Abstract
Objective
The purpose of this study was to introduce and evaluate the early clinical outcomes of the full-endoscopic posterior lumbar interbody fusion (Endo-PLIF) technique with epidural anesthesia (EA) for single-segment lumbar degenerative diseases.
Methods
In this retrospective case series study, we explored the feasibility and effectiveness of the Endo-PLIF with EA for single-segment lumbar degenerative diseases. Between March 2018 and January 2019, a series of 24 patients with single-segment lumbar degenerative diseases underwent Endo-PLIF surgery and were followed up for a minimum of 12 months (15.21±2.27 months). Clinical outcomes including visual analog scale (VAS) scores for back and leg pain, Oswestry Disability Index (ODI) scores, and the Short Form-36 health survey questionnaire (SF-36) were evaluated preoperatively, and postoperatively at 3 days and at 3, 6, and 12-months.
Results
All patients underwent successful single-segment Endo-PLIF surgery. The mean operation time was 209.17±39.49 min, and average amount of bleeding was 43.33±14.87 mL. The VAS for lower extremity pain and back pain significantly improved at 3 days, and at 3, 6, 12 months compared with preoperative, respectively. The ODI scores decreased from 42.04±3.96 to 12.75±2.71 (P<0.001) at preoperative and 12 months postoperatively, respectively. The SF-36 Physical Component Scores (PCS) improved from 34.96±4.63 preoperatively to 52.08±6.05 (P<0.001) at 12 months postoperatively. Additionally, the SF-36 Mental Component Scores (MCS) improved from 39.38±5.70 at preoperative to 53.13±5.97 (P<0.001) at 12 months postoperatively. Two patients experienced dysesthesia, and one patient had a wound infection.
Conclusion
Endo-PLIF with EA is a feasible and valuable technique for the treatment of single-segment lumbar degenerative diseases in selected patients.
Introduction
Lumbar degenerative diseases (LDD) are common, and the age of onset has been trending downwards over the years. LDD has been considered as the main cause of chronic low back pain (LBP) and sciatica, including lumbar disc herniation, lumbar spinal stenosis, and lumbar spondylolisthesis.Citation1,Citation2 Currently, while ladder-like therapy is recommended to treat with LDD, surgery is the most effective treatment in the final step.Citation1 Open surgical procedures, including anterior lumbar interbody fusion (ALIF), posterior lumbar interbody fusion (PLIF), lateral lumbar interbody fusion (LLIF) and transforaminal lumbar interbody fusion (TLIF), have been regarded as effective interventions that can achieve ideal clinical outcomes by addressing the pathology thoroughly.Citation3 Meanwhile, there are some limitations that should not be ignored, such as extensive soft tissue destruction and long period of recovery. In addition, enhanced recovery after surgery (ERAS) has become of importance to spinal surgeons.Citation4,Citation5 Therefore, minimally invasive techniques have been explored to perform lumbar interbody fusion in recent years.
A large body of evidence has demonstrated that minimally invasive techniques can safely and effectively perform LIF.Citation6,Citation7 In 2002, Khoo et alCitation8 validated minimally invasive PLIF (MIS-PLIF) in three cadaveric torsos and then applied it in three patients. After that, MIS-PLIF was used by more spinal surgeons and obtained excellent clinical outcomes.Citation9–Citation11 LIF has shifted from conventionally open to minimally invasive procedures to date. For example, compared with the conventional TLIF, MIS-TLIF can achieve equivalent clinical outcomes with less destruction and a faster postoperative recovery.Citation12,Citation13 Furthermore, several studies have reported endoscopic spinal surgery techniques that have proven to be safe and less invasive.Citation14–Citation16 Endoscopic transforaminal lumbar interbody fusion (Endo-TLIF), has been employed extensively as a representative procedure.Citation17–Citation19 With the updates in surgical technique and minimally invasive concept, endoscopic-assisted spinal surgery will be attractive for surgeons to conduct decompression and LIF for patients with LDD. However, the safety and effectiveness of Endo-PLIF have remained unclear.
In this study, we introduced a minimally invasive technique to perform PLIF using a full endoscopic technique, and collected and analyzed a series of patients’ clinical data. The purpose of this study was to evaluate the safety and effectiveness of the Endo-PLIF for the treatment of single-segment LDD.
Materials and Methods
Patients
From March 2018 to January 2019, 24 consecutive patients (14 females and 10 males; mean age: 59.54±7.37 years) suffering from LDD underwent Endo-PLIF.
All operations were performed by the same surgeon (H Du). Surgical indications met the inclusion criteria as the following:Citation1 patients who complained about low back pain and sciatica;Citation2 patients who were diagnosed with lumbar degenerative diseases by combining medical history and imaging data, especially lumbar spinal canal stenosis (the central and lateral recess stenosis) and spondylolisthesis (lower than grade II);Citation3 single-segment that was involved; andCitation4 non-surgical treatments that failed or were more than 6 months. Exclusion criteria were as follows:Citation1 patients who had no symptoms and signs;Citation2 patients who suffered from spinal infection or coagulation abnormalities;Citation3 patients who had previously undergone lumbar surgical treatment; andCitation4 patients who were unwilling to receive surgery or unable to complete follow-up. Clinical baseline and perioperative characteristics of patients were collected, including age, weight, gender, diagnosis, examination findings, operative details, follow-up time, postoperative complications and functional scores. This study conformed to the Declaration of Helsinki and was approved by the ethics committee of the First Affiliated Hospital of Xi’an Jiaotong University (Number:2020G28). Informed consent was obtained from every patient after explanation of the study.
Outcome Assessment and Follow-Up
Outcome assessment included functional and radiological results. Functional outcomes were determined by comparing preoperative measurements with follow-up data, including visual analog scale (VAS) scores for back and leg pain, Oswestry Disability Index (ODI) scores, and the Short Form-36 health survey questionnaire (SF-36). Follow-up was a minimum of 12 months after surgery. CT scans and X-rays were taken at 3, 6 and 12 months after operation, which were used to evaluate the fusion rate according to Brantigan and Steffee criteria ().Citation20,Citation21 Grade 4 and grade 5 were considered fusion.
Table 1 The Fusion Grade of Brantigan and Steffee Criteria
Surgical Techniques
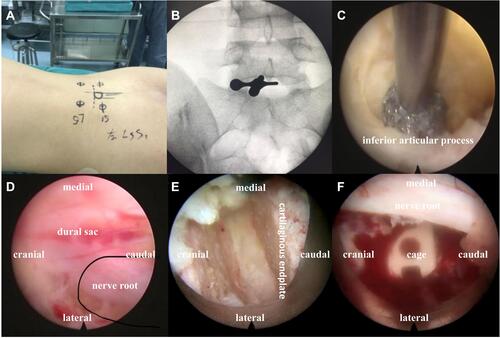
The schematic diagram of the procedure is shown in . The patient was placed prone on a radiolucent table, which was convenient for surgeons to obtain X-ray images by C-arm during the procedure. Spinous process, bilateral pedicles and intervertebral space horizontal line at vertebral bodies of the adjacent lesion level were confirmed by C-arm fluoroscopic control, and the corresponding positions were marked on the skin (). In order to obtain live feedback from the patients when nerve roots decompressed, the procedure was performed with epidural anesthesia (EA). The anesthetic scheme was the following: 0.1–0.2% ropivacaine + dexmedetomidine (micro-infusion pump–sedation loading dose: 0.5 μg/kg (10–15 min); maintenance doses: 0.2–0.5 μg/kg.h). Further, it was necessary to maintain a catheter for adding anesthetics (ropivacaine 7–10 mL) intraoperatively. After anesthesia, an incision was made at the skin entry point from the outer edge of spinous process (8 mm) to the inner edge of articular process, parallel to midline of the intervertebral space. The needle was inserted into the junction of articular process and lamina, which was kept parallel to the space. The dilating and protective cannula were carried out along the needle, and C-arm was employed to confirm the correct position (). Next, the dilating cannula was removed, and the endoscopic system (SPINEENDOS, Germany) was installed. The parameters of endoscopic system are 7.0 mm (outer diameter), 4.3 mm (inner diameter) and 2.5 mm (endoscopic drill).
Figure 1 The schematic diagram of the procedure. (A) Placing work cannula targeting to the junction of spinous space and lamina; (B) Removing the inferior articular process; (C) Exposing and decompressing the nerve root; (D) Implanting allograft bone and PEEK cage.
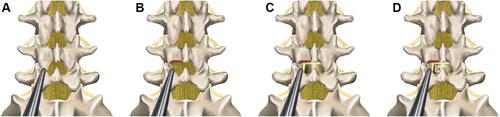
Figure 2 Intraoperative endoscopic images. (A) Confirming and marking skin entry points by C-arm; (B) Placing work cannula targeting to the junction of spinous space and lamina under C- arm fluoroscopic control; (C) Removing the inferior articular process, partial lamina, and the partial superior articular process by endoscopic drill under the full visualization; (D) Exposing nerve root after removing ligamentum flavum and hypertrophied tissues; (E) Scrapping away adequately cartilaginous endplate; (F) Checking cage position by full endoscopic visualization.
Under full endoscopic visualization, the articular processes of surgical level and ligamentum flavum were exposed, and the inferior articular process and partial lamina of upper vertebral body were removed by endoscopic drill (). Additionally, the part superior articular process (SAP) of the lower vertebral body was also removed. We initially removed the inferior articular process and reserved partial SAP. The distance was about 8–10 mm from the nerve root to the remaining SAP, which guaranteed a safe working space for completing surgery. After that, the dural sac and nerve root were exposed by removing ligamentum flavum and hypertrophied tissues (). The ipsilateral nerve root was sufficiently decompressed and released under the endoscopic system. If the patient suffered from bilateral symptoms, the contralateral nerve root decompression was performed by adjusting the cannula direction.
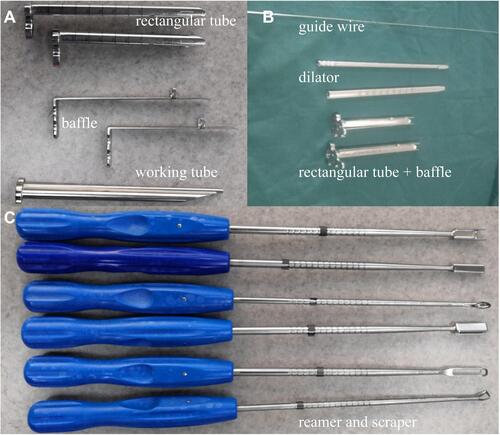
Subsequently, under the C-arm fluoroscopic control and neuromonitoring, the ZELIF® system (Sanyou, Inc., Shanghai, China)Citation13 was installed via dilating layer by layer and then applied to perform discectomy and endplate preparation, which provided a safe and accessible approach to disc space ( and ). The ZELIF® system is a caudally expandable rectangular tubular system with the dimensions of 100 mm (length) x 9 mm (width) x 14 mm (height) ( and ). The disc tissue was removed through reamers and scrapers of different sizes (). After removal of the disc tissue, the cartilaginous endplate was scrapped away with curettes under endoscopic visualization, which ensured adequate endplate preparation (). The model cages height (8mm/10mm/12mm) and length (23mm/26mm) were implanted through the cannula under the C-arm fluoroscopic control and neuromonitoring. After confirming the optimal size, the model was removed, and the PLIF cage (PEEK material, Sanyou, Inc., Shanghai, China) filled with allograft bone was implanted under the C-arm fluoroscopic control ( and ). Full endoscopic view checking found that the dural sac and nerve root were adequately decompressed without injury (). The endoscopic system and working cannula were withdrawn after meticulous hemostasis.
Figure 3 Intraoperative C-arm fluoroscopic control. (A–C) Installing expandable tubular, dilating intervertebral space and implanting allograft bone and PEEK cage under the C-arm fluoroscopic control; (D) Lateral X-rays showing satisfactory cage position; (E and F) Anteroposterior and lateral X-rays showing correct implant position.
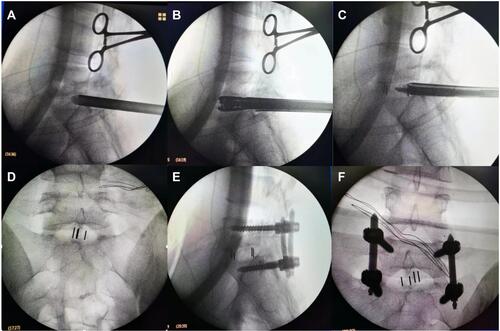
Figure 4 Instruments of ZELIF. (A and B) Guide wire, dilator, working tube and baffle of ZELIF® system; (C) Reamers and scrapers.
After local anesthesia, four 15 mm incisions were made in the previously marked skin point entry, and percutaneous pedicle screws were implanted through the incision under the C-arm fluoroscopic control. Bilateral connecting rods were installed percutaneously and tightened screw-rod after confirming the ideal position by C-arm ( and ). All instruments were removed, and the skin incisions sutured. There was no drainage after the operation.
Statistical Analysis
Data were analyzed using the statistical software SPSS 23.0 (SPSS, IBM Inc, American). Continuous variables were represented as mean±standard deviation (SD). Categorical variables were shown as number and percent. Differences between preoperative and postoperative variables were analyzed by using the repeated measures analysis of variance. For all tests, the significance was set at a P value of <0.05 (two-tailed).
Results
Baseline Clinical and Intraoperative Detail of Patients
In this study, 24 patients were included with ages ranging from 45 to 75 (59.54±7.37) years. All patients underwent a single-segment Endo-PLIF procedure, with 12 cases at L4/5, 10 cases at L5/S1 and two cases at L3/4. Baseline clinical and perioperative characteristics of patients are shown in . All patients’ diagnosis was confirmed before surgery without contraindications. There were 10 cases with lumbar spinal stenosis, eight cases with lumbar disc herniation accompanying instability, and six cases with lumbar spondylolisthesis. The average operation time was 209.17±39.49 min (range 158–320 min), the mean intraoperative blood loss was 43.33±14.87 mL (range 25–80 mL), and the mean hospitalization duration was 8.67±3.59 days (range 5–22 d). No patient was lost to follow-up and the average follow-up time was 15.21±2.27 months (range 12–20 m).
Table 2 Baseline Clinical and Perioperative Characteristics of Patients
Clinical Outcomes
Compared with preoperative clinical assessments, lower back pain VAS dramatically decreased from 5.83±1.09 to 3.54±0.72, 2.42±0.65, 1.71±0.75, 0.96±0.69 at postoperative 3 days and at 3, 6 and 12 months, respectively. Likewise, lower extremity pain VAS dramatically decreased from 6.54±1.22 to 4.33±0.92, 2.58±0.83, 1.42±0.78, 0.71±0.62 at postoperative 3 days and at 3, 6 and 12 months. Further, ODI dramatically improved from 42.04±3.96 to 35.33±5.25, 25.17±4.26, 17.67±4.38, and 12.75±2.71 postoperative 3 days and at 3, 6, and 12 months, respectively. Of note, SF-36 PCS improved from 34.96±4.63 to 39.88±4.92, 43.79±4.84, 47.25±4.57, 52.08±6.05 at postoperative 3 days and at 3, 6, and 12 months, respectively. Similarly, SF-36 MCS improved from 39.38±5.70 to 42.29±5.77, 45.21±6.21, 48.92±5.48, 53.13±5.97 at postoperative 3 days and at 3, 6, and 12 months, respectively ().
Table 3 Preoperative and Follow-Up Functional Scores
Radiological Outcomes
There were no observed cases of nonunion, cage migration and subsidence in our initial 24 patients at 12 months after operation, including 4 cases with fusion grade 4 (16.67%) and 20 cases with fusion grade 5 (83.33%) ().
Complications
Three patients (12.5%) experienced complications after surgery (). Two patients suffered from paresthesia without dyskinesia. The distributions of paresthesia corresponded to the surgical site traversing the nerve root, which was caused by a pressed nerve root in the procedure. They were treated with conservative therapy, including physical treatment and medicine. Symptoms were gradually relieved and completely recovered by the last follow-up. There was no severe nerve injury in any patient. Surgical site infection occurred in one patient on the fifth day after surgery, which was superficial in nature with a negative bacterial culture. The surgical wound healed with dressing changes and antibiotics within a month. No patient is required to undergo revision or debridement surgery.
Representative Cases
Two representative cases of patients in this study are shown in and .
Figure 5 Images were obtained from a 48-year-old male patient with the degenerative spondylolisthesis at L5-S1. (A and B) Sagittal and axial CT images showing degenerative spondylolisthesis at L5-S1; (C and D) Postoperative anteroposterior and lateral X-rays images showing correct cage and pedicle screws after full Endo-PLIF; (E–H) Postoperative sagittal CT images at 3, 6, 9, 12 months showing interbody fusion.
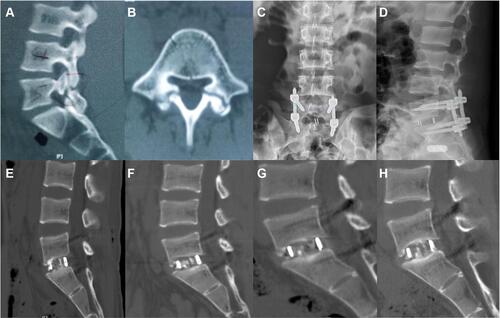
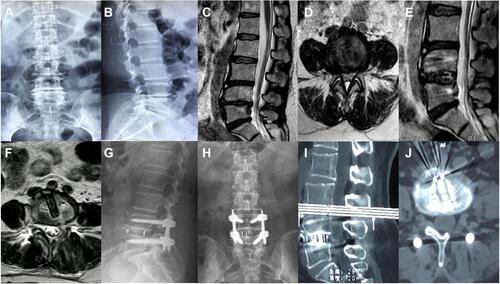
Figure 6 Images were obtained from a 51-year-old female patient with the lumbar spinal stenosis at L4-5. (A–D) Preoperative X-rays and MRI images showing lumbar spinal stenosis at L4-5; (E and F) Postoperative sagittal and axial MRI images showing complete decompression after full Endo-PLIF; (G and H) Postoperative anteroposterior and lateral X-rays at 3 months showing correct position; (I and J) Postoperative sagittal and axial CT images at 6 months showing interbody fusion.
Discussion
In the present study, we demonstrate that full-endoscopic posterior lumbar interbody fusion (Endo-PLIF) is a feasible and effective technique in initial 24 cases. Previous publications have demonstrated that interbody fusion surgery is an effective treatment for unbearable LBP caused by LDD, especially for patients with nonsurgical therapy failure.Citation1,Citation2 In the past decades, conventional open LIF has been regarded as the gold standard for treatment of this patient population due to complete decompression, satisfactory fusion rate, solid internal fixation and excellent clinical outcomes at long-time follow-up.Citation3,Citation6 Various procedures for open LIF have been developed to improve the surgical results and minimize morbidity, yet significant surgical destruction still occurs, such as long surgical incision and high intraoperative blood loss.Citation6 Additionally, ERAS programs have been paid widespread attention in multiple surgical fields. Wang et alCitation5 provided the first ERAS protocol for interbody fusion and conducted a retrospective study, which reduced care cost, recovery time and morbidity after surgery. Regardless of which procedure has been employed to perform LIF, the final aims are similar: achieving optimal outcomes and minimal complications. Endo-PLIF, a newly emerging technique owing to iterative instruments and innovative concepts, takes advantages of less invasive surgery to obtain complete decompression and satisfactory fusion.
In our study, patients who suffered from LDD, including lumbar spinal stenosis, disc herniation with instability and spondylolisthesis, and were immune to nonsurgical treatments underwent single-segment Endo-PLIF. Decompression and fusion play a critical role in addressing LDD for most patients. Of note, endoscopic indications and applications have been evolving in spinal surgery. Foley et alCitation22 first reported and evaluated the feasibility of micro-endoscopy discectomy (MED) in treating lumbar disc herniation. Since then, endoscopic technique has been gaining attraction to address more spinal diseases. At present, endoscopic techniques have been widely applied in spinal surgery ranging from the cervical to lumbar spine.Citation23 Meanwhile, initial studies mainly focus on one- or two-segment fusion with endoscopy,Citation23 but multiple-segments will be explored as instruments improve and experiences accumulate. With this understanding, we performed Endo-PLIF with single-segment.
Regarding operative details, operation time and hospitalization duration were longer in our study compared to similar reports.Citation19,Citation24 Preoperative examination may be an important aspect, which usually took two or three days in our hospital. The other reason for this may be that although the surgeon had experience in spinal surgery with endoscopy, it was the first time performing fully endoscopic fusion. Theoretically, the learning curve is essential for spinal surgeons to master a novel technique;Citation3 therefore, those drawbacks will be inevitable for the novice, but will eventually be overcome with skilled technique. We also took more time to evaluate the recovery condition of patient after surgery. Finally, postoperative complications, especially one case with surgical site infection, increased mean hospitalization duration.
The push to develop minimally invasive techniques has played a vital role in driving spinal surgery technique evolution.Citation23 However, how to obtain optimal outcomes and minimal complications has been a challenge for surgeons, especially those with elderly and comorbid patients. On the one hand, surgeon should address patients’ complaints as completely as possible. On the other hand, they must consider perioperative risk, complications and costs. Wang MY and colleaguesCitation25 reported 10 consecutive patients who underwent endoscopic TLIF without general anesthesia. In the present study, we made full use of the advantages of EA compared to general anesthesia to reduce risks in higher risk populations. Compared with general anesthesia, EA can provide sufficient pain relief with minimal influences on cardiovascular and respiratory systems. It also keeps patients conscious to cooperate with the surgery, which is helpful to decrease the risk of nerve injury. Moreover, EA not only increases the degree of satisfaction but also improves the effectiveness of the operation. Along with faster recovery and lower cost, EA enhances surgical patients’ safety and comfort. Therefore, the aging population, especially those with cardiopulmonary dysfunction, could undergo Endo-PLIF, which may allow for more patients to obtain surgical benefits.
Since the endoscope-assisted TLIF was introduced in 2008,Citation23 many scholars have attempted and designed special instruments to perform TLIF with the endoscope.Citation14,Citation17,Citation19,Citation25,Citation26 Although many spinal procedures share the endoscopic labels, the surgical techniques can mainly be grouped into three categories in terms of the endoscopic system used: percutaneous endoscopic (or full-endoscopic) TLIF, biportal endoscopic TLIF and micro-endoscopic TLIF.Citation7 Compared with the minimally invasive TILF (MIS-TLIF), Endo-TLIF demonstrated better outcomes and faster recovery time after operation, owing to less invasion.Citation13,Citation27 In fact, the transforaminal approach must face the following challenges: exiting nerve roots injury and inadequately contralateral decompression, which have been determined by the anatomical structure. In a retrospective research, the TESSYS-ISEE technique was employed to remove the dorsal and ventral structures around the involved nerve root, which achieved 270-degree spinal canal decompression.Citation24 Moreover, the coronal imbalance and lumbar lordosis cannot be completely addressed through Endo-TLIF or MIS-TLIF because of the narrow surgical corridor. Therefore, the expandable cage has been used to overcome this drawback.Citation28 However, there is still controversy surrounding the late outcome of the expandable cage. The expandable cage is superior to the conventional PEEK cage in biomechanical stability but may lead to endplate fracture when bracing in intervertebral space due to excessive extrusion.Citation29,Citation30 A recent monocentric study reported that the expandable cage did not show better clinical outcome and improved sagittal alignment in the MIS-TLIF, and even increased the risk of late subsidence after operation.Citation31 In our patients, we used similar PLIF surgical access to achieve complete decompression and fusion under the endoscope. Only part of the superior articular process was removed, which is less invasive and helps maintain the stability of spine. Meanwhile, this technique is helpful to perform contralateral decompression and conforms to anatomical structure for orthopedic surgeons. Additionally, the whole procedure was performed with full visualization, which reduces tissue injury and performs thorough endplate preparation. For interbody fusion, short-term outcomes are determined by decompression and long-term outcomes are determined by endplate preparation. In order to achieve satisfactory decompression and fusion, we took the advantages of ZELIF® in our series. This exquisite design provides enough working room and can reach the intervertebral space, which is helpful to perform adequate decompression and insert the conventional PEEK cage. There were no observed cases of nonunion, cage migration or subsidence in our initial 24 patients at 12 months after operation. Importantly, postoperative follow-up indicated remarkable improvement in VAS, ODI score, SF-36 PCS and MCS. Compared with previous studies, postoperative outcomes were similar and less invasive to our design.Citation11,Citation14
In summary, we utilized the endoscope and ZELIF® to conduct posterior lumbar interbody fusion with epidural anesthesia. First, we achieved sufficient decompression, especially for central canal and contralateral decompression. Second, a conventional PEEK cage could be inserted by ZELIF®. Finally, the aging patients, especially those with cardiopulmonary dysfunction, could obtain surgical benefits with EA. Based on the preliminary experience, the major Endo-PLIF indications include single-level lumbar degenerative diseases, such as lumbar spinal canal stenosis (the central and lateral recess stenosis) and spondylolisthesis (lower than grade II).
The complications and limitations of the present study should be brought to attention. Postoperative complications have negative effects, such as prolonging hospitalization and increasing cost. Paresthesia occurred in two patients after surgery, and after analysis, we found that insufficient articular process removal was the main cause; the nerve root was disturbed when cage inserted into disc space through narrow channel. Certainly, the traversing nerve root and epidural may be injured when the posterior access is taken, which are the disadvantages of this technique. One patient suffered from an incision infection and the resultant increased hospitalization stay. Several limitations were as follows: First, this was a retrospective case series, and the sample size was small. Compared with randomized controlled trials (RCTs), the reliability of the results was limited. Second, only the selected patients underwent this novel procedure; therefore, whether this procedure was suitable for a greater population requires further study. Third, more potential complications were not exposed due to the short follow-up and small sample; multi-center studies consisting of a larger sample size should be performed in the future.
Conclusion
In summary, Endo-PLIF with EA is proven to be a feasible and effective technique for the treatment of single-segment lumbar with degenerative diseases in selected patients. However, Endo-PLIF technique has relatively limited indications, and requires surgeon mastery of the technique. In addition, narrow working channels and large cage insertion remain huge challenges. Future improvement of technique and instrument is needed for application of Endo-PLIF in the treatment of lumbar degenerative diseases.
Abbreviations
EA, epidural anesthesia; Endo-PLIF, Full-endoscopic Posterior Lumbar Interbody Fusion; VAS, visual analog scale; ODI, Oswestry Disability Index; SF-36, Short Form-36 health survey questionnaire; MCS, Mental Component Scores; PCS, Physical Component Scores; LDD, Lumbar degenerative diseases; LBP, low back pain; ALIF, anterior lumbar interbody fusion; PLIF, posterior lumbar interbody fusion; LLIF, lateral lumbar interbody fusion; TLIF, transforaminal lumbar interbody fusion; ERAS, enhanced recovery after surgery; MIS-PLIF, minimally invasive PLIF; Endo-TLIF, Endoscopic transforaminal lumbar interbody fusion; MED, micro-endoscopy discectomy.
Data Sharing Statement
All the data will be available upon motivated request to the corresponding author.
Ethics Approval and Consent to Participate
This study conformed to the Declaration of Helsinki and was approved by the ethics committee of the First Affiliated Hospital of Xi’an Jiaotong University (Number:2020G28). Informed consent was obtained from every patient after explanation of the study.
Consent for Publication
Informed consent was obtained from every patient before publishing their data.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval for the version to be published; and agreed to be accountable for all aspects of the work.
Acknowledgments
The authors thank AiMi Academic Services (www.aimieditor.com) for the English language editing and review services.
Disclosure
The authors declare that they have no competing interests.
Additional information
Funding
References
- Phillips FM, Slosar PJ, Youssef JA, et al. Lumbar spine fusion for chronic low back pain due to degenerative disc disease: a systematic review. Spine. 2013;38(7):E409–422. doi:10.1097/BRS.0b013e3182877f11.
- Xiao L, Zhao Q, Sun X, et al. Relationship between alterations of spinal/pelvic sagittal parameters and clinical outcomes after oblique lumbar interbody fusion. World Neurosurg. 2020;133:e156–e164. doi:10.1016/j.wneu.2019.08.158
- Wu J, Liu H, Ao S, et al. Percutaneous endoscopic lumbar interbody fusion: technical note and preliminary clinical experience with 2-year follow-up. Biomed Res Int. 2018;2018:5806037. doi:10.1155/2018/5806037
- Kehlet H, Wilmore DW. Multimodal strategies to improve surgical outcome. Am J Surg. 2002;183(6):630–641. doi:10.1016/S0002-9610(02)00866-8
- Wang MY, Chang PY, Grossman J. Development of an Enhanced Recovery After Surgery (ERAS) approach for lumbar spinal fusion. J Neurosurg Spine. 2017;26(4):411–418. doi:10.3171/2016.9.SPINE16375
- Xu DS, Walker CT, Godzik J, et al. Minimally invasive anterior, lateral, and oblique lumbar interbody fusion: a literature review. Ann Trans Med. 2018;6(6):104. doi:10.21037/atm.2018.03.24.
- Ahn Y, Youn MS, Heo DH. Endoscopic transforaminal lumbar interbody fusion: a comprehensive review. Expert Rev Med Devices. 2019;16(5):373–380. doi:10.1080/17434440.2019.1610388
- Khoo LT, Palmer S, Laich DT, et al. Minimally invasive percutaneous posterior lumbar interbody fusion. Neurosurgery. 2002;51(5 Suppl):S166–181.
- Park Y, Ha JW. Comparison of one-level posterior lumbar interbody fusion performed with a minimally invasive approach or a traditional open approach. Spine. 2007;32(5):537–543. doi:10.1097/01.brs.0000256473.49791.f4
- Fan S, Hu Z, Zhao F, et al. Multifidus muscle changes and clinical effects of one-level posterior lumbar interbody fusion: minimally invasive procedure versus conventional open approach. EUR Spine J. 2010;19(2):316–324. doi:10.1007/s00586-009-1191-6.
- Sidhu GS, Henkelman E, Vaccaro AR, et al. Minimally invasive versus open posterior lumbar interbody fusion: a systematic review. Clin Orthop Relat Res. 2014;472(6):1792–1799. doi:10.1007/s11999-014-3619-5.
- Lee KH, Yue WM, Yeo W, et al. Clinical and radiological outcomes of open versus minimally invasive transforaminal lumbar interbody fusion. Eur Spine J. 2012;21(11):2265–2270. doi:10.1007/s00586-012-2281-4.
- Ao S, Zheng W, Wu J, et al. Comparison of Preliminary clinical outcomes between percutaneous endoscopic and minimally invasive transforaminal lumbar interbody fusion for lumbar degenerative diseases in a tertiary hospital: is percutaneous endoscopic procedure superior to MIS-TLIF? A prospective cohort study. Int J Surg. 76;2020:136–143. doi:10.1016/j.ijsu.2020.02.043
- Ruetten S, Komp M, Merk H, et al. Full-endoscopic interlaminar and transforaminal lumbar discectomy versus conventional microsurgical technique: a prospective, randomized, controlled study. Spine. 2008;33(9):931–939. doi:10.1097/BRS.0b013e31816c8af7.
- Cong L, Zhu Y, Tu G, A meta-analysis of endoscopic discectomy versus open discectomy for symptomatic lumbar disk herniation. Eur Spine J. 2016;25(1):134–143. doi:10.1007/s00586-015-3776-6
- Ruan W, Feng F, Liu Z, et al. Comparison of percutaneous endoscopic lumbar discectomy versus open lumbar microdiscectomy for lumbar disc herniation: a meta-analysis. Int J Surg. 31;2016:86–92. doi:10.1016/j.ijsu.2016.05.061
- Jacquot F, Gastambide D. Percutaneous endoscopic transforaminal lumbar interbody fusion: is it worth it? Int Orthop. 2013;37(8):1507–1510. doi:10.1007/s00264-013-1905-6
- Lee S-H, Erken HY, Bae J. Percutaneous transforaminal endoscopic lumbar interbody fusion: clinical and radiological results of mean 46-month follow-up. Biomed Res Int. 2017;2017:3731983. doi:10.1155/2017/3731983
- Kim JE, Choi DJ. Biportal Endoscopic transforaminal lumbar interbody fusion with arthroscopy. Clin Orthop Surg. 2018;10(2):248–252. doi:10.4055/cios.2018.10.2.248
- Brantigan JW, Steffee A. A carbon fiber implant to aid interbody lumbar fusion. two-year clinical results in the first 26 patients. Spine. 1993;18(14):2106–2117. doi:10.1097/00007632-199310001-00030
- Heo DH, Park CK. Clinical results of percutaneous biportal endoscopic lumbar interbody fusion with application of enhanced recovery after surgery. Neurosurg Focus. 2019;46(4):E18. doi:10.3171/2019.1.FOCUS18695
- Foley K, Smith M, Rampersaud Y. Microendoscopic approach to far-lateral lumbar disc herniation. Neurosurg Focus. 1999;7(5):e5. doi:10.3171/foc.1999.7.5.8
- Brusko GD, Wang MY. Endoscopic lumbar interbody fusion. Neurosurg Clin N Am. 2020;31(1):17–24. doi:10.1016/j.nec.2019.08.002
- Xiong C, Li T, Kang H, et al. Early outcomes of 270-degree spinal canal decompression by using TESSYS-ISEE technique in patients with lumbar spinal stenosis combined with disk herniation. Eur Spine J. 2019;28(1):78–86. doi:10.1007/s00586-018-5655-4.
- Wang MY, Grossman J. Endoscopic minimally invasive transforaminal interbody fusion without general anesthesia: initial clinical experience with 1-year follow-up. Neurosurg Focus. 2016;40(2):E13. doi:10.3171/2015.11.FOCUS15435
- Wu P, Kim H, Lee Y, et al. Uniportal full endoscopic posterolateral transforaminal lumbar interb\ody fusion with endoscopic disc drilling preparation technique for symptomatic foraminal stenosis secondary to severe collapsed disc space: a clinical and Computer tomographic study with technical note. Brain Sci. 2020;10(6). doi:10.3390/brainsci10060373.
- Zhang H, Zhou C, Wang C, et al. Percutaneous endoscopic transforaminal lumbar interbody fusion: technique note and comparison of early outcomes with minimally invasive transforaminal lumbar interbody fusion for lumbar spondylolisthesis. Int J Gen Med. 2021;14:549–558. doi:10.2147/IJGM.S298591
- Kim CW, Doerr TM, Luna IY, et al. Minimally invasive transforaminal lumbar interbody fusion using expandable technology: a clinical and radiographic analysis of 50 patients. World Neurosurgery. 2016;90:228–235. doi:10.1016/j.wneu.2016.02.075
- Gonzalez-Blohm SA, Doulgeris JJ, Aghayev K, et al. Biomechanical analysis of an interspinous fusion device as a stand-alone and as supplemental fixation to posterior expandable interbody cages in the lumbar spine. J Neurosurg Spine. 2014;20(2):209–219. doi:10.3171/2013.10.SPINE13612
- Mantell M, Cyriac M, Haines CM, et al. Biomechanical analysis of an expandable lateral cage and a static transforaminal lumbar interbody fusion cage with posterior instrumentation in an in vitro spondylolisthesis model. J Neurosurg Spine. 2016;24(1):32–38. doi:10.3171/2015.4.SPINE14636
- Armocida D, Pesce A, Proietti L, et al. Minimally invasive transforaminal lumbar interbody fusion (MIS-TLIF) using expandable cages: increased risk of late post-operative subsidence without a real improve of perioperative outcomes. A clinical mono-centric study. World Neurosurg. 2021;04:e1–e7.
