 year prospective studyClin Rehab2010245444453
- EphraimPLWegenerSTMacKenzieEJDillinghamTRPezzinLEPhantom pain, residual limb pain, and back pain in amputees: results of a national surveyArch Phys Med Rehabil200586101910191916213230
- SchleyMTWilmsPToepfnerSPainful and non-painful phantom and stump sensations in acute traumatic amputeesJ Trauma200865485886418849803
- BorghiBD’AddabboMWhitePFThe use of prolonged peripheral neural blockade after lower extremity amputation: the effect on symptoms associated with phantom limb syndromeAnesth analg201011151308131520881281
- KaranikolasMArethaDTsolakisIOptimized perioperative analgesia reduces chronic phantom limb pain intensity, prevalence, and frequency: a prospective, randomized, clinical trialAnesthesiology201111451144115421368651
- CohenLGBandinelliSFindleyTWHallettMMotor reorganization after upper limb amputation in man. A study with focal magnetic stimulationBrain1991114Pt 1B6156272004259
- FlorHElbertTKnechtSPhantom-limb pain as a correlate of cortical reorganization following arm amputationNature199537565314824847777055
- BirbaumerNLutzenbergerWMontoyaPeffects of regional anesthesia on phantom limb pain are mirrored in changes in cortical reorganizationJ Neurosci19971714550355089204932
- WeeksSRAnderson-BarnesVCTsaoJWPhantom limb pain: theories and therapiesNeurologist201016527728620827116
- FlorHPhantom-limb pain: characteristics, causes, and treatmentsLancet Neurol20021318218912849487
- NystromBHagbarthKEMicroelectrode recordings from trans-sected nerves in amputees with phantom limb painNeurosci Lett19812722112167322453
- WallPDGutnickMOngoing activity in peripheral nerves: the physiology and pharmacology of impulses originating from a neuromaExp Neurol19744335805934827166
- WolpawJRTennissenAActivity-dependent spinal cord plasticity in health and diseaseAnnu Rev Neurosci20012480784311520919
- DoubellTPMannionRJWoolfCJThe dorsal horn: state dependent sensory processing, plasticity and the generation of painWallPDMelzackRTextbook of Pain4th edEdinburgh, UKChurchill Livingstone1999165181
- BaronRMechanisms of disease: neuropathic pain – a clinical perspectiveNat Clin Pract Neurol2006229510616932531
- BuonomanoDVMerzenichMCortical plasticity: from synapse to mapsAnnu Rev Neurosci1998211491869530495
- LotzeMMontoyaPErbMActivation of cortical and cerebellar motor areas during executed and imagined hand movements: an fMRI studyJ Cogn Neurosci199911549150110511638
- LotzeMFlorHGroddWLarbigWBirbaumerNPhantom movements and pain. An fMRI study in upper limb amputeesBrain2001124Pt 112268227711673327
- KarlABirbaumerNLutzenbergerWCohenLGFlorHReorganization of motor and somatosensory cortex in upper extremity amputees with phantom limb painJ Neurosci200121103609361811331390
- KarlAMühlnickelWKurthRFlorHNeuroelectric source imaging of steady-state movement-related cortical potentials in human upper extremity amputees with and without phantom limb painPain20041101–29010215275756
- AlviarMJHaleTDungcaMPharmacologic interventions for treating phantom limb painCochrane Database Syst Rev201112CD00638022161403
- O’ConnorABDworkinRHTreatment of neuropathic pain: an overview of recent guidelinesAm J Med200912210 Suppl 1S22S32
- ByrantBKnightsKSalernoEPharmacology for Health ProfessionalsAmsterdam, HollandElsevier2007270
- AttalNCruccuaGBaronREFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revisionEur J Neurol20101791113e8820402746
- RobinsonLRCzernieckiJMEhdeDMTrial of amitriptyline for relief of pain in amputees: results of a randomized controlled studyArch Phys Med Rehabil20048511614970960
- SatoKHiguchiHHishikawaYManagement of phantom limb pain and sensation with milnacipranJ Neuropsychiatry Clin Neurosci200820336818806244
- NikolajsenLFinnerupNBKrampSVimtrupASKellerJJensenTSA randomized study of the effects of gabapentin on postamputation painAnesthesiology200610551008101517065896
- SpiegelDRLappinenEGottliebMA presumed case of phantom limb pain treated successfully with duloxetine and pregabalinGen Hosp Psychiatry2010322228.e5228e720303003
- HackworthRJTokarzKAFowlerIMWallaceSCStedje-LarsenETProfound pain reduction after induction of memantine treatment in two patients with severe phantom limb painAnesth Analg200810741377137918806054
- SchleyMTopfnerSWiechKContinuous brachial plexus blockade in combination with the NMDA receptor antagonist memantine prevents phantom pain in acute traumatic upper limb amputeesEur J Pain200711329930816716615
- ZunigaRESchlichtCRAbramSEIntrathecal baclofen is analgesic in patients with chronic painAnesthesiology200092387688010719971
- OmoteKOhmoriHKawamataMMatsumotoMNamikiAIntrathecal buprenorphine in the treatment of phantom limb painAnesth Analg1995805103010327726400
- BergmansLSnijdelaarDGKatzJCrulBJMethadone for phantom limb painClin J Pain200218320320512048424
- CrucianiRAStradaEAKnotkovaHNeuropathic painBrueraEPortenoyRKCancer Pain – Assessment and ManagementNew York, NYCambridge University Press2010478505
- Wilder-SmithCHHillLTLaurentSPostamputation pain and sensory changes in treatment-naive patients: characteristics and responses to treatment with tramadol, amitriptyline, and placeboAnesthesiology2005103361962816129989
- KuikenTASchechtmanLHardenRNPhantom limb pain treatment with mirtazapine: a case seriesPain Pract20055435636017177770
- KaranikolasMArethaDTsolakisIOptimized perioperative analgesia reduces chronic phantom limb pain intensity, prevalence, and frequency: a prospective, randomized, clinical trialAnesthesiology201111451144115421368651
- RasmussenSKehletHManagement of nerves during leg amputation – a neglected area in our understanding of the pathogenesis of phantom limb painActa Anaesthesiol Scand20075181115111617697308
- HayesCArmstrong-BrownABurstalRPerioperative intravenous ketamine infusion for the prevention of persistent post-amputation pain: a randomized, controlled trialAnaesth Intens Care2004323330338
- WilsonJANimmoAFFleetwood-WalkerSMColvinLAA randomized double blind trial of the effect of preemptive epidural ketamine on persistent pain after lower limb amputationPain20081351–210811817583431
- FrommGHTerrenceCFChatthaASBaclofen in the treatment of trigeminal neuralgia: double-blind study and long-term follow upAnn Neurol19841532402446372646
- PandeyCKSinghNSinghPKGabapentin for refractory idiopathic trigeminal neuralgiaIndian Med Assoc20081062124125
- EhdeDMJensenMPEngelJTurnerJAHoffmanAJCardenasDDChronic pain secondary to disability: a reviewClin J Pain200319131712514452
- PerezMACohenLGPrinciples and mechanisms of transcranial direct current stimulationKnotkovaHCrucianiRAMerrrickJBrain Stimulation in the Treatment of PainNew York, NYNova Science Publishers Inc2010113129
- CanaveroSBonicalziVDottaMVighettiSAsteggianoGCocitoDTranscranial magnetic cortical stimulation relieves central painStereotact Funct Neurosurg2002783–419219612652043
- Andre-ObadiaNPeyronRMertensPMauguiereFLaurentBGarcia-LareaLTranscranial magnetic stimulation for pain control. Double-blind study of different frequencies against placebo, and correlation with motor cortex stimulation efficacyClin Neurophysiol200611771536154416753335
- LefaucheurJHatemSNinebASomatotopic organization of the analgesic effects of motor cortex rTMS in neuropathic painNeurology200667111998200417159107
- LefaucheurJPDrouotXMenard-LefaucheurINeurogenic pain relief by repetitive transcranial magnetic cortical stimulation depends on the origin and the site of painJ Neurol Neurosurg Psychiatry200475461261615026508
- HirayamaASaitohYKishimaHReduction of intractable deaf-ferentation pain by navigation-guided repetitive transcranial magnetic stimulation of the primary motor cortexPain20061221–2222716495011
- TopperRFoltysHMeisterIGSparingRBoroojerdiBRepetitive transcranial magnetic stimulation of the parietal cortex transiently ameliorates phantom limb pain-like syndromeClin Neurophysiol200311481521153012888036
- KhedrEMKotbHKamelNFAhmedMASadekRRothwellJCLonglasting antalgic effects of daily sessions of repetitive transcranial magnetic stimulation in central and peripheral neuropathic painJ Neurol Neurosurg Psychiatry200576683383815897507
- Di RolloAPallantiSPhantom limb pain: low frequency repetitive transcranial magnetic stimulation in unaffected hemisphereCase Report Med2011201113075121629848
- MacIverKLloydDMKellySRobertsNNurmikkoTPhantom limb pain, cortical reorganization and the therapeutic effects of mental imageryBrain2008131Pt 82181219118567624
- MacLachlanMMcDonaldDWalochJMirror treatment of lower limb phantom pain: a case studyDisabil Rehabil20042614–1590190415497919
- BrodieEEWhyteANivenCAAnalgesia through the looking-glass? A randomized controlled trial investigating the effect of viewing a “virtual” limb upon phantom limb pain, sensation and movementEuro J Pain2007114428436
- MoseleyGLGallaceASpenceCIs mirror therapy all it is cracked to be? Current evidence and future directionsPain2008138171018621484
- ChanBLWittRCharrowAPMirror therapy for phantom limb painN Engl J Med2007357212206220718032777
- RamachandranVSRogers-RamachandranDSynaesthesia in phantom limbs induced with mirrorsProc Bilo Sci19962631369377386
- HashimotoRRothwellJCDynamic changes in corticospinal excitability during motor imageryExp Brain Res19991251758110100979
- DiersMChristmannCKoeppeCRufMFlorHMirrored, imagined and executed movements differentially activate sensorimotor cortex in amputees with and without phantom limb painPain2010149229630420359825
- MoseleyGLGraded motor imagery for pathologic pain: a randomised controlled trialNeurology200667122129213417082465
- McCabeCMirror visual feedback therapy. A practical approachJ Hand Ther201124217017821106347
- BeaumontGMercierCMichonPEMalounFJacksonPLDecreasing phantom limb pain through observation of action and imageryCase Series2011122289299
- UlgerOEffectiveness of phantom exercises for phantom limb pain: a pilot studyJ Rehab Med2009417582584
- BrownCALidoCReflexology treatment for patients with lower limb amputations and phantom limb pain: an exploratory pilot studyComplement Ther Clin Pract200814212413118396256
- GiuffridaOSimpsonLHalliganPWContralateral stimulation, using TENS, of phantom limb pain: two confirmatory casesPain Med201011113314119788713
- MulveyMRBagnallAMJohnsonMIMarchantPRTranscutaneous electrical nerve stimulation (TENS) for phantom pain and stump pain following amputation in adultsCochrane Database Syst Rev20105CD00726420464749
- SumitaniMYozuATomiokaTYamadaYMiyauchiSUsing the intact hand for objective assessment of phantom hand-perceptionEur J Pain201014326126519477667
- DietrichCWalter-WalshKPreißlerSSensory feedback prosthesis reduces phantom limb pain: proof of a principleNeurosci Lett201250729710022085692
- LotzeMGroddWBirbaumerNErbMHuseEFlorHDoes use of a myoelectric prosthesis prevent cortical reorganization and phantom limb pain?Nat Neurosci19992650150210448212
- De RoosCVeenstraACDeJonghATreatment of chronic phantom limb pain using a trauma focused psychological approachPain Res Manag2010152657120458374
- DieckmannGWitzmannAInitial and long-term results of deep brain stimulation for chronic intractable painAppl Neurophysiol1982451–21671726977317
- HeathRGMickleWAEvaluation of 7-years’ experience with depth electrode studies in human patientsO’DohertyDSElectrical Studies on the Unanesthetized BrainNew York, NYP Hoeber1960214247
- HosobuchiYIntracerebral stimulation for the relief of chronic painYoumansJRNeurological SurgeryPhiladelphia, PAWB Saunders199041284143
- HosobuchiYAdamsJELinchitzRPain relief by electrical stimulation of the central gray matter in human and its reversal by naloxoneScience19771974299183186301658
- HosobuchiYAdamsJERutkinBChronic thalamic stimulation for the control of facial anaesthesia dolorosaArch Neurol19732931581614591720
- KumarKTothCNathRKDeep brain stimulation for intractable pain: a 15-year experienceNeurosurgery19974047367469092847
- MazarsGRugeRMazarsYRésultats de la stimulation du faisceau spino-thalamique et leur incidence sur la pathophysiologie de la douleurRev Neurol1960103136138
- MazarsGMerienneLCiolocaCTreatment of certain types of pain by implantable thalamic stimulatorsNeurochirurgie19742021171244418054
- MazarsGJIntermittent stimulation of nucleus ventralis posterolateralis for intractable painSurg Neurol19754193951080908
- MeyersonBABoethiusJCarlssonAMAlleviation of malignant pain by electrical stimulation in the periventricular-periaqueductal region. Pain relief as related to stimulation sitesAlbe-FessardDAdvances in Pain Research and TherapyNew York, NYRaven Press1979525533
- MeyersonBALindblomULinderothBLindGHerregodtsPMotor cortex stimulation as treatment of trigeminal neuropathic painActa Neurochir Suppl (Wien)1993581501538109279
- RichardsonDEAkilHPain reduction by electrical brain stimulation in man. Part 1: Acute administration in periaqueductal and periventricular sitesJ Neurosurg1977472178183327030
- RichardsonDEAkilHPain reduction by electrical stimulation in man. Part 2: Chronic self-administration in the periventricular gray matterJ Neurosurg1977472184194301558
- KringelbachMLAzizTZNeuroethical principles of deep-brain stimulationWorld Neurosurg201176651852922251494
- RichardsonDEAkilHLong term results of periventricular gray self-stimulationNeurosurgery197712199202308192
- SpiegelEAWycisHTMesencephalotomy in treatment of intractable facial painAMA Arch Neurol Psychiat1953691113
- TsubokawaTKatayamaYYamamotoTHirayamaTDeafferentation pain and stimulation of the thalamic sensory relay nucleus: clinical and experimental studyAppl Neurophysiol1985481–61661713017207
- SiegfriedJCetinalpENeurosurgical treatment of phantom limb painZimmermannMPhantom and Stump PainBerlin, GermanySpringer-Verlag1981148155
- WalshNERogersJNInjection proceduresDeLisaJAGansBMWalshNEPhysical Medicine and Rehabilitation: Principles and Practice1Philadelphia, PALippincott Williams & Wilkins2005311361
- AdamsJENaloxone reversal of analgesia produced by brain stimulation in humanPain197622161166800249
- RascheDRuppoltMStippichCUnterbergATronnierVMMotor cortex stimulation for long-term relief of chronic neuropathic pain: a 10 year experiencePain20061212435216480828
- NguyenJPNizardJKeravelYLefaucheurJPInvasive brain stimulation for the treatment of neuropathic painNat Rev Neurol201171269970921931348
- Garcia-LarreaLPeyronRMertensPPositron emission tomography during motor cortex stimulation for pain controlStereotact Funct Neurosurg1997681–4 Pt 11411489711707
- Garcia-LarreaLPeyronRMertensPElectrical stimulation of motor cortex for pain control: a combined PET-scan and electrophysiological studyPain199983225927310534598
- MertensPNutiCSindouMGuenotMPeyronRGarcia-LarreaLBPrecentral cortex stimulation for the treatment of central neuropathic pain: results of a prospective study in a 20-patient seriesStereotact Funct Neurosurg1999731–412212510853116
- RouxFEIbarrolaDLazorthesYBerryIChronic motor cortex stimulation for phantom limb pain: a functional magnetic resonance imaging study: technical case reportNeurosurgery200148368168811270562
- SaitohYShibataMHiranoSIHirataMMoshimoTYoshimineTMotor cortex stimulation for central and peripheral deafferentation painJ Neurosurg200092115015510616094
- KatayamaYYamamotoTKobayashiKKasaiMOshimaHFukayaCMotor cortex stimulation for phantom limb pain: Comprehensive therapy with spinal cord and thalamic stimulationStereotact Funct Neurosurg2001771–415916212378068
- LimaMCFregniFMotor cortex stimulation for chronic pain. Systematic review and meta-analysis of the literatureNeurology200870242329233718541887
- RascheDTronnierVMInvasive treatment of chronic neuropathic pain syndromes: epidural stimulation of the motor cortexKnotkovaHCrucianiRMerrickJBrain Stimulation in the Treatment of PainNew York, NYNova Science Publishers Inc20106987
- RascheDRinaldiPCYoungRFTronnierVMDeep brain stimulation for the treatment of various chronic pain syndromesNeurosurg Focus2006216E817341052
- Fagundes-PereyaWJTeixeiraMJReynsNMotor cortex stimulation for the treatment of neuropathic painArq Neuropsiquiatr201068692392921243254
- FontaineDHamaniCLozanoAEfficacy and safety of motor cortex stimulation for chronic neuropathic pain: critical review of the literatureJ Neurosurg2009110225125618991496
- CanaveroSBonicalziTherapeutic extradural cortical stimulation for central and neuropathic pain: a reviewClin J Pain2002181485511803303
- OakleyJPragerJSpinal cord stimulation: mechanisms of actionSpine (Phila Pa 1976)200227222574258312435996
- ViswanathanAUse of spinal cord stimulation in the treatment of phantom limb pain: case series and review of literaturePain Pract201010547948420412499
- KrainickJUThoderUSpinal cord stimulation in post-amputation painSiegfriedJZimmermanMPhantom and Stump PainBerlin, GermanySpringer-Verlag2002527535
- NitscheMAPaulusWExcitability changes induced in the human motor cortex by weak transcranial direct current stimulationJ Physiol2000527363363910990547
- FregniFGimenesRValleASA randomized, shamcontrolled, proof of principle study of transcranial direct current stimulation for the treatment of pain in fibromyalgiaArthritis Rheum200654123988399817133529
- FregniFBoggioPSLimaMCA sham-controlled, phase II trial of transcranial direct current stimulation for the treatment of central pain in traumatic spinal cord injuryPain20061221–219720916564618
- GrünerUEggersCAmeliMSarfeldASFinkGRNowakDA1 Hz rTMS preconditioned by tDCS over the primary motor cortex in Parkinson’s disease: effects on bradykinesia of arm and handJ Neural Transm2010117220721620033235
- FentonBFanningJBoggioPFregniFA pilot efficacy trial of tDCS for the treatment of refractory chronic pelvic painBrain Stimulation200813260
- KnotkovaHFeldmanDDvorkinECrucianiRATranscranial direct current stimulation (tDCS): A novel strategy to alleviate neuropathic painJ Pain Manag200813271275
- WangLGChiaoJWProstate cancer chemopreventive activity of phenethyl isothiocyanate through epigenetic regulation (review)Int J Oncol201033753353920664922