Abstract
Attention-deficit/hyperactivity disorder (ADHD) is a chronic psychiatric disorder characterized by hyperactivity and/or inattention and is often associated with a substantial impact on psychosocial functioning. Methylphenidate (MPH), a central nervous system stimulant, is commonly used for pharmacological treatment of adults and children with ADHD. Current practice guidelines recommend optimizing MPH dosage to individual patient needs; however, the clinical benefits of individual dose optimization compared with fixed-dose regimens remain unclear. Here we review the available literature on MPH dose optimization from clinical trials and real-world experience on ADHD management. In addition, we report safety and efficacy data from the largest MPH modified-release long-acting Phase III clinical trial conducted to examine benefits of dose optimization in adults with ADHD. Overall, MPH is an effective ADHD treatment with a good safety profile; data suggest that dose optimization may enhance the safety and efficacy of treatment. Further research is required to establish the extent to which short-term clinical benefits of MPH dose optimization translate into improved long-term outcomes for patients with ADHD.
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is one of the most common chronic childhood-onset psychiatric disorders, with an estimated global prevalence of 5.9%–7.1% among children/adolescents and 1.2%–7.3% among adults.Citation1–Citation3 ADHD was initially considered a childhood disorder that dissipated by adolescence or adulthoodCitation4; however, it is now defined as a chronic disorder that is reported to persist into adulthood in ~30%–80% of those who retrospectively reported childhood ADHD.Citation5–Citation7 Children with ADHD are often characterized as hyperactive, and adults with ADHD are more likely to experience inner restlessness, an inability to relax, overtalkativeness, inattention, poor planning, and impulsivity.Citation6,Citation8,Citation9 ADHD has a significant impact on psychosocial functioning (lower level of education, higher level of unemployment, and higher rates of unsuccessful marriages, criminality, and road traffic accidents).Citation10,Citation11
Treatment strategies for ADHD include both pharmacological and psychological therapies aimed to improve the core symptoms of ADHD (hyperactivity, impulsivity, and inattention) and associated functional impairments. Psychostimulants, including methylphenidate (MPH) and amphetamine-based treatments, are the most commonly prescribed pharmacological treatments for adults and children with ADHD.Citation12–Citation14 Despite being one of the mainstay treatments for ADHD, MPH use remains off-label for adults in some countries.Citation15,Citation16
Based on the efficacy data from randomized controlled trials and meta-analyses, MPH is now a widely accepted treatment for ADHD.Citation17,Citation18 Moreover, MPH is also reported to reduce the social, health, economical, and functional impairments experienced by ADHD patients.Citation6,Citation19–Citation22 However, response to MPH treatment varies significantly between patients.Citation17,Citation23,Citation24 Thus, certain patients may not achieve adequate symptom control, while others are unable to tolerate the same dose of MPH because of adverse events (AEs).Citation25–Citation28 Recent guidelines recommend tailoring MPH dosage to individual patient’s needs (referred to as dose optimization, dose individualization, or flexible dose; ).Citation29–Citation31 However, no head-to-head studies have directly compared benefits of flexible-versus fixed-dose treatment regimens.
Table 1 Methylphenidate Treatment Guidelines: International and National ADHD Treatment recommendationsTable Footnotea
The present review examines the recommendations for dose optimization in recent ADHD treatment guidelines, and the use of dose optimization in randomized clinical trials, as well as naturalistic studies. In addition, previously unpublished analysis from the largest MPH modified-release long-acting (MPH-LA) trial is presented to highlight the potential efficacy and safety benefits of dose optimization versus randomized fixed-dose regimens.
Dose optimization in clinical practice
Dose optimization is used routinely in general medicine and psychiatry to ensure optimal clinical effect, while minimizing the risk of AEs. Dose optimization is common for almost all psychotropic medications and may be crucial, particularly when considering clinical dose–response relationships with high interindividual variation, such as the use of stimulants for treatment of ADHD.Citation32,Citation33 Various factors can influence the need for dose optimization including genetic variability, patient weight, age, sex, drug-induced tolerance, and interactions with other medications or medical conditions. The length of time required and the method used to optimize dosage can vary extensively between pharmacological treatments.
MPH has a wide interindividual variability in dose response. In a meta-analysis of 18 randomized controlled trials, Castells et al found a positive dose–response relationship between MPH and ADHD symptoms in adults; however, in large clinical trials investigating a range of various MPH formulations, no dose–response relationship was found in adults with ADHD.Citation17,Citation34–Citation36
Moreover, the dose–response relationship of MPH has also been debated in pediatric studies. Reportedly, a dose–response relationship was observed with osmotic-controlled release oral delivery system methylphenidate (OROS-MPH) in children with combined-type ADHD, whereas such a relationship was not noted in children with ADHD of a predominantly inattentive subtype.Citation37 Furthermore, the association between dose optimization and weight remains inconclusive; some studies have reported no relationship between the final optimal dose and body weight, while others have indicated weight as a reliable clinical parameter for dose optimization.Citation38–Citation40 Douglas et al reported a linear dose–response relationship between dose (0.15, 0.30, and 0.60 mg/kg) and improvements in performance.Citation41 This substantial intersubject variability has prompted the use of dose optimization in ADHD management.
MPH dose optimization: current recommendations
A number of European guidelines, including Swedish ADHD and National Institute for Health and Care Excellence (NICE) guidelines, recommend MPH as the first-line pharmacological treatment for adults and children with severe impairment (or moderate impairment but nonresponsive to nonpharmacological interventions).Citation29,Citation42 MPH is available as an immediate-release (MPH-IR) tablet and in a variety of modified-release formulations including long-acting formulations, topical patches, and extended-release formulations.Citation43–Citation48 In accordance with most ADHD treatment guidelines (eg, NICE), product guidelines recommend that MPH-naïve patients must be initially treated at low doses, and the doses should be increased on an individual basis until optimal response is achieved without an AE.Citation43–Citation48 Several ADHD treatment guidelines recommend that long- or short-acting stimulant medications should be prescribed based on individual patient needs.Citation16,Citation29,Citation49 However, other guidelines recommend the use of long-acting stimulants as first-line treatment.Citation30
European, Australasian, and North American ADHD guidelines support the use of pharmacotherapy for children with severe ADHD, children with moderate ADHD who are nonresponsive to behavioral therapy, and adults with moderate or severe ADHD. Collectively, these guidelines also highlight the need for dose titration and optimization of pharmacological therapy ().Citation6,Citation29–Citation31 Moreover, the Canadian Attention Deficit Hyperactivity Disorder Resource Alliance guidelines recommend regular follow-ups to ensure treatment efficacy and tolerability.Citation30 It is important to note that treatment guidelines vary regarding how dose optimization should be managed. Certain guidelines define optimal treatment as the dose above which there is no further improvement, whereas other guidelines recommend using the lowest dose necessary to achieve optimal therapeutic response.Citation30,Citation49 Therefore, methods of optimization tend to vary between clinical studies. In certain trials, doses have been increased until the patient responds or until the maximum dose is reached.Citation50 In other studies, doses have been increased until the occurrence of an AE, whereby the dose was then decreased to the previously tolerated dose.Citation34 Another way to find an optimal dose is the use of a blinded N=1 strategy, where different doses are given to the patient in a blinded manner to find the best balance between symptom control and possible AEs. Although blinded N=1 trials are a promising way of dose optimization at the individual patient level, they are not widely used as they are considered time-consuming and no standards are established on possible ranges, duration of drug or placebo exposure, and titration schedules on the N=1 level.
Despite a high level of consistency between national and international treatment guidelines () and MPH product labels, in the real-world clinical treatment of ADHD, health care providers may not adhere to these recommendations.Citation43–Citation48 Goodman et alCitation51 reported that a high proportion of family physicians are not confident with diagnosing and treating adult ADHD. A survey of 1,216 primary care physicians (PCPs) revealed that 31% of PCPs rated themselves as “not confident” when diagnosing adult ADHD, and 36% were “not confident” regarding treatment. In the same survey, psychiatrists were more confident with diagnosis and treatment of ADHD. Furthermore, a recently published US study of over 1,500 patient charts from 188 pediatricians across 50 different practices, from August 2002 through December 2012, demonstrated that community-based ADHD care was not consistent with the guidelines.Citation52 Overall, only 47.4% of children prescribed medication had contact (phone contact or visit) with their pediatrician within the first month of their prescription, and parent- and teacher-rating scales were rarely collected to monitor treatment response or side effects. These data indicate the need for improved awareness of how best to manage pharmacological dose titration to achieve optimal dosage.
Dose optimization in clinical trials and naturalistic studies
MPH dose optimization in clinical trials
Although MPH dose optimization is recommended in the ADHD treatment guidelines, and clinical trials report flexible-dose therapy, fixed-dose therapy, or a combination of both, analyses of the clinical benefits of dose optimization versus fixed-dose regimens for patients are limited. A recent meta-regression analysis of 18 randomized, placebo-controlled studies compared the outcomes of MPH clinical trials that included flexible-dose regimens (12 studies; 1,327 adults with ADHD) to those that included fixed-dose regimens (6 studies; 718 adults with ADHD).Citation17 However, none of the trials included in their analysis directly compared fixed-versus flexible-dose regimens.
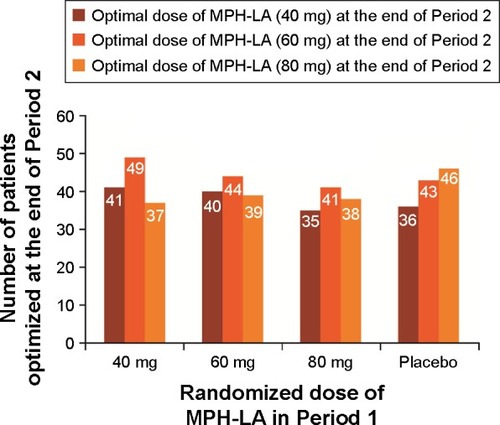
Recent randomized, controlled ADHD trials that have incorporated a flexible-dose regimen have reported that a range of MPH doses are required to effectively treat patients.Citation38,Citation50,Citation53–Citation56 In a randomized, placebo-controlled trial (), Huss et alCitation53 reported that several doses of MPH-LA (40, 60, and 80 mg) were determined to be optimal for a similar number of ADHD patients (n=152, n=177, and n=160, respectively). Further analysis of the MPH-LA study data showed that this was irrespective of the initial dose administered during Period 1 (; previously unpublished data). Similarly, other clinical trials have also reported that multiple doses of MPH are required for adequate treatment of adults and children with ADHD. Buitelaar et alCitation54 reported that a range of OROS-MPH doses are required; overall, 7.9% of patients completed the study on 18 mg of OROS-MPH, 29.3% on 36 mg, 34.4% on 54 mg, 19.8% on 72 mg, and 8.7% on 90 mg. In another study wherein children and adolescents treated with MPH extended-release, a number of doses were also required for optimal treatmentCitation38; overall, 27.7% of pediatric patients had received a final open-label dose of 30 mg, 25.2% had 40 mg, 17.8% had 50 mg, 16.8% had 20 mg, 8.9% had 60 mg, 2.0% had 15 mg, and 1.5% had 10 mg. The need for multiple doses of MPH to achieve effective symptom control further highlights the importance of an individualized or tailored treatment approach for ADHD. Recently, Chermá et alCitation40 reported a large variation in the optimal treatment dose of 59 patients with ADHD; however, blood sample analysis revealed that the median daily dose per body weight was similar (~1.0 mg/kg). As per the available literature, genetic differences may lead to significant variability in pharmacokinetic profiles across the day at an individual level, which may result in the large variation observed.Citation58
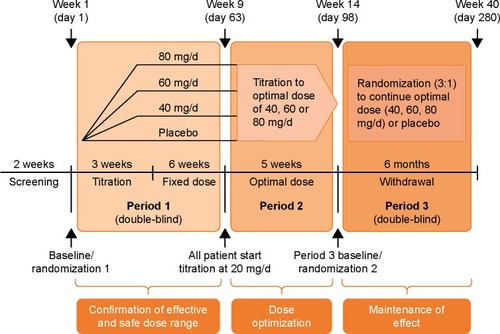
Figure 1 The MPH-LA trial design includes a combination of fixed-dose (Period 1) and flexible-dose (Period 2 and Period 3) periods in a single study to assess the efficacy of MPH-LA in adult ADHD and to identify the individualized optimal dose for patients. Reproduced from Huss M, Ginsberg Y, Tvedten T, et al. Methylphenidate hydrochloride modified-release in adults with attention deficit hyperactivity disorder: a randomized double-blind placebo-controlled trial. Adv Ther. 2014;31(1):44–65.Citation34
MPH dose optimization in naturalistic and open-label extension studies
Recent noninterventional or naturalistic studies have examined the efficacy and/or safety of MPH in children, adolescents, or adults with ADHD.Citation57 Fredriksen et al conducted a naturalistic study to assess the 1-year efficacy of ADHD treatment regimens in adults with ADHD.Citation59 All patients received MPH as the first-line pharmacological treatment in addition to psychosocial interventions. The study was conducted at a specialized outpatient clinic in Norway and involved a 6-week MPH titration phase (up to 60 mg/day) followed by a dose-optimization phase (maximum dose 120 mg/day). Under these real-world conditions, ~70% of patients completed the 1-year study phase without discontinuing pharmacological treatment: 128 patients continued to receive MPH, 25 had switched to dextroamphetamine, and 10 had switched to atomoxetine. In line with randomized, controlled clinical trials, AEs and lack of efficacy were reported as the most common reasons for treatment discontinuation.Citation59,Citation60 Moreover, the study revealed that approximately half of the patients preferred immediate-release MPH over extended-release formulations, highlighting the need for clinicians to consider alternative MPH formulations on an individual patient basis.
Cortese et al have recently published long-term MPH-IR safety data from the Italian National ADHD RegistryCitation61; similar to the study conducted by Fredriksen et al, treatment was individualized based on clinical response and tolerability (maximum dose 0.6 mg/kg/dose).Citation59 The safety profile was favorable as 25.9% of MPH-treated patients had experienced at least one mild AE and 4.5% had experienced at least one severe AE.Citation61
Open-label extension studies of randomized controlled trials have provided evidence regarding the long-term benefits and safety of dose-optimized MPH treatment.Citation53,Citation63–Citation65 Buitelaar et al evaluated the long-term efficacy and tolerance of OROS-MPH, wherein no evidence of withdrawal or rebound was reported after treatment discontinuation.Citation64 As previously reported in randomized controlled trials, the most common reason reported for treatment discontinuation was AE.Citation60 Ginsberg et al reported a lower rate (2.3%) of AE-induced discontinuation during the extension phase of the MPH-LA study compared with other open-label extension studies such as OROS-MPH (18.5%).Citation55,Citation63 They suggested that this was because of the use of flexible-dose regimens, which was allowed during the extension phase of the study.
MPH-LA clinical trial: design and analysis
Huss et al designed the only clinical trial (NCT01259492) including both fixed- and flexible-dose phases that directly investigated the benefits of flexible-dose regimens.Citation34,Citation53 This MPH-LA trial encompassed both fixed- and flexible dosing in a single patient cohort ().Citation34,Citation53 This MPH-LA trial was a 40-week, randomized, double-blind, placebo-controlled, multicenter, efficacy and safety study of MPH-LA in the treatment of adult patients with childhood-onset ADHD. The primary objectives of the trial were to 1) confirm the clinically effective dose range; 2) confirm the maintenance effect of MPH-LA; and 3) evaluate safety profile of MPH-LA vs placebo. The three-phase study design also allowed for post hoc analysis of the benefits of dose optimization.Citation53 Symptomatic and functional responses to MPH-LA were evaluated using validated instruments, including the Diagnostic and Statistical Manual of Mental Disorders, 4th edition Attention-Deficit/Hyperactivity Disorder Rating Scale (DSM-IV ADHD RS), and Sheehan Disability Scale (SDS).Citation66,Citation67 Published results demonstrate that dose optimization of MPH-LA is possible, sudden switching between doses is safe, dose optimization with MPH-LA (40, 60, or 80 mg/day) improves symptomatic and functional treatment outcomes, and numerous doses of MPH-LA are required for optimal treatment of adult ADHD.Citation34,Citation53
Few other studies have also included an initial fixed-dose period followed by MPH dose optimization; however, these studies have not assessed the benefits of dose optimization. Wigal et al reported that pediatric patients continued to improve during an 11-week dose-optimization phase (open-label period).Citation38 In addition, Buitelaar et al reported that following a 7-week open-label extension of the fixed-dose LAMDA study with a flexible dose of OROS-MPH dose (18–90 mg/day), ADHD patients experienced improvements in symptoms, ability to function, and quality of life.Citation54,Citation68 This was irrespective of whether they received placebo or OROS-MPH during the fixed-dose phase.
Optimized dosing: clinical efficacy and functional improvement in the MPH-LA trial
Here we present previously unpublished post hoc analysis from the MPH-LA clinical trial ( and ; ).Citation34 This post hoc analysis was conducted using the analysis of covariance model, with treatment and center as factors and baseline score as a covariate, to assess the benefits of dose optimization. DSM-IV ADHD RS and SDS scores were retrospectively analyzed for Period 1 based on patients’ optimal dose, as determined during Period 2. Patients were then divided into four groups: placebo, patients who received their optimal dose, patients who received more than their optimal dose, and patients who received less than their optimal dose during Period 1 ().
Figure 3 LS mean change in (A) DSM-IV ADHD RS (n=119, n=116, n=119, and n=118, respectively) and (B) SDS total (n=113, n=115, n=117, and n=113, respectively) score from baseline 1 to end of Period 1 by optimal dose achieved in Period 2. Data were analyzed using an analysis of covariance model with treatment and center as factors, and baseline score as a covariate. P-values correspond to each group versus placebo.
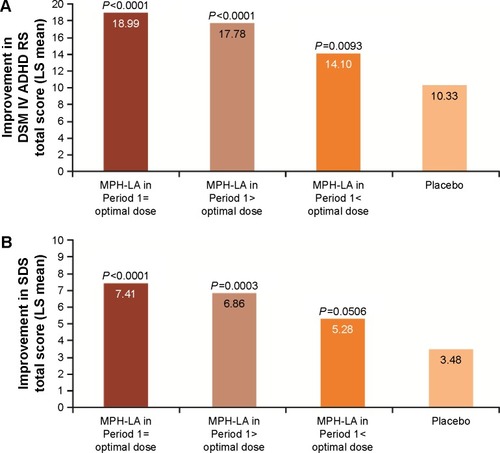
Table 2 Frequency of adverse events in Period 1 based on optimal dose achieved in Period 2
Patients who received their optimal dose of MPH-LA during Period 1 exhibited the highest average improvements in DSM-IV ADHD RS and SDS total scores from baseline (, respectively). The highest improvement in DSM-IV ADHD RS () was seen in patients who received their optimal dose (18.99: P<0.0001 vs placebo), followed by patients who received greater than their optimal dose (17.78: P<0.0001 vs placebo), and then those who received less than their optimal dose (14.10: P=0.0093 vs placebo). The improvement from baseline in SDS total score () was greater in patients who received their optimal dose (7.41: P<0.0001 vs placebo) compared with those who received more than their optimal dose (6.86: P=0.0003 vs placebo) and those who received less than their optimal dose (5.28: P=0.0506 vs placebo) during Period 1.
Optimized dose: safety profile of MPH in the MPH-LA trial
MPH treatment is associated with several challenges, including high rates of mild-to-moderate AEs, poor compliance, high treatment discontinuation rates, and high rates of intersubject variability in response. A systematic review of literature revealed that AEs are one of the most common reasons for treatment discontinuation.Citation60 The most common AEs reported with MPH included headaches, decreased appetite, dry mouth, nasopharyngitis, nausea, irritability, and insomnia.Citation34,Citation54 A recent meta-analysis revealed that MPH treatment was associated with an increased risk of nonserious AEs compared to placebo, but no increase in the risk of serious AEs.Citation69 As with treatment efficacy, the relationship between dose and AEs remains unclear. In numerous MPH trials, a similar incidence of AEs, with the exception of decreased appetite and insomnia, was observed at all treatment doses.Citation34,Citation37 Moreover, the safety profile of MPH seems to be similar in pediatric and adult populations.Citation70
For the purpose of this review, AEs in Period 1 of the Huss et al MPH-LA study were reanalyzed based on patients’ known optimal dose from Period 2.Citation34 Descriptive statistics were used for the AE data, and summarizes the proportion of AEs that occurred in ≥5% of patients. Patients who received more than their optimal dose experienced the highest rate of AEs (82.8% of patients) compared with those who received their optimal dose (68.3%) or less than their optimal dose (68.0%). Moreover, the rate of cardiac disorders (palpitations and tachycardia) seemed to be associated with the individually optimized dose: 19.8% of patients who received greater than their optimal dose experienced cardiac AEs compared with 10.6% of patients who received their optimal dose and 4.8% of patients who received less than their optimal dose (). In addition, the proportion of patients who experienced decreased appetite was higher for those who received more than their optimal dose (35.3%) than for patients who received their optimal dose (25.2%) or less than their optimal dose (19.2%). There was no association between AEs and optimal dose for gastrointestinal disorders, general disorders (fatigue and irritability), infections and infestations, musculoskeletal and connective tissue disorders, skin disorders, or central nervous system disorders ().
Discussion and limitations
There are limited data available regarding stimulant dose optimization versus fixed-dose regimens for ADHD. The MPH-LA study provides valuable insights into the clinical efficacy and safety benefits of optimized dosing; however, it was not specifically designed to assess this objective, and further research is needed to confirm the efficacy and safety benefits of dose optimization with MPH.Citation34 Although dose optimization is reported in the majority of clinical trials and naturalistic studies, there is currently no data available regarding the use of dose optimization in general practice. Additionally, although the once-daily dosing regimen used in the MPH-LA adult study is optimal for scientifically investigating dosage, it does not mimic naturalistic real-world treatment regimens. As a result, there is a need to generate real-world data on the use of flexible dose in routine clinical practice. Murray and colleagues identified 13 electronic databases of ADHD health care records in Europe.Citation62 In the future, these databases may provide additional insight into the long-term outcomes of ADHD patients.
This review has a number of potential limitations including that it was not a systematic literature review, and hence, the quality of the studies included in the review was not analyzed systematically. Although an extensive database was searched to identify relevant articles for inclusion, study inclusion may have been subject to the author and publication bias. Moreover, the methods of treatment optimization varied in different studies, which may impact the results; however, this was not considered while summarizing results in this review.
Conclusion
Dose optimization may improve patient satisfaction with stimulant therapy and could also enhance treatment effectiveness, patient compliance, and tolerability. The authors recommend that dose individualization should continue to be used (or implemented if not yet used) to improve patient functioning and reduce the social and economic burden of disease, in the pharmacological treatment of ADHD.
Acknowledgments
Medical writing support was provided by Áine Abautret-Daly (Novartis Ireland Ltd., Dublin, Ireland) and Anupama Tamta (Novartis Heathcare Pvt. Ltd., India). This service was supported by Novartis Pharma AG, Basel, Switzerland.
Disclosure
MH has received unrestricted grants to conduct Investigator Initiated Trials (IIT) with products of Engelhard Arzeimittel and Medice. He has received speaker honoraria and travel support and served as an advisor for Lilly, Shire, Medice, Novartis, Engelhard Arzneimittel, Acelion, and Lundbeck. He also holds an international patent on Doppler radar to assess ADHD. PD is an employee of Novartis Healthcare Pvt Ltd and has been working as Medical Affairs Director for the Established Medicines Franchise of Novartis Healthcare Pvt Ltd. PG is an employee of Novartis Pharma AG and has been working as Medical Director for the Established Medicines Franchise of Novartis Pharma AG. CWC is an employee of Novartis Pharmaceuticals Corporation. CS is a full-time employee of Novartis Pharma AG. VK is an employee of Novartis Pharmaceuticals Corporation and owns stocks in the company.
References
- MoffittTEHoutsRAshersonPIs Adult ADHD a childhood-onset neurodevelopmental disorder? Evidence from a four-decade longitudinal cohort studyAm J Psychiatry201517296797725998281
- FaraoneSVAntshelKMDiagnosing and treating attention-deficit/hyperactivity disorder in adultsWorld Psychiatry20087313113618836579
- PolanczykGVWillcuttEGSalumGAKielingCRohdeLAADHD prevalence estimates across three decades: an updated systematic review and meta-regression analysisInt J Epidemiol201443243444224464188
- HillJCSchoenerEPAge-dependent decline of attention deficit hyperactivity disorderAm J Psychiatry19961539114311468780416
- TurgayAGoodmanDWAshersonPLifespan persistence of ADHD: the life transition model and its applicationJ Clin Psychiatry201273219220122313720
- KooijSJBejerotSBlackwellAEuropean consensus statement on diagnosis and treatment of adult ADHD: the European Network Adult ADHDBMC Psychiatry2010106720815868
- LaraCFayyadJde GraafRChildhood predictors of adult attention-deficit/hyperactivity disorder: results from the World Health Organization World Mental Health Survey InitiativeBiol Psychiatry2009651465419006789
- European Medicines Agency EGuideline on the clinical investigation of medicinal products for the treatment of attention deficit hyperactivity disorder (ADHD)2008 Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/08/WC500095686.pdfAccessed February, 2016
- American Psychiatric AssociationDiagnostic and Statistical Manual of Mental Disorders5th edDSM-5. American Psychiatric Publishing2012 Available from: http://www.dsm5.orgAccessed February, 2017
- GoodmanDWThe consequences of attention-deficit/hyperactivity disorder in adultsJ Psychiatr Pract200713531832717890980
- BiedermanJFaraoneSVSpencerTJMickEMonuteauxMCAleardiMFunctional impairments in adults with self-reports of diagnosed ADHD: a controlled study of 1001 adults in the communityJ Clin Psychiatry200667452454016669717
- MortonWAStocktonGGMethylphenidate abuse and psychiatric side effectsPrim Care Companion J Clin Psychiatry20002515916415014637
- SaferDJRecent trends in stimulant usageJ Atten Disord201620647147726486603
- RenouxCShinJYDell’AnielloSFergussonESuissaSPrescribing trends of attention-deficit hyperactivity disorder (ADHD) medications in UK primary care, 1995–2015Br J Clin Pharmacol201682385886827145886
- TrenqueTHerlemEAbou TaamMDrameMMethylphenidate off-label use and safetySpringerplus2014328625279275
- HodgkinsPShawMCoghillDHechtmanLAmfetamine and methylphenidate medications for attention-deficit/hyperactivity disorder: complementary treatment optionsEur Child Adolesc Psychiatry201221947749222763750
- CastellsXRamos-QuirogaJARigauDEfficacy of methylphenidate for adults with attention-deficit hyperactivity disorder: a meta-regression analysisCNS Drugs201125215716921254791
- ChanEFoglerJMHammernessPGTreatment of attention-deficit/hyperactivity disorder in adolescents: a systematic reviewJAMA2016315181997200827163988
- LichtensteinPHalldnerLZetterqvistJMedication for attention deficit-hyperactivity disorder and criminalityN Engl J Med20123672006201423171097
- KonsteniusMJayaram-LindstromNGuterstamJBeckOPhilipsBFranckJMethylphenidate for attention deficit hyperactivity disorder and drug relapse in criminal offenders with substance dependence: a 24-week randomized placebo-controlled trialAddiction2014109344044924118269
- ManKKChanEWCoghillDMethylphenidate and the risk of traumaPediatrics20151351404825511122
- ShawMHodgkinsPCaciHA systematic review and analysis of long-term outcomes in attention deficit hyperactivity disorder: effects of treatment and non-treatmentBMC Med2012109922947230
- MeszarosACzoborPBalintSKomlosiSSimonVBitterIPharmacotherapy of adult attention deficit hyperactivity disorder (ADHD): a metaanalysisInt J Neuropsychopharmacol20091281137114719580697
- WilensTEDrug therapy for adults with attention-deficit hyperactivity disorderDrugs200363222395241114609347
- FroehlichTEEpsteinJNNickTGPharmacogenetic predictors of methylphenidate dose-response in attention-deficit/hyperactivity disorderJ Am Acad Child Adolesc Psychiatry2011501111291139.e112222024001
- GreenhillLLAbikoffHBArnoldLEMedication treatment strategies in the MTA Study: relevance to clinicians and researchersJ Am Acad Child Adolesc Psychiatry19963510130413138885584
- VitielloBSevereJBGreenhillLLMethylphenidate dosage for children with ADHD over time under controlled conditions: lessons from the MTAJ Am Acad Child Adolesc Psychiatry200140218819611211367
- CoghillDBanaschewskiTZuddasAPelazAGaglianoADoepfnerMLong-acting methylphenidate formulations in the treatment of attention-deficit/hyperactivity disorder: a systematic review of head-to-head studiesBMC Psychiatry20131323724074240
- National Institute for Health and Care Excellence [NICE] guidelines for the treatment of adult ADHD2008 Available from: https://www.nice.org.uk/guidance/cg72Accessed February, 2016
- Canadian Attention Deficit Hyperactivity Disorder Resource Alliance (CADDRA): Canadian ADHD Practice Guidelines2014 Available from: https://caddra.ca/pdfs/caddraGuidelines2011.pdfAccessed February, 2016
- WolraichMBrownLBrownRTADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescentsPediatrics201112851007102222003063
- American Psychiatric AssociationPractice Guideline for the Treatment of Patients with Major Depressive Disorder3rd ednWashington, DCAmerican Psychiatric Publishing2010
- AshersonPManorIHussMAttention-deficit/hyperactivity disorder in adults: update on clinical presentation and careNeuropsychiatry-Lond20144109128
- HussMGinsbergYTvedtenTMethylphenidate hydrochloride modified-release in adults with attention deficit hyperactivity disorder: a randomized double-blind placebo-controlled trialAdv Ther2014311446524371021
- MedoriRRamos-QuirogaJACasasMA randomized, placebo-controlled trial of three fixed dosages of prolonged-release OROS methylphenidate in adults with attention-deficit/hyperactivity disorderBiol Psychiatry2008631098198918206857
- SpencerTJAdlerLAMcGoughJJEfficacy and safety of dexmethylphenidate extended-release capsules in adults with attention-deficit/hyperactivity disorderBiol Psychiatry200761121380138717137560
- SteinMASarampoteCSWaldmanIDA dose-response study of OROS methylphenidate in children with attention-deficit/hyperactivity disorderPediatrics20031125e40414595084
- WigalSBNordbrockEAdjeiALChildressAKupperRJGreenhillLEfficacy of methylphenidate hydrochloride extended-release capsules (Aptensio XR™) in children and adolescents with attention-deficit/hyperactivity disorder: a phase III, randomized, double-blind studyCNS Drugs201529433134025877989
- RapportMDDuPaulGJKellyKLAttention deficit hyperactivity disorder and methylphenidate: the relationship between gross body weight and drug response in childrenPsychopharmacol Bull19892522852902602521
- ChermaMDJosefssonMRydbergIMethylphenidate for treating ADHD: a naturalistic clinical study of methylphenidate blood concentrations in children and adults with optimized dosageEur J Drug Metab Pharmacokinet201742229530727220743
- DouglasVIBarrRGAminKO’NeillMEBrittonBGDosage effects and individual responsivity to methylphenidate in attention deficit disorderJ Child Psychol Psychiatry19882944534753063718
- Swedish Medical Products AgencyADHD drugs – treatment guide-linesThe Swedish Medical Products Agency201621324
- Ritalin LA [package insert]Novartis Pharmaceuticals CorporationEast Hanover, NJ62015 Available from: https://www.pharma.us.novartis.com/product/pi/pdf/ritalin_la.pdfAccessed February, 2016
- Aptensio XR [package insert]Patheon Manufacturing Services LLCGreenville, NC42015 Available from: http://www.aptensioxr.com/resources/full-prescribing-information.pdfAccessed February, 2016
- CONCERTA [package insert]ALZA CorporationMountain View, CA32007 Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2007/021121s014lbl.pdfAccessed February, 2016
- Daytrana [package insert]Shire Pharmaceuticals Ireland Limited32007 Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2007/021514s005lbl.pdfAccessed February, 2016
- Metadate ER [package insert]Mallinckrodt IncHazelwood, MO62015 Available from: www2.mallinckrodt.com/WorkArea/DownloadAsset.aspx?id=2147483760Accessed February, 2016
- Metadate CD [package insert]UCB, IncSmyrna, GA82008 http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/021259s021lbl.pdfAccessed February, 2016
- Health NZMoNew Zealand Guidelines for the Assessment and Treatment of Attention-Deficit/Hyperactivity DisorderWellingtonMinistry of Health2001
- TakahashiNKohTTominagaYSaitoYKashimotoYMatsumuraTA randomized, double-blind, placebo-controlled, parallel-group study to evaluate the efficacy and safety of osmotic-controlled release oral delivery system methylphenidate HCl in adults with attention-deficit/hyperactivity disorder in JapanWorld J Biol Psychiatry20141548849824456065
- GoodmanDWSurmanCBSchererPBSalinasGDBrownJJAssessment of physician practices in adult attention-deficit/hyperactivity disorderPrim Care Companion CNS Disord2012144
- EpsteinJNKelleherKJBaumRVariability in ADHD care in community-based pediatricsPediatrics201413461136114325367532
- HussMGinsbergYArngrimTOpen-label dose optimization of methylphenidate modified release long acting (MPH-LA): a post hoc analysis of real-life titration from a 40-week randomized trialClin Drug Investig2014349639649
- BuitelaarJKRamos-QuirogaJACasasMSafety and tolerability of flexible dosages of prolonged-release OROS methylphenidate in adults with attention-deficit/hyperactivity disorderNeuropsychiatr Dis Treat2009545746619777067
- AdlerLAOrmanCStarrHLLong-term safety of OROS methylphenidate in adults with attention-deficit/hyperactivity disorder: an open-label, dose-titration, 1-year studyJ Clin Psychopharmacol201131110811421192153
- GreenhillLLSwansonJMVitielloBImpairment and deportment responses to different methylphenidate doses in children with ADHD: the MTA titration trialJ Am Acad Child Adolesc Psychiatry200140218018711211366
- FredriksenMHalmoyAFaraoneSVHaavikJLong-term efficacy and safety of treatment with stimulants and atomoxetine in adult ADHD: a review of controlled and naturalistic studiesEur Neuropsychopharmacol201323650852722917983
- BonviciniCFaraoneSVScassellatiCAttention-deficit hyperactivity disorder in adults: a systematic review and meta-analysis of genetic, pharmacogenetic and biochemical studiesMol Psychiatry201621787288427217152
- FredriksenMDahlAAMartinsenEWKlungsoyrOHaavikJPeleikisDEEffectiveness of one-year pharmacological treatment of adult attention-deficit/hyperactivity disorder (ADHD): an open-label prospective study of time in treatment, dose, side-effects and comorbidityEur Neuropsychopharmacol201424121873188425453480
- GajriaKLuMSikiricaVAdherence, persistence, and medication discontinuation in patients with attention-deficit/hyperactivity disorder – a systematic literature reviewNeuropsychiatr Dis Treat2014101543156925187718
- CorteseSPaneiPArcieriRSafety of methylphenidate and atomoxetine in children with attention-deficit/hyperactivity disorder (ADHD): data from the Italian National ADHD RegistryCNS Drugs20152986587726293742
- MurrayMLInsukSBanaschewskiTAn inventory of European data sources for the long-term safety evaluation of methylphenidateEur Child Adolesc Psychiatry2013221060561823508655
- GinsbergYArngrimTPhilipsenALong-term (1 year) safety and efficacy of methylphenidate modified-release long-acting formulation (MPH-LA) in adults with attention-deficit hyperactivity disorder: a 26-week, flexible-dose, open-label extension to a 40-week, double-blind, randomised, placebo-controlled core studyCNS Drugs2014281095196225183661
- BuitelaarJKTrottGEHofeckerMLong-term efficacy and safety outcomes with OROS-MPH in adults with ADHDInt J Neuropsychopharmacol201215111321798108
- FindlingRLWigalSBBuksteinOGLong-term tolerability of the methylphenidate transdermal system in pediatric attention-deficit/hyperactivity disorder: a multicenter, prospective, 12-month, open-label, uncontrolled, phase III extension of four clinical trialsClin Ther20093181844185519808143
- ZhangSFariesDEVowlesMMichelsonDADHD Rating Scale IV: psychometric properties from a multinational study as a clinician-administered instrumentInt J Methods Psychiatr Res200514418620116395872
- LeonACOlfsonMPorteraLFarberLSheehanDVAssessing psychiatric impairment in primary care with the Sheehan Disability ScaleInt J Psychiatry Med1997272931059565717
- BuitelaarJKCasasMPhilipsenAFunctional improvement and correlations with symptomatic improvement in adults with attention deficit hyperactivity disorder receiving long-acting methylphenidatePsychol Med201242119520421733214
- StoreboOJKroghHBRamstadEMethylphenidate for attention-deficit/hyperactivity disorder in children and adolescents: cochrane systematic review with meta-analyses and trial sequential analyses of randomised clinical trialsBMJ2015351h520326608309
- GinsbergYArngrimTPhilipsenASafety profile of methylphenidate hydrochloride-modified release (MPH-LA) in adults and children with attention deficit hyperactivity disorderEur Psychiat201429Suppl 11
- NuttDJFoneKAshersonPEvidence-based guidelines for management of attention-deficit/hyperactivity disorder in adolescents in transition to adult services and in adults: recommendations from the British Association for PsychopharmacologyJ Psychopharmacol2007211104117092962
- Bolea-AlamanacBNuttDJAdamouMEvidence-based guidelines for the pharmacological management of attention deficit hyperactivity disorder: update on recommendations from the British Association for PsychopharmacologyJ Psychopharmacol201428317920324526134
- TaylorEDöpfnerMSergeantJEuropean clinical guidelines for hyperkinetic disorder–first upgradeEur Child Adolesc Psychiatry200413Suppl 11730
- LeeTCHashimAHKuanCSClinical practice guidelines. Management of attention deficit hyperactivity disorder in children and adolescents2008 Available from: http://www.acadmed.org.my/index.cfm?&menuid=67#Mental_Health
- National Health and Medical Research CouncilClinical Practice Points on the Diagnosis, Assessment and Management of Attention Deficit Hyperactivity Disorder in Children and AdolescentsCommonwealth of Australia2012
- Scottish Intercollegiate Guidelines NetworkManagement of Attention Deficit and Hyperactivity Disorders in Children and Young People – A National Clinical GuidelineScottish Intercollegiate Guidelines Network (SIGN)2009
- Development Group of the Clinical Practice Guideline on Attention Defi cit Hyperactivity Disorder (ADHD) in Children and Adolescents. Fundació Sant Joan de Déu, coordinator. Clinical Practice Guideline on Attention Deficit Hyperactivity Disorder (ADHD) in Children and AdolescentsQuality Plan for the National Health System of the Ministry of Health, Social Policies and Equality. Agència dinformació, Avaluació i Qualitat (AIAQS) of CataloniaClin Practice Guidelines Spanish NHS (SNS)2010