Abstract
Purpose
Chronic low back pain (cLBP) is a recurring and intractable disease that is often accompanied by emotional and cognitive disorders such as depression and anxiety. The nucleus accumbens (NAc) plays an important role in mediating emotional and cognitive processes and analgesia. This study investigated the resting-state functional connectivity (rsFC) and effective connectivity (EC) of NAc and its subregions in cLBP.
Methods
Thirty-four cLBP patients and 34 age- and sex-matched healthy controls (HC) underwent resting-state functional magnetic resonance imaging (rs-fMRI). Seed-based rsFC and Dynamic Causal Modelling (DCM) were used to examine the alteration of the rsFC and EC of the NAc.
Results
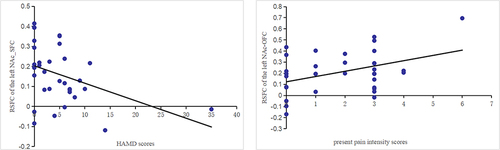
Our results showed that the cLBP group had increased rsFC of the bilateral NAc-left superior frontal cortex (SFC), orbital frontal cortex (OFC), left angular gyrus, the left NAc-bilateral middle temporal gyrus, as well as decreased rsFC of left NAc-left supramarginal gyrus, right precentral gyrus, left cerebellum, brainstem (medulla oblongata), and right insula pathways compared with the HC; the results of the subregions were largely consistent with the whole NAc. In addition, the rsFC of the left NAc-left SFC was negatively correlated with Hamilton’s Depression Scale (HAMD) scores (r = −0.402, p = 0.018), and the rsFC of left NAc-OFC was positively correlated with present pain intensity scores (r = 0.406, p = 0.017) in the cLBP group. DCM showed that the cLBP group showed significantly increased EC from the left cerebellum to the right NAc (p = 0.012) as compared with HC.
Conclusion
Overall, our findings demonstrate aberrant rsFC and EC between NAc and regions that are associated with emotional regulation and cognitive processing in individuals with cLBP, underscoring the pivotal roles of emotion and cognition in cLBP.
Introduction
Low back pain (LBP) is the leading cause of disability worldwide, and its prevalence has increased over the years.Citation1–5 Chronic LBP (cLBP) is characterized by continued pain or frequency of pain for a duration exceeding 3 months. Because of the lack of effective treatment, patients are often accompanied by emotional dysregulation such as depression, anxiety, and fear.Citation3,Citation6 Recent studies have showed that some conservative treatments are effective in reducing pain intensity, improving disability and pain sensitivity variables related to chronic pain, such as exercise therapy and orthopedic manual therapy.Citation7–9 A previous study showed that biopsychosocial intervention therapeutic approach is effective for recovery from cLBP. Certain patients’ expectations could be related to a better recovery outcomes,Citation10 and positive verbal can activate the same brain areas as those related to pain relief and have favorable effects on osteoarthritis pain and LBP.Citation11,Citation12
A previous study showed that cognitive and emotional factors strongly influence the connectivity of brain regions that modulate pain perception, emotional states, attention, and expectations.Citation13,Citation14 A growing body of research has suggested that alterations in the brain play an important role in the maintenance and development of pain. Therefore, we studied the central regulatory mechanisms of cLBP, with the aim to identify new targets for effective treatment of cLBP.
The nucleus accumbens (NAc) is a key component of reward processing,Citation15 and it mainly mediates the hedonic perception of rewards, which is related to reward evaluation and expectation.Citation16–20 Previous studies have shown that reward-associated circuits around the NAc appear to promote the transition from acute to chronic pain.Citation21,Citation22 Furthermore, the altered volume and activity of the NAc may confer further risk of developing chronic pain.Citation20 Multiple studies have suggested that negative emotions and pain are closely related and cognitive-affective integration–related brain regions in patients with chronic pain are now recognized as key components of pain.Citation23–25 Dopamine in the NAc is critical for pain relief and to regulate emotion perception.Citation26,Citation27 Recent research has shown that the NAc can be divided into two subregions, namely the core-like part and shell-like part, each with different function and connectivity patterns.Citation16,Citation28,Citation29
The FC of spatially distinct regions is one of the most widely used resting-state functional magnetic resonance imaging (rs-fMRI),Citation30 which has been widely used to explore the brain regulation mechanism of pain. Dynamic causal modelling (DCM) has been widely used to infer the effective connectivity (EC) of distinct regions, as it can indicate the specific intensity and reflect the direction of information communication between brain regions.Citation31,Citation32
Studies on the subregions of the NAc in cLBP are relatively limited, and their findings lack consistency. Furthermore, most previous studies have relied on resting-state functional connectivity (rsFC), which fails to provide directional information regarding interregional communication within the brain. Thus, in this study, we combined the methods of seed-based rsFC and DCM with the aim to investigate the rsFC and EC of the NAc and its subregions in cLBP and clarify the regulatory mechanisms of the NAc in cLBP.
Materials and Methods
Participants
GPower software (version 3.1) was used to estimate the sample size. In response to the power analysis (α = 0.05, β = 0.1, effect size = 0.8),Citation33 the calculated sample size was found to be 34 patients and 34 HC. A total of 34 cLBP patients (29 female and 5 male; mean age (±SD): 40.1 ± 9.6 years) and 34 age- and sex-matched healthy controls (HC) (29 female and 5 male; mean age: 38.2 ± 12.8 years) were recruited in this study. The participants were mainly recruited from the Department of Orthopedics of the Second Affiliated Hospital of Xi’an Jiaotong University and the community. This study was approved by the Research Ethics Committee of the Second Affiliated Hospital of Xi’an Jiaotong University (number: 2023512). All participants provided written informed consent prior to the MRI scan. All participants were equipped with disposable silent earplugs before the examination to reduce noise, and they were instructed to remain relaxed and awake during the examination. The inclusion criteria were as follows: (1) age between 18 and 60 years; (2) right handedness; (3) LBP duration of at least one year; (4) pain intensity of at least 4 on the 0–10 visual analog scale (VAS). The exclusion criteria were as follows: (1) specific causes of back pain (eg, cancer, fractures, infections); (2) history of nervous system, cardiac, or respiratory disease (eg, stroke, asthma, diabetes); (3) history of psychotic and/or mood disorders (eg, depression, schizophrenia); (4) presence of any contraindications to MRI scanning (eg, claustrophobia, cardiac pacemaker, or metal implants).
Questionnaire
All participants completed the following scales: Edinburgh Handedness Inventory, which was used to judge handedness; Montreal Cognitive Assessment (MoCA) to assess cognitive states; and the 17-item Hamilton Depression Scale (HAMD) and Hamilton Anxiety Scale (HAMA), which were used to assess depressive and anxious states. Furthermore, all cLBP patients completed the Short-Form McGill Pain Questionnaire, including Visual Analog Scale (VAS) and present pain intensity scores, which were used to evaluate the pain intensity of the subjects.Citation34
Image Acquisition
All subjects underwent functional scanning on a 3.0-T MR scanner (GE Architect) using a 48-channel head coil. T1-weighted images were obtained for each participant by using a 3-dimensional MP-RAGE sequence with the following parameters: repetition time (TR) = 900 ms, echo time (TE)=3.1 ms, field of view (FOV) = 256 mm, slice thickness = 1.0 mm (no gaps), flip angle = 8, and slices = 150. The rs-fMRI used the following parameters: TR = 2000 ms, TE = 35 ms, matrix = 80 × 80, FOV = 240 mm, flip angle = 90, slice thickness = 3 mm, number of slices = 49, and 198 volumes per participant.
fMRI Data Analysis
Image Preprocessing
The CONN toolbox version 22a (http://www.nitrc.org/project/conn/) in MATLAB (MathWorks, Inc., Natick, MA, USA) was used to perform the fMRI data preprocessing. The first four volumes of the fMRI data were discarded. The FC data of functional connectivity were all preprocessed using a default preprocessing pipeline for volume-based analyses (direct normalization to MNI-space) in the CONN toolbox. The specific steps including functional Slice-Timing correction (correction for inter-slice differences in acquisition time), functional Realignment & unwarp (subject motion estimation and correction), functional Outlier detection (ART-based identification of outlier scans for scrubbing), functional Direct Segmentation & Normalization (simultaneous Grey/White/Cerebrospinal Fluid (CSF) segmentation and MNI normalization), structural Segmentation & Normalization (simultaneous Grey/White/CSF segmentation and MNI normalization) and functional smoothing (spatial convolution with Gaussian kernel; full-width-at-half maximum [FWHM] = 6 mm). The DCM data were preprocessed using the above steps without smoothing.
rsFC Analysis of the NAc
A “seed-to-voxel” rsFC analysis was performed using the CONN toolbox. The left and right NAc as the seed regions were defined using the Harvard–Oxford subcortical atlas, and the subregions as the seed regions were obtained from the website (http://atlas.brainnetome.org/download.html) and combined literature.Citation35 The first-level FC analysis yielded Fisher’s r-to-z transformed bivariate correlations between the NAc and other voxels of the brain. Then, second-level analyses were performed to determine group-based differences in rsFC of the NAc and the whole brain. Age, sex, and HAMD score were included as covariates. The results were considered significant at a threshold of cluster-level p<0.05. Family Wise Error (FWE) was corrected for between-group comparisons.
EC of the NAc
Spectral DCM analyses were conducted using DCM 12 implemented in SPM12 software (Wellcome Department of Imaging Neuroscience, London, UK; https://www.fil.ion.ucl.ac.uk/spm/). The region of interest (ROI) used in DCM analysis was obtained from the rsFC analyses and combined physiological functions of brain regions. Four ROIs were selected, namely the left SFC (x = −12, y = 56, z = 34, radius = 5 mm); left NAc (x = −10, y = 14, z = −6, radius = 3 mm); right NAc (x = 8, y = 14, z = −6, radius = 3 mm); and left cerebellum (Cer, x = −12, y = −38, z = −32, radius = 5 mm). Then, the time series of these four ROIs were extracted. Five models were established, including a fully connected model, a model that did not consider the interactions of the bilateral NAc, a model that did not consider the interactions of the left SFC and right NAc, a model that excluded the left cerebellum, and a model that excluded the SFC. Fixed effects (FFX) Bayesian model selection (BMS)Citation32 was used to determine the best model for each subject. Bayesian model averaging (BMA)Citation36 was conducted to analyze the connectivity parameters at the group level to identify the best model.
Statistical Analyses
Statistical analysis was performed using SPSS statistics version 18.0 (IBM Corporation, Armonk, NY, USA). The age, MoCA scores, HAMD, and HAMA scores between the two groups were compared by two-sample t-tests. Sex differences were characterized by the chi-square test, and one-sample t-test and independent two-samples t-test were performed for effective connectivity parameters. Significance was set at p<0.05.
Pearson’s correlation analysis was performed to assess the associations between network parameters and clinical features in the cLBP group. P<0.05 (FDR corrected) was considered to indicate statistically significant differences.
Results
Demographic and Clinical Data
The demographic data of all participants are presented in . No significant differences were observed in age (p = 0.49), sex (p = 1), MoCA scores (p = 0.31) and HAMA scores (p = 0.12) between the two groups, the HAMD scores (p = 0.049) have significant differences between the two groups. The mean of HAMD and HAMA scores in the cLBP patients were higher than in the HC group (4.76 ± 6.55 and 3.65 ± 7.63, respectively), indicating low depressive and anxiety symptoms in these patients. The VAS scores, present pain intensity scores and pain duration of the cLBP patients are also detailed in .
Table 1 Demographic and Clinical Data of Patients
rsFC Results
Compared with HC, patients with cLBP showed increased rsFC of the left NAc-left SFC (p < 0.001, PWE corrected); left NAc-left middle temporal gyrus (p = 0.002, PWE corrected); left NAc-left angular gyrus (p = 0.005, PWE corrected); left NAc-right middle temporal gyrus (p = 0.043, PWE corrected); left NAc-OFC (p = 0.011, PWE corrected); right NAc-left SFC (p < 0.001, PWE corrected); right NAc-OFC (p = 0.028, PWE corrected); and right NAc-left angular gyrus (p = 0.03, PWE corrected). Patients with cLBP showed decreased rsFC of the left NAc-left supramarginal gyrus (p < 0.001, PWE corrected); left NAc-right precentral gyrus (p = 0.001, PWE corrected); left NAc-left cerebellum (p = 0.007, PWE corrected); left NAc-brainstem (p = 0.01, PWE corrected); and left NAc-right insula (p = 0.008, PWE corrected) (, and ).
Table 2 Resting-State Functional Connectivity of the Nucleus Accumbens
Figure 1 Group-differences of the resting-state functional connectivity of the left NAc. Higher (blue) and lower (red) resting-state functional connectivity was found in the patients with cLBP compared to the HC.
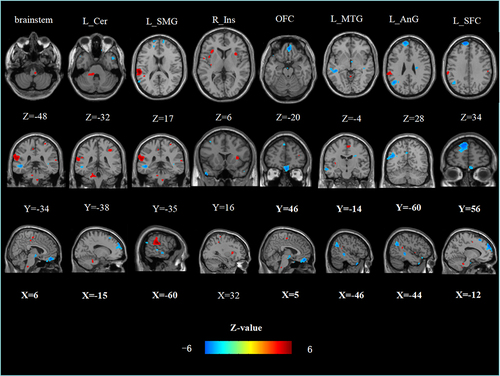
Figure 2 Group-differences of the resting-state functional connectivity of the right NAc. Higher (blue) and lower (red) resting-state functional connectivity was found in the patients with cLBP compared to the HC.
Compared with HC, patients with cLBP showed increased rsFC of left core-left middle temporal gyrus (p = 0.001, PWE corrected); left core-left temporal pole (p = 0.037, PWE corrected); left core-left angular gyrus (p = 0.002, PWE corrected); left core-right middle temporal gyrus (p = 0.005, PWE corrected); left core-left SFC (p = 0.021, PWE corrected); left core-right thalamus proper (p = 0.017, PWE corrected); left shell-OFC (p = 0.008, PWE corrected); left shell-left SFC (p = 0.013, PWE corrected); right shell-left angular gyrus (p = 0.002, PWE corrected); and right shell-left SFC (p = 0.003, PWE corrected). Patients with cLBP showed decreased rsFC of left shell-left supramarginal gyrus (p < 0.001, PWE corrected); shell-left cerebellum (p = 0.007, PWE corrected); left shell-right supramarginal gyrus (p = 0.04, PWE corrected); left shell-left SFC (p < 0.001, PWE corrected); and left shell-right posterior insula (p = 0.007, PWE corrected) (, Figures S1 and S2).
Table 3 Resting-State Functional Connectivity of the Nucleus Accumbens Subregions
Results from DCM Analysis
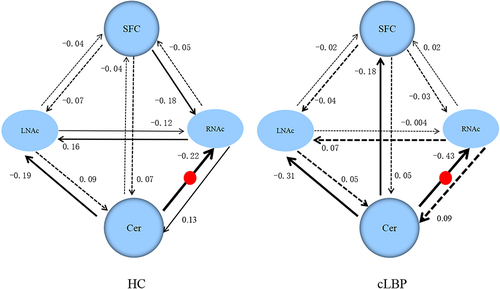
The five models used in DCM are shown in . The results from BMS revealed that the fully connected model was the best model in both the cLBP and HC groups, and it was the best model for 28 of 34 subjects in the HC group and for 31 of 34 patients in the cLBP group. For HC, models 2–4 were the best models for four, one, and one subjects, respectively. For patients with cLBP, models 2 and 4 were the best models for two and one subjects, respectively. The EC values in the cLBP and HC groups are shown in . Patients showed significantly increased EC from the left cerebellum to the right NAc (p = 0.012) compared with HC ().
Table 4 The Effective Connectivity of the Nucleus Accumbens
Figure 3 Effective connectivity of the NAc. (A) Locations of brain regions used in the dynamic causal modelling. (B) Five models used in the DCM analysis. (C) Results from Bayesian model selection; the single-group level t-test showed that the fully connected model was the best model for the two groups.
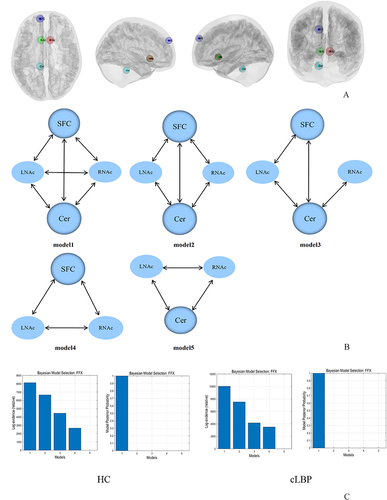
Figure 4 The winning model and the connectivity parameters for the two groups. The solid lines represent connectivity values greater than 0.1 Hz, the dotted lines represent the connectivity values below 0.1 Hz, and their thickness reflects the size of the value. The red round represents the significant group differences between cLBP and HC.
Correlation Analysis
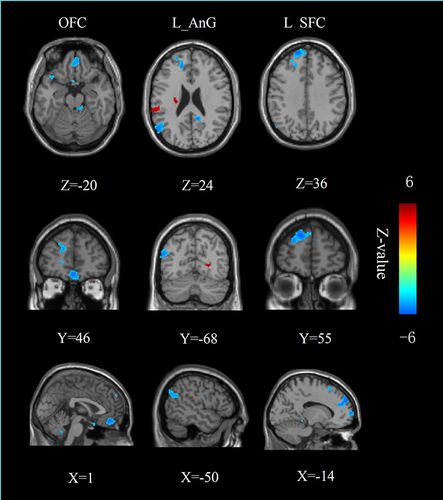
The rsFC of left NAc-left SFC was negatively correlated with HAMD scores (r = −0.402, p = 0.018) in patients with cLBP. The rsFC of left NAc-OFC was positively correlated with the present pain intensity scores (r = 0.406, p = 0.017) in patients with cLBP (). There were no significant correlations between the EC of NAc and pain characteristics, depression, or anxiety scores.
Figure 5 Results of the correlation analysis. There was a significant negative correlation between the rsFC values of the left NAc-SFC and HAMD scores (r = −0.402, p = 0.018), a significant positively correlation between the rsFC values of the left NAc-OFC and the present pain intensity scores (r = 0.406, p = 0.017) in patients group.
Discussion
In this study, we combined rs-fMRI and DCM studies to investigate the functional alterations in the NAc and its subregions in cLBP. We found that the rsFC of bilateral NAc and its subregions with SFC, OFC, left angular gyrus, bilateral middle temporal gyrus, right thalamus showed an increase in cLBP, while that of the bilateral NAc and its subregions with bilateral supramarginal gyrus, right insula, right SFC, right precentral gyrus, brainstem, left cerebellum were decreased in cLBP; the left NAc-left SFC pathway was negatively correlated with HAMD scores, while the left NAc-OFC pathway was positively correlated with present pain intensity scores. In addition, patients showed significantly increased EC from the left cerebellum to the right NAc compared with HC.
The NAc is one of the most commonly activated brain regions in response to painCitation37 and receives both direct input from excitatory glutamatergic projections and indirect input from dopaminergic projections.Citation18,Citation26,Citation38–41 Dopamine holds a central position in reward circuitry.Citation42 A study showed that the baseline dopamine metabolism is reduced in cLBP patients;Citation43 it can also integrate cognitive and affective information processed by frontal and temporal areas,Citation44 as well as can produce m-opioids via activating the descending pain inhibitory system to alleviate pain.Citation45 The changes in NAc-cortical connectivity are regarded as one of the known risk factors for the transition from acute to chronic pain.Citation46 Studies have suggested that the shell and core play prominent roles in reward and aversion processing, respectively.Citation28,Citation35 Pain relief is associated with increased dopamine levels in the NAc shell.Citation17
We found that the rsFC was increased in the pathway of NAc-SFC/OFC, a finding that is consistent with previous studies, who found that the connection between the PFC and the NAc increases in patients who experience the cLBP.Citation47,Citation48 In addition, the rsFC of the left NAc-SFC pathway was negatively correlated with HAMD scores, while the rsFC of left NAc-OFC was positively correlated with present pain intensity scores in the cLBP group. The PFC and NAc are important compositions of the corticolimbic system,Citation14,Citation17,Citation25,Citation46,Citation49 which plays a crucial role in the development, amplification, and prediction of chronic pain and its emotional-affective dimension.Citation20,Citation25,Citation46 A study showed that the corticolimbic system plays a crucial role in the chronic transformation of pain in patients with primary dysmenorrhea.Citation50 The PFC affects several structures involved in pain perception, motivational drive, substance seeking, and anxiodepressive states.Citation17,Citation40 The PFC-NAc pathway is an important node in the reward circuitry and plays an important role in regulating affective and motivational components of pain.Citation51 A longitudinal study found that the increased FC of PFC-NAc pathway can predict pain persistence.Citation47 Another study reported that the OFC is of vital importance for the adaptive regulation of emotional states and pain,Citation38,Citation52 as it mediates pain inhibition.Citation53 Therefore, we hypothesized that the abnormalities in the FC of PFC-NAC pathway may be one of the most critical reasons for the persistence of pain and negative emotions in cLBP.
We also found that rsFC of the left NAc-right brainstem (medulla oblongata), left cerebellum pathway decreased in the cLBP group, and the EC increased from the left cerebellum to the right NAc. The brainstem is the origin of the descending pain inhibitory system,Citation54 and it is a critical area for nociception and pain processing that also plays an important role in relaying and coordinating signaling between the cerebrum, cerebellum, and spinal cord.Citation39,Citation55 The cerebellum is crucially implicated involved in the modulation of sensorimotor responses and higher level cognitive and affective function.Citation31,Citation56,Citation57 The neocortex-cerebellum and basal ganglia-cerebellum circuit are important in reward-related cognitive and emotion processing.Citation58–60 A study found a positive relationship between the local pontine network activity and the intensity of cLBP,Citation61 wherein compared with HC, patients with cLBP showed significantly decreased rsFC between the habenula and pons compared with HC.Citation32 Another study reported significantly altered EC in the cerebellum-neocortex and cerebellum-basal ganglia circuits in patients with major depressive disorder,Citation31 and decreased rsFC between bilateral cerebellar and cortical brain regions in patients with cLBP after motor control exercise.Citation62 Both the brainstem and the cerebellum have important roles in the regulation of pain and emotion; therefore, the abnormalities of the rsFC and EC between them and NAC may be an important reason for the persistence of pain and negative emotions in cLBP.
The thalamus relays nociceptive information to the insula, PFC, and cerebellum to process sensory-discriminative properties and affective components of the pain sensation and the formation of pain memory.Citation37,Citation63 The role of the thalamus in pain has been extensively studied, and numerous studies have confirmed the abnormality in thalamic structure and function with cLBP.Citation2,Citation64,Citation65 Previous studies have shown that compared to the control group, patients with diabetic peripheral neuropathy who had pain for >2 years showed decreased rsFC between the primary somatosensory cortex, ventral posterior lateral thalamic nucleus, and medial dorsal thalamic nucleus compared to the control group.Citation66 The insula integrates sensory with emotional and cognitive processes and is involved in aversive motivational salience,Citation67 that plays critical roles in pain perception and chronic pain.Citation68–72 Another previous study showed that the PAG-bilateral posterior insular rsFC is reduced in patients with chronic neck and shoulder pain.Citation73
The angular gyrus is a major posterior component of the default-mode networks (DMNs), which may participate in regulation of memory and emotion.Citation74 The temporal lobe has an important influence on higher neural activities such as memory and emotion.Citation75 Therefore, we hypothesized that the abnormalities in the rsFC of NAC-angular gyrus and middle temporal gyrus pathway may likely mainly contribute to the dysfunction of emotions in cLBP.
Clinical Implications
Our study highlights the important role of NAc in cLBP, which has contributed to understanding the brain’s regulatory role in cLBP and provided a theoretical basis for understanding the relationship between emotions and chronic pain. Our study is important for understanding the underlying mechanisms that positive emotions improve symptoms in patients with cLBP and provide a theoretical basis for identifying effective treatments.
Limitations
Our study has some limitations. First, the sample size is relatively small; thus, we plan to include a larger sample of patients and controls in future studies, as this study is still ongoing. Second, this study used a cross-sectional design, and the causal relationship between brain changes and cLBP is still unclear. A longitudinal study is necessary to understand the causal relationships of NAc alterations and the pathogenesis underlying cLBP. Third, the scales describing the cognitive states are not sufficient. In future studies, more cognitive and emotional scales will be included.
Conclusion
Our results show that the altered rsFC and EC of the NAc were mainly located at regions that were related to both pain and emotion processing, which suggests that the reward system is involved in the regulation of cLBP. Our results suggest that NAc plays an important role in relieving pain and improving the prognosis of patients with cLBP. The NAc may be an important target for pain relief and to improve the emotional discomfort caused by pain.
Ethics Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Disclosure
The authors declare no conflicts of interest in this work.
Acknowledgments
The authors thank all the participants for their participation in the study.
Data Sharing Statement
The data of the 68 participants are in-house dataset and are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Buchbinder R, van Tulder M, Öberg B, et al. Low back pain: a call for action. Lancet. 2018;391(10137):2384–2388. doi:10.1016/s0140-6736(18)30488-4
- Tu Y, Fu Z, Mao C, et al. Distinct thalamocortical network dynamics are associated with the pathophysiology of chronic low back pain. Nat Commun. 2020;11(1):3948. doi:10.1038/s41467-020-17788-z
- Knezevic NN, Candido KD, Vlaeyen JWS, Van Zundert J, Cohen SP. Low back pain. Lancet. 2021;398(10294):78–92. doi:10.1016/s0140-6736(21)00733-9
- Murray K, Lin Y, Makary MM, Whang PG, Geha P. Brain structure and function of chronic low back pain patients on long-term opioid analgesic treatment: a preliminary study. Article. Molecular Pain. 2021;171744806921990938. doi:10.1177/1744806921990938
- Wu A, March L, Zheng X, et al. Global low back pain prevalence and years lived with disability from 1990 to 2017: estimates from the Global Burden of Disease Study 2017. Ann Translat Med. 2020;8(6):299.
- Zhao X, Boersma K, Gerdle B, Molander P, Hesser H. Fear network and pain extent: interplays among psychological constructs related to the fear-avoidance model. J Psychosom Res. 2023;167:111176. doi:10.1016/j.jpsychores.2023.111176
- Martinez-Pozas O, Sánchez-Romero EA, Beltran-Alacreu H, et al. Effects of orthopedic manual therapy on pain sensitization in patients with chronic musculoskeletal pain. Am J Phys Med Rehabilit. 2023;102(10):879–885. doi:10.1097/phm.0000000000002239
- Cuenca-Zaldívar JN, Fernández-Carnero J, Sánchez-Romero EA, et al. Effects of a therapeutic exercise protocol for patients with chronic non-specific back pain in primary health care: a single-group retrospective cohort study. J Clin Med. 2023;12(20):6478. doi:10.3390/jcm12206478
- Chuhuai Wang M, Li Y, Zhang Z. Motor control exercise modulates the neural plasticity of the default mode network in patients with chronic low back pain. Pain Physician. 2024;27(1):E55–E64.
- Ballestra E, Battaglino A, Cotella D, Rossettini G, Romero EAS, Villafañe H. Do patients’ expectations influence conservative treatment in Chronic Low Back Pain? A narrative review. Retos. 2022;46:395–403. doi:10.47197/retos.v46.93950
- Romero ESA, Lim T, Villafañe JH, et al. The influence of verbal suggestion on post-needling soreness and pain processing after dry needling treatment: an experimental study. Int J Environ Res Public Health. 2021;18(8):4206. doi:10.3390/ijerph18084206
- Malfliet A, Lluch Girbés E, Pecos‐Martin D, Gallego‐Izquierdo T, Valera‐Calero A. The influence of treatment expectations on clinical outcomes and cortisol levels in patients with chronic neck pain: an experimental study. Pain Pract. 2019;19(4):370–381. doi:10.1111/papr.12749
- Amaro-Diaz L, Montoro CI, Fischer-Jbali LR, Galvez-Sanchez CM. Chronic pain and emotional stroop: a systematic review. J Clin Med. 2022;11(12):3259. doi:10.3390/jcm11123259
- Vachon-Presseau E, Tétreault P, Petre B, et al. Corticolimbic anatomical characteristics predetermine risk for chronic pain. Brain. 2016;139(7):1958–1970. doi:10.1093/brain/aww100
- Ding Y-D, Chen X, Chen Z-B, et al. Reduced nucleus accumbens functional connectivity in reward network and default mode network in patients with recurrent major depressive disorder. Transl Psychiatry. 2022;12(1):236. doi:10.1038/s41398-022-01995-x
- Hu Y, Zhao C, Zhao H, Qiao J. Abnormal functional connectivity of the nucleus accumbens subregions mediates the association between anhedonia and major depressive disorder. BMC Psychiatry. 2023;23(1):282. doi:10.1186/s12888-023-04693-0
- Serafini RA, Pryce KD, Zachariou V. The mesolimbic dopamine system in chronic pain and associated affective comorbidities. Biol Psychiatry. 2020;87(1):64–73. doi:10.1016/j.biopsych.2019.10.018
- Salgado S, Kaplitt MG. The nucleus accumbens: a comprehensive review. Stereotact Function Neurosurg. 2015;93(2):75–93. doi:10.1159/000368279
- Löffler M, Levine SM, Usai K, et al. Corticostriatal circuits in the transition to chronic back pain: the predictive role of reward learning. Cell Rep Med. 2022;3(7):100677. doi:10.1016/j.xcrm.2022.100677
- Makary MM, Polosecki P, Cecchi GA, et al. Loss of nucleus accumbens low-frequency fluctuations is a signature of chronic pain. Proc Natl Acad Sci. 2020;117(18):10015–10023. doi:10.1073/pnas.1918682117
- Rizvi SJ, Gandhi W, Salomons T. Reward processing as a common diathesis for chronic pain and depression. Review. Neurosci Biobehav Rev. 2021;127:749–760. doi:10.1016/j.neubiorev.2021.04.033
- Noursadeghi E, Haghparast A. Modulatory role of intra-accumbal dopamine receptors in the restraint stress-induced antinociceptive responses. Article. Brain Res Bull. 2023;195:172–179. doi:10.1016/j.brainresbull.2023.03.003
- Chae Y, Park HJ, Lee IS. Pain modalities in the body and brain: current knowledge and future perspectives. Neurosci Biobehav Rev. 2022;139:104744. doi:10.1016/j.neubiorev.2022.104744
- Gilam G, Gross JJ, Wager TD, Keefe FJ, Mackey SC. What is the relationship between pain and emotion? Bridging constructs and communities. editorial material. Neuron. 2020;107(1):17–21. doi:10.1016/j.neuron.2020.05.024
- Barroso J, Branco P, Apkarian AV. Brain mechanisms of chronic pain: critical role of translational approach. Transl Res. 2021;238:76–89. doi:10.1016/j.trsl.2021.06.004
- Yan H, Shlobin NA, Jung Y, et al. Nucleus accumbens: a systematic review of neural circuitry and clinical studies in healthy and pathological states. Review. J Neurosurg. 2022;138(2):337–346. doi:10.3171/2022.5.Jns212548
- Li CS, Liu SF, Lu XH, Tao F. Role of descending dopaminergic pathways in pain modulation. Curr Neuropharmacol. 2019;17(12):1176–1182. doi:10.2174/1570159x17666190430102531
- Liu R, Wang Y, Chen XY, Zhang ZF, Xiao L, Zhou Y. Anhedonia correlates with functional connectivity of the nucleus accumbens subregions in patients with major depressive disorder. Neuroimage Clin. 2021;30:102599. doi:10.1016/j.nicl.2021.102599
- Zhao X, Yang R, Wang K, et al. Connectivity-based parcellation of the nucleus accumbens into core and shell portions for stereotactic target localization and alterations in each NAc subdivision in mTLE patients. Article. Human Brain Mapp. 2018;39(3):1232–1245. doi:10.1002/hbm.23912
- Frässle S, Harrison SJ, Heinzle J, et al. Regression dynamic causal modeling for resting-state fMRI. Human Brain Mapp. 2021;42(7):2159–2180. doi:10.1002/hbm.25357
- Dai PS, Zhou XY, Xiong T, et al. Altered effective connectivity among the cerebellum and cerebrum in patients with major depressive disorder using multisite resting-state fMRI. Cerebellum. 2023;22(5):781–789. doi:10.1007/s12311-022-01454-9
- Mao CP, Wu Y, Yang HJ, et al. Altered habenular connectivity in chronic low back pain: an fMRI and machine learning study. Article. Human Brain Mapp. 2023;44(11):4407–4421. doi:10.1002/hbm.26389
- Yilmaz H, Güler H. Can video‐assisted and three‐dimensional (3D) anatomy teaching be an alternative to traditional anatomy teaching? Randomized controlled trial on muscular system anatomy. Clin Anat. 2024;37(2):227–232. doi:10.1002/ca.24088
- Zhang H, Xia D, Wu X, et al. Abnormal intrinsic functional interactions within pain network in cervical discogenic pain. Front Neurosci. 2021;15:671280. doi:10.3389/fnins.2021.671280
- Xia X, Fan L, Cheng C, et al. Multimodal connectivity-based parcellation reveals a shell-core dichotomy of the human nucleus accumbens. Review. Human Brain Mapp. 2017;38(8):3878–3898. doi:10.1002/hbm.23636
- Mao CP, Yang HJ, Yang QX, Sun HH, Zhang GR, Zhang QJ. Altered amygdala-prefrontal connectivity in chronic nonspecific low back pain: resting-state fMRI and dynamic causal modelling study. Neuroscience. 2022;482:18–29. doi:10.1016/j.neuroscience.2021.12.003
- Kummer KK, Mitric M, Kalpachidou T, Kress M. The medial prefrontal cortex as a central hub for mental comorbidities associated with chronic pain. Review. Int J Mol Sci. 2020;21(10):3440. doi:10.3390/ijms21103440
- Ong WY, Stohler CS, Herr DR. Role of the prefrontal cortex in pain processing. Mol Neurobiol. 2019;56(2):1137–1166. doi:10.1007/s12035-018-1130-9
- Gamal-Eltrabily M, Martínez-Lorenzana G, González-Hernández A, Condés-Lara M. Cortical modulation of nociception. Neuroscience. 2021;458:256–270. doi:10.1016/j.neuroscience.2021.01.001
- Wang X-Q, Mokhtari T, Zeng Y-X, L-p Y, Hu L, Wang X-Q. The distinct functions of dopaminergic receptors on pain modulation: a narrative review. Neural Plast. 2021;2021:1–11. doi:10.1155/2021/6682275
- Seminowicz DA, Remeniuk B, Krimmel SR, et al. Pain-related nucleus accumbens function: modulation by reward and sleep disruption. Pain. 2019;160(5):1196–1207. doi:10.1097/j.pain.0000000000001498
- Lewis RG, Florio E, Punzo D, Borrelli E. The Brain’s Reward System in Health and Disease. Springer; 2021.
- Martikainen IK, Nuechterlein EB, Pecina M, et al. Chronic back pain is associated with alterations in dopamine neurotransmission in the ventral striatum. J Neurosci. 2015;35(27):9957–9965. doi:10.1523/JNEUROSCI.4605-14.2015
- Chen L, Wang J, Xia M, et al. Altered functional connectivity of nucleus accumbens subregions associates with non‐motor symptoms in Parkinson’s disease. CNS Neurosci Ther. 2022;28(12):2308–2318. doi:10.1111/cns.13979
- Konno S-I, Sekiguchi M. Association between brain and low back pain. J Orthop Sci. 2018;23(1):3–7. doi:10.1016/j.jos.2017.11.007
- Thompson JM, Neugebauer V. Cortico-limbic pain mechanisms. Neurosci Lett. 2019;702:15–23. doi:10.1016/j.neulet.2018.11.037
- Baliki MN, Petre B, Torbey S, et al. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat Neurosci. 2012;15(8):1117–1119. doi:10.1038/nn.3153
- Martinez E, Lin HH, Zhou H, Dale J, Liu K, Wang J. Corticostriatal regulation of acute pain. Front Cell Neurosci. 2017;11:146. doi:10.3389/fncel.2017.00146
- Park SH, Baker AK, Krishna V, Mackey SC, Martucci KT. Altered resting-state functional connectivity within corticostriatal and subcortical-striatal circuits in chronic pain. Sci Rep. 2022;12(1):12683. doi:10.1038/s41598-022-16835-7
- Liu N, Li Y, Hong Y, et al. Altered brain activities in mesocorticolimbic pathway in primary dysmenorrhea patients of long-term menstrual pain. Front Neurosci. 2023;17:1098573. doi:10.3389/fnins.2023.1098573
- Tan LL, Kuner R. Neocortical circuits in pain and pain relief. Nat Rev Neurosci. 2021;22(8):458–471. doi:10.1038/s41583-021-00468-2
- Liu N, Huo J, Li Y, et al. Changes in brain structure and related functional connectivity during menstruation in women with primary dysmenorrhea. Article. Quant Imaging Med Surg. 2023;13(2):1071–1082. doi:10.21037/qims-22-683
- Zhou F, Gu L, Hong S, et al. Altered low-frequency oscillation amplitude of resting state-fMRI in patients with discogenic low-back and leg pain. Article. J Pain Res. 2018;11:165–176. doi:10.2147/jpr.S151562
- Lv Q, Wu F, Gan X, et al. The involvement of descending pain inhibitory system in electroacupuncture-induced analgesia. Review. Front Integr Neurosci. 2019;13:38. doi:10.3389/fnint.2019.00038
- Napadow V, Sclocco R, Henderson LA. Brainstem neuroimaging of nociception and pain circuitries. Pain Rep. 2019;4(4):e745. doi:10.1097/PR9.0000000000000745
- Van Overwalle F, Van de Steen F, Mariën P. Dynamic causal modeling of the effective connectivity between the cerebrum and cerebellum in social mentalizing across five studies. Cognit Affect Behav Neurosci. 2019;19(1):211–223. doi:10.3758/s13415-018-00659-y
- Fastenrath M, Spalek K, Coynel D, et al. Human cerebellum and corticocerebellar connections involved in emotional memory enhancement. Proc Natl Acad Sci. 2022;119(41):e2204900119. doi:10.1073/pnas.2204900119
- Wagner MJ, Luo L. Neocortex–cerebellum circuits for cognitive processing. Trends Neurosci. 2020;43(1):42–54. doi:10.1016/j.tins.2019.11.002
- Pierce JE, Péron J. The basal ganglia and the cerebellum in human emotion. Soc Cognit Affect Neurosci. 2020;15(5):599–613. doi:10.1093/scan/nsaa076
- Yoshida J, Oñate M, Khatami L, Vera J, Nadim F, Khodakhah K. Cerebellar contributions to the basal ganglia influence motor coordination, reward processing, and movement vigor. J Neurosci. 2022;42(45):8406–8415. doi:10.1523/JNEUROSCI.1535-22.2022
- Jahn P, Deak B, Mayr A, et al. Intrinsic network activity reflects the ongoing experience of chronic pain. Sci Rep. 2021;11(1):21870. doi:10.1038/s41598-021-01340-0
- Zhang C, Zhang Z, Li Y, et al. Alterations in functional connectivity in patients with non-specific chronic low back pain after motor control exercise: a randomized trial. Eur J Phys Rehabil Med. 2024;60(2). doi:10.23736/S1973-9087.24.08087-0
- Groh A, Krieger P, Mease RA, Henderson L. Acute and chronic pain processing in the thalamocortical system of humans and animal models. Neuroscience. 2018;387:58–71. doi:10.1016/j.neuroscience.2017.09.042
- Mao CP, Wilson G, Cao J, Meshberg N, Huang Y, Kong J. Abnormal anatomical and functional connectivity of the thalamo-sensorimotor circuit in chronic low back pain: resting-state functional magnetic resonance imaging and diffusion tensor imaging study. Neuroscience. 2022;487:143–154. doi:10.1016/j.neuroscience.2022.02.001
- Riegner G, Posey G, Oliva V, Jung Y, Mobley W, Zeidan F. Disentangling self from pain: mindfulness meditation–induced pain relief is driven by thalamic–default mode network decoupling. Pain. 2023;164(2):280–291. doi:10.1097/j.pain.0000000000002731
- Chitneni A, Rupp A, Ghorayeb J, Abd-Elsayed A. Early detection of diabetic peripheral neuropathy by fMRI: an evidence-based review. Brain Sci. 2022;12(5):557. doi:10.3390/brainsci12050557
- Gogolla N. The insular cortex. Curr Biol. 2017;27(12):R580–R586. doi:10.1016/j.cub.2017.05.010
- Lee J-HA, Chen Q, Zhuo M. Synaptic plasticity in the pain-related cingulate and insular cortex. Biomedicines. 2022;10(11):2745. doi:10.3390/biomedicines10112745
- Lu C, Yang T, Zhao H, et al. Insular cortex is critical for the perception, modulation, and chronification of pain. Review. Neurosci Bull. 2016;32(2):191–201. doi:10.1007/s12264-016-0016-y
- Wang N, Zhang Y-H, Wang J-Y, Luo F. Current understanding of the involvement of the insular cortex in neuropathic pain: a narrative review. Review. Int J Mol Sci. 2021;22(5):2648. doi:10.3390/ijms22052648
- Bastuji H, Frot M, Perchet C, Hagiwara K, Garcia-Larrea L. Convergence of sensory and limbic noxious input into the anterior insula and the emergence of pain from nociception. Article. Sci Rep. 2018;8(1):3360. doi:10.1038/s41598-018-31781-z
- Labrakakis C. The role of the insular cortex in pain. Int J Mol Sci. 2023;24(6):5736. doi:10.3390/ijms24065736
- Xu H, Chen Y, Tao Y, et al. Modulation effect of acupuncture treatment on chronic neck and shoulder pain in female patients: evidence from periaqueductal gray-based functional connectivity. Article; Early Access. CNS Neurosci Ther. 2022;28(5):714–723. doi:10.1111/cns.13803
- Lan F, Lin G, Cao G, et al. Altered intrinsic brain activity and functional connectivity before and after knee arthroplasty in the elderly: a resting-state fMRI study. Article. Front Neurol. 2020:11556028. doi:10.3389/fneur.2020.556028
- Feng L, Wu D, Ma S, et al. Resting-state functional connectivity of the cerebellum-cerebrum in older women with depressive symptoms. Article. BMC Psychiatry. 2023;23(1):732. doi:10.1186/s12888-023-05232-7
