Abstract
This article highlights the evidence linking depression to increased inflammatory drive and explores putative mechanisms for the association by reviewing both preclinical and clinical literature. The enzyme indoleamine 2,3-dioxygenase is induced by proinflammatory cytokines and may form a link between immune functioning and altered neurotransmission, which results in depression. Increased indoleamine 2,3-dioxygenase activity may cause both tryptophan depletion and increased neurotoxic metabolites of the kynurenine pathway, two alterations which have been hypothesized to cause depression. The tryptophan-kynurenine pathway is comprehensively described with a focus on the evidence linking metabolite alterations to depression. The use of immune-activated groups at high risk of depression have been used to explore these hypotheses; we focus on the studies involving chronic hepatitis C patients receiving interferon-alpha, an immune activating cytokine. Findings from this work have led to novel strategies for the future development of antidepressants including inhibition of indoleamine 2,3-dioxygenase, moderating the cytokines which activate it, or addressing other targets in the kynurenine pathway.
Introduction
Clinical depression is extremely common and debilitating. It is ranked by the World Health Organization as the fourth largest cause of burden amongst all diseases and the leading nonfatal disease burden.Citation1 Current treatments have only moderate efficacy, with around 35% remission after initial treatment and approximately 70% remission after four cumulative treatment trials.Citation2 Therefore it is necessary to look beyond currently characterized neurotransmitter systems to understand the pathophysiology of depression in order to produce more effective treatments in the long-term.
Emerging evidence demonstrates that: a) major depression is associated with increased inflammatory drive;Citation3–Citation5 and b) provoking an acute inflammatory response in healthy humans can result in depression-like behaviors and symptoms.Citation6,Citation7 The nature of these associations has yet to be delineated with respect to causality. Determining a plausible biological mechanism remains an important step. In this article we review a putative mechanism by which increased inflammation may affect mood, by altering activity of the enzyme indoleamine 2,3-dioxygenase (IDO).
Depression, mood, and immune functioning
There is a growing body of literature that suggests that major depression is associated with an increased inflammatory drive. People with depression display increased plasma concentrations of pro-inflammatory cytokines such as: interleukin-1 (IL-1)Citation3,Citation8 (also increased in cerebrospinal fluid [CSF]Citation9), interleukin-6 (IL-6),Citation3,Citation4,Citation9–Citation11 tumor necrosis factor (TNF)Citation4,Citation12 and other acute phase proteins, such as C-reactive protein (CRP),Citation3 haptoglobinCitation11 and neopterin.Citation13 There have been some negative findings,Citation14,Citation15 but the overall picture is sufficient to support both a positive meta-analysis exploring the associations of CRP, IL-1, IL-6 and depressionCitation3 and the suggestion that plasma IL-6 and soluble IL-2-receptor should be considered biomarkers of depression.Citation16
Treatment of depression with antidepressants may reverse derangements in these inflammatory markers.Citation17 Fluoxetine treatment for depression reduces serum IL-6 in patients.Citation18 Imipramine, clomipramine, venlafaxine, fluoxetine, sertraline and trazodone have been shown to reduce the interferon-gamma (INF-γ)/IL-10 ratio of in vitro human blood samples (a ratio of pro-inflammatory/anti-inflammatory drive), consistent with an anti-inflammatory effect.Citation19–Citation21 In addition, nonresponders to selective serotonin reuptake inhibitor (SSRI) medication continue to exhibit raised IL-6 levels, raising the possibility that response to treatment is linked to a reduction of IL-6.Citation22 Preliminary evidence also exists that an increased body temperature may also be present in depression and reversed by successful treatment.Citation23
Abnormalities of plasma cytokines may occur in various psychiatric disorders. In bipolar disorder, increased IL-1, IL-6 and TNF have been reported at differing stages of the illness.Citation24 In schizophrenia, less consistent results have been found, but a recent meta-analysis reported increased plasma IL-6 and IL-1 receptor antagonist levels.Citation25 However, exploring these illnesses in detail is beyond the scope of this review, which focuses upon the changes seen in major depression.
“Sickness behavior” is a characteristic constellation of symptoms (hypomotility, hyperthermia, hypophagia, hyperalgesia, decreased interest in exploration, decreased sexual activity, increased sleep) observed in animals following immune activationCitation26–Citation28 that has been proposed to be a model of depression.Citation29 Activating an immune response by injecting lipopolysaccharide (LPS),Citation30 IL-1,Citation31 or IFNCitation32 results in characteristic sickness behavior. In addition, an acute inflammatory challenge has been reported to produce depression-like responses in two other animal models of depression, the tail suppression and sucrose consumption tests, after the initial illness behaviors have subsided.Citation33 The biochemical and behavioral effects of challenges like these may also be augmented by social stress,Citation34 analogous to social risk factors for depression.Citation35 Pretreatment with the antidepressant imipramine has been found to attenuate LPS-induced sickness behavior.Citation30
Provoking an acute inflammatory response in healthy humans, for example via injection of endotoxin,Citation6,Citation7 IL-6,Citation36 or IFN-β,Citation37 also produces symptoms similar to those seen in depression (such as fatigue, lack of motivation, anorexia, poor sleep). Although these symptoms are short-lived, subtle cognitive symptoms similar to those seen in depression are also present. These include feelings of social isolationCitation6,Citation38 and psychomotor slowing.Citation37 The symptoms produced by challenge tests such as these resolve quickly and are not prolonged as is seen in depression.
Immune effects on indoleamine 2,3-dioxygenase
Indoleamine 2,3-dioxygenase (IDO) and its hepatic equivalent tryptophan 2,3-dioxygenase (TDO) oxygenate tryptophan to form kynurenineCitation39 (, tryptophan metabolic pathway). The majority of dietary tryptophan is metabolized through this pathway with less than 1% eventually being available for conversion (via hydroxylation by tryptophan hydroxylase, TPH, and decarboxylation) into 5HT in the brain.Citation40 Under normal circumstances, TDO is the dominant enzyme, but IDO is subject to induction during immune activation. At such times the effect of increasing the combined availability of IDO and TDO means that the overall capacity of the kynurenine pathway is much increased. Therefore serum tryptophan concentration can be reduced by 25%–50%, leaving proportionally less tryptophan available for conversion to serotonin.Citation41–Citation43
Figure 1 Tryptophan metabolic pathway.
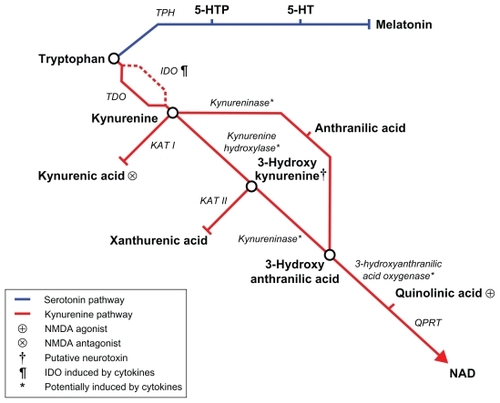
IDO is ubiquitous throughout the organs and present in human immune cells including macrophages and microglia.Citation44 Interferons are important in the induction of IDO. The sites of action are two IFN-stimulated response elements (ISREs) and IFN-γ activated site (GAS) element sequences found in the 5′ promoter region of the IDO gene.Citation45,Citation46 IDO can be stimulated by INF-γ in macrophages and microglia.Citation47,Citation48 However, other cytokines such as TNF in combination with IL-6 or IL-1 can induce IDO via signal transducer and activator of transcription protein (STAT)-independent pathways involving p38 mitogen-activated protein kinase (p38 MAPK) and nuclear factor-kappa B (NF-κB).Citation49
A proxy measure for in vivo IDO activity, like many enzymes, is the ratio of product:substrate (in this case kynurenine:tryptophan). Thus, an increase in the ratio reflects greater enzyme activity, a decrease indicates lower activity and no change implies the same activity.Citation50
Animal models of IDO activation and mood
Animal models support the hypothesis that immune-related sickness behavior may be related to increased activity of IDO. IDO activity, measured by either the plasma concentration of kynurenine pathway metabolites or IDO mRNA expression, is increased in animal sickness behavior.Citation51 This activation is partly mediated by IFN-γ and TNF, since IFN-γ knockout mice, and animals with prior treatment with the TNF antagonist etanercept, both show reduced IDO activation and depressive behaviors (in the forced swim and tail suspension tests).Citation52 IDO knock-out mice lack the expected depressive behaviors secondary to an immune challenge, despite normal cytokine responses.Citation51 In addition, inhibition of IDO blocks the depressive behavior in these modelsCitation51,Citation53 and administration of kynurenine induces depressive behavior in a dose-dependent manner.Citation53
A human model of IDO activation and depression: hepatitis C cohorts treated with IFN-α
As described above, acute inflammatory challenges reproduce sickness behavior and depressive cognitions in healthy humans. Clearly, it is ethically difficult to continue challenges like these for prolonged periods due to the high degree of morbidity they cause. Therefore, an alternative is to study patient cohorts who require long-term pro-inflammatory treatments for an underlying condition.
An ideal high-risk population that can be used to assess the effects of increased inflammatory drive is chronic hepatitis C (HCV) patients being treated with IFN-α therapy. HCV is a common illness, affecting approximately 170 million people worldwide.Citation54,Citation55 Without treatment, it causes considerable morbidity and mortality; it leads to chronic infection in approximately 85% of cases, cirrhosis in 15%–20%, and in cirrhosis patients, 1%–4% progress to hepatocellular carcinoma.Citation56 HCV patients undergo an IFN-α-based treatment regime for between 6 and 12 months. During this time there are high rates of depression, estimated at approximately 25% Citation57,Citation58 and 33%Citation59,Citation60 (although some studies report higher prevalences, a precise figure is difficult to determine due to methodological differences between studies; those reporting higher rates report self-rated symptoms rather than utilizing standardized objective depression scalesCitation61).
In HCV, IFN-α increases inflammatory drive with elevations in pro-inflammatory cytokines (eg, IL-1, IL-6, IL-8 and TNFCitation62,Citation63) similar to that observed in depression.Citation3 Although IFN-α is peripherally administered, increases in IFN-α, IL-6 and monocyte chemoattractant protein-1 have been observed in the CSF of this group, providing evidence of central immunomodulatory effects. In conjunction with the increase in pro-inflammatory cytokines, the kynurenine:tryptophan ratio is increased (reflecting increased IDO activity) in both the blood and CSF.Citation64,Citation65
There are two current hypotheses regarding the mechanism of how increased inflammatory drive and IDO activation may cause depression in the HCV group – tryptophan depletion and kynurenine toxicity.
Tryptophan depletion
Increased IDO activity should reduce the availability of its substrate, the dietary essential amino acid tryptophan. Citation66 Serotonin (5HT) is produced from tryptophan via 5-hydroxytryptophan (5HTP). Under normal conditions the rate-limiting enzyme tryptophan hydroxylase is only about 50% saturated. Therefore 5HT synthesis varies with tryptophan availability.Citation67 The evidence linking 5HT dysfunction to depressive illness has been well described. Many effective antidepressants (such as SSRIs) work primarily on increasing serotonin availability in the synaptic cleft.Citation68 This antidepressant effect can be temporarily reversed using the acute tryptophan depletion (ATD) technique, which acutely lowers 5HT by lowering the brain availability of its precursor tryptophan.Citation69,Citation70 Lower concentrations of plasma tryptophan have also been reported in depression.Citation71 Imaging studies have also reported central changes in the 5HT system in depression including reduced 5HT transporters,Citation72,Citation73 reduced 5HT-1a receptors,Citation74,Citation75 and reduced 5HT-2a receptors.Citation76
In the HCV cohort, reductions in plasma tryptophan (and 5HT, although this is an inexact measure, as much plasma 5HT is stored by platelets and released when they are stimulated, such as in venepuncture, resulting in inconsistent resultsCitation77) have been observed.Citation78 SSRIs are highly effective at treating or preventing IFN-α associated depression.Citation79,Citation80 However, tryptophan does not have clear access to the brain from the plasma: 95% is protein-bound in the plasma, leaving 5% free to access the CNS.Citation81 It is transported across the blood–brain barrier via active transport in competition with the other large neutral amino acids (LNAAs): valine, leucine, isoleucine, methionine, phenylalanine and tyrosine.Citation82 Plasma tryptophan concentrations correlate poorly with those of the CSF.Citation83 Thus, a more accurate measure of brain tryptophan availability is the tryptophan:LNAA ratio.Citation84 This ratio remains unchanged in IFN-α therapy for hepatitis C and does not appear to vary with depressive symptoms.Citation64 In keeping with this, CSF levels of tryptophan do not change during interferon treatment.Citation65 However, this does not entirely disprove the 5HT reduction theory, as the 5HT metabolite 5-hydroxyindoleacetic acid (5HIAA) is reduced and this reduction correlates with depressive symptoms.Citation85 Therefore although absolute tryptophan levels appear not to be altered, an overall reduction in brain 5HT turnover may still be related to depression.
Different measures of brain 5HT functioning are required to further delineate these changes. A sensitive method is polysomnography. 5HT is important in the regulation of sleepCitation86 and sleep disturbances prior to interferon treatment have been suggested to predict later depression.Citation87 Serotonergic compounds (such as SSRIs) increase time until onset of rapid eye movement (REM) sleep (increased REM latency). Decreasing serotonin availability, by ATD, has the opposite effect, decreased REM latency.Citation88–Citation90 Alterations in REM latency have been utilized by our group to detect differences in physiological potency between different SSRIs,Citation91 proving this technique’s sensitivity to alterations in central 5HT functioning. Using a within-subjects design we observed no significant alteration in REM latency after 6 weeks IFN-α treatment (Pers comm, David N Christmas, 2011). Our finding of no decrease in REM latency is similar to an independent study by a different group that observed an REM latency increase during IFN-α treatment.Citation92 The difference between these studies has yet to be explained, but importantly neither observed the decrease in REM latency predicted by the 5HT depletion hypothesis.
An alternative explanation for the lack of decrease in REM latency is that IDO activation may also affect REM sleep via a different mechanism to 5HT depletion. Preclinical evidence suggests that glutamatergic neurons are important in the genesis of REM sleep: indeed kynurenic acid (which is produced from kynurenine and is an N-methyl-D-aspartate [NMDA] antagonist) can abolish experimentally induced REM sleep.Citation93 The control of REM sleep is complex, with 5HT, acetylcholine, glutamate and gamma-amino hydroxybutyric acid (GABA) all playing important roles.Citation94 At risk of oversimplification, REM-on neurons appear to be glutamatergic (under tonic inhibition by GABA neurons) and REM-off neurons serotonergic or noradrenergic. Therefore, altering the balance between kynurenic acid and quinolinic acid (also on the kynurenine metabolic pathway and an NMDA agonist []) may also alter both REM latency and duration. However, IFN-α does not alter the CSF kynurenic acid:quinolinic acid ratio.Citation65 Therefore the preliminary conclusion is that neither central 5HT functioning nor NMDA activation is altered during IFN-α treatment. However, further research is required to form a conclusive picture.
Kynurenine excess
The products of IDO activation have also been hypothesized to cause depression. Under normal circumstances the liver enzyme TDOCitation95 metabolizes tryptophan into kynurenine. TDO is not induced by immune activation, but is constitutively active and is induced by tryptophan, tyrosine, histidine, glucocorticoids and kynurenine. TDO primarily serves nicotinamide adenine dinucleotide synthesis () and is the rate-limiting enzyme of the pathway. Under circumstances of immune activation, IDO activity is increased, causing detectable increases in kynurenine and decreases in tryptophan.Citation41,Citation43,Citation96 Kynurenine is mostly hydroxylated (kynurenine hydroxylase) into 3-hydroxykynurenine (3-HK). Kynureninase acts upon both 3-HK and kynurenine; on 3-HK to form 3-hydroxyanthranilic acid (3-HAA); and on kynurenine to form anthranilic acid (although the latter conversion accounts for only a minority of kynureninase activity). 3-HAA is converted into quinolinic acid by 3-hydroxyanthranilic acid oxygenase. Kynurenine can also be converted into kynurenic acid by kynurenine aminotransferase I and 3-HK into xanthurenic acid by kynurenine aminotransferase II.
Some of these kynurenine metabolites modulate neurotransmission and some may be directly neurotoxic. As mentioned above, quinolinic acid is an NMDA agonist and kynurenic acid an NMDA antagonist. 3-HK is believed to be neurotoxic due to increased formation of reactive oxygen species involved in neuronal apoptosis.Citation97,Citation98 Quinolinic acid may also be neurotoxic due to increased oxidative stress,Citation99,Citation100 whereas kynurenic acid has been postulated to be neuroprotective.Citation101 Under conditions of immune activation, preclinical evidence suggests kynurenine aminotransferase activity is unchanged whereas, in addition to IDO, kynurenine 3-hydroxylase, kynureninase and 3-hydroxyanthranilic acid oxygenase activity may be increasedCitation51,Citation102 (although the evidence for induction of the last enzyme is contradictory, possibly due to species differences). Therefore, kynurenine metabolism is shifted toward the 3-HK/quinolinic acid pathway and away from the kynurenic acid pathway, which should result in greater neurotoxic and reduced neuroprotective metabolites. The relative balance of neurotoxic and neuroprotective pathways of kynurenine metabolism can be assessed indirectly in vivo by the kynurenine:kynurenic acid ratio.Citation64,Citation103
During IFN-α treatment, the plasma kynurenine:kynurenic acid ratio is increased and this correlates with depressive symptoms.Citation64 CSF kynurenine and quinolinic acid also increase and these increases correlate with increases in depressive symptoms. However, the kynurenine:kynurenic acid ratio does not alter as CSF kynurenic acid also rises.Citation65
One cross-sectional study observed increased IDO activity and a decreased kynurenic acid:kynurenine ratio (reflecting a shift towards neurotoxicity) in otherwise healthy major depression sufferers compared to controls.Citation104 However, as yet no studies have been undertaken to identify whether these differences resolve once the depressive episode has been successfully treated.
Future drug targets
Some preliminary studies have already reported possible efficacy of anti-inflammatory drugs in depression. A double-blind, randomized clinical trial reported an advantage of reboxetine and the cyclo-oxygenase-2 inhibitor celecoxib over reboxetine and placebo.Citation105 In addition, an open pilot study reported a benefit of augmentation with aspirin in depressed patients with no early response to an SSRI.Citation106 However, both these cases require larger more robust trials to prove their efficacy. A further problem may occur with this route; there is a large increased risk of gastrointestinal bleeding when SSRIs and nonsteroidal anti-inflammatory drugs are combinedCitation107 and cyclo-oxygenase-2 inhibitors alone have been associated with increased cardiovascular and all-cause mortality above other anti-inflammatory drugs.Citation108,Citation109 Therefore alternative strategies may be required to maintain a favorable risk–benefit ratio.
Utilizing the above evidence, it is possible to identify future novel pharmacological targets for antidepressants. The first target could be antagonizing or reducing IDO activity. The drug 1-methlytryptophan can inhibit IDO and has been successful in reducing depressive behaviors following inflammatory challenges in animal models.Citation51 Clinical trials using 1-methyltryptophan have commenced in humans as a putative anticancer agent (trial identifier NCT00567931, http://clinicaltrials.gov). However, there is some debate as to whether it inhibits human IDO in vivo.Citation110 In addition, IDO may have immunosuppressive actions in itself, highlighting the complexity of immune functioning.Citation110
A different avenue may be to block the pro-inflammatory cytokines that are raised in depression and known to induce IDO. Monoclonal antibodies are available for human use, to treat rheumatoid arthritis or inflammatory bowel disease, to block both TNF (such as infliximab or etanercept) and IL-6 (tocilizumab). Indeed, a clinical trial at Emory University evaluating the efficacy of infliximab in treatment resistant depression is approaching completion (trial identifier NCT00463580, http://clinicaltrials.gov).
A third approach may be to block the downstream actions of excess kynurenine metabolites. As the ratio of NMDA receptor agonism:antagonism appears to be shifted towards agonism in depression, NMDA antagonists may have antidepressant effects. Unfortunately human subjects exposed to direct NMDA antagonists have experienced serious side effects such as sedation, memory impairment and psychosis.Citation111,Citation112 Thus design of NMDA manipulating compounds may require novel strategies, such as targeting NMDA cotransmitters. Despite this, several small studies have shown promising results for the use of ketamine, an NMDA receptor antagonist, for treatment-resistant depression.Citation113–Citation117
Summary
In summary, major depression appears to be accompanied by increases in some pro-inflammatory cytokines. In keeping with this, inducing increased inflammation in animals or humans results in characteristic sickness behavior, or full-blown major depression in the high-risk HCV cohort. In tandem with markers of increased inflammation, IDO is activated, both peripherally and centrally. Although the evidence falls short of proving a causative link between inflammation, IDO and mood, the diversity and congruence of evidence suggests this pathway is a promising field for future drug targets.
A presentation relating to this manuscript was made by Dr David Christmas at the 9th International Meeting on Clinical Pharmacology in Psychiatry (9th IMCPP) in Copenhagen, Denmark in September 2010
Disclosure
The authors report no conflicts of interest in this work.
References
- UstunTBAyuso-MateosJLChatterjiSMathersCMurrayCJGlobal burden of depressive disorders in the year 2000Br J Psychiatry200418438639215123501
- RushAJTrivediMHWisniewskiSRAcute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D reportAm J Psychiatry2006163111905191717074942
- HowrenMBLamkinDMSulsJAssociations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysisPsychosom Med2009711217118619188531
- KimYKNaKSShinKHJungHYChoiSHKimJBCytokine imbalance in the pathophysiology of major depressive disorderProg Neuropsychopharmacol Biol Psychiatry20073151044105317433516
- MaesMBosmansEMeltzerHYImmunoendocrine aspects of major depression. Relationships between plasma interleukin-6 and soluble interleukin-2 receptor, prolactin and cortisolEur Arch Psychiatry Clin Neurosci199524531721787669825
- EisenbergerNIInagakiTKMashalNMIrwinMRInflammation and social experience: an inflammatory challenge induces feelings of social disconnection in addition to depressed moodBrain Behav Immun201024455856320043983
- ReichenbergAYirmiyaRSchuldACytokine-associated emotional and cognitive disturbances in humansArch Gen Psychiatry2001585544545211343523
- OwenBMEcclestonDFerrierINYoungAHRaised levels of plasma interleukin-1beta in major and postviral depressionActa Psychiatr Scand2001103322622811240580
- LevineJBarakYChengappaKNRapoportARebeyMBarakVCerebrospinal cytokine levels in patients with acute depressionNeuropsychobiology199940417117610559698
- MaesMMeltzerHYBosmansEIncreased plasma concentrations of interleukin-6, soluble interleukin-6, soluble interleukin-2 and transferrin receptor in major depressionJ Affect Disord19953443013098550956
- ZorrillaEPLuborskyLMcKayJRThe relationship of depression and stressors to immunological assays: a meta-analytic reviewBrain Behav Immun200115319922611566046
- HestadKATonsethSStoenCDUelandTAukrustPRaised plasma levels of tumor necrosis factor alpha in patients with depression: normalization during electroconvulsive therapyJ ECT200319418318814657769
- MaesMScharpeSMeltzerHYIncreased neopterin and interferon-gamma secretion and lower availability of L-tryptophan in major depression: further evidence for an immune responsePsychiatry Res19945421431607761549
- BrambillaFMaggioniMBlood levels of cytokines in elderly patients with major depressive disorderActa Psychiatr Scand19989743093139570493
- CarpenterLLHeningerGRMalisonRTTyrkaARPriceLHCerebrospinal fluid interleukin (IL)-6 in unipolar major depressionJ Affect Disord2004791328528915023475
- MossnerRMikovaOKoutsilieriEConsensus paper of the WFSBP Task Force on Biological Markers: biological markers in depressionWorld J Biol Psychiatry20078314117417654407
- MaesMYirmyiaRNorabergJThe inflammatory and neurodegenerative (I&ND) hypothesis of depression: leads for future research and new drug developments in depressionMetab Brain Dis2009241275319085093
- SluzewskaARybakowskiJKLaciakMMackiewiczASobieskaMWiktorowiczKInterleukin-6 serum levels in depressed patients before and after treatment with fluoxetineAnn N Y Acad Sci19957624744767668562
- LinASongCKenisGThe in vitro immunosuppressive effects of moclobemide in healthy volunteersJ Affect Disord2000581697410760560
- MaesMSongCLinAHNegative immunoregulatory effects of antidepressants: inhibition of interferon-gamma and stimulation of interleukin-10 secretionNeuropsychopharmacology199920437037910088138
- KuberaMLinAHKenisGBosmansEvanBDMaesMAnti- Inflammatory effects of antidepressants through suppression of the interferon-gamma/interleukin-10 production ratioJ Clin Psychopharmacol200121219920611270917
- O’BrienSMScullyPFitzgeraldPScottLVDinanTGPlasma cytokine profiles in depressed patients who fail to respond to selective serotonin reuptake inhibitor therapyJ Psychiatr Res2007413432633116434055
- SzubaMPGuzeBHBaxterLRJrElectroconvulsive therapy increases circadian amplitude and lowers core body temperature in depressed subjectsBiol Psychiatry19974212113011379426883
- BerkMKapczinskiFAndreazzaACPathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factorsNeurosci Biobehav Rev201135380481720934453
- PotvinSStipESepehryAAGendronABahRKouassiEInflammatory cytokine alterations in schizophrenia: a systematic quantitative reviewBiol Psychiatry200863880180818005941
- HartBLBiological basis of the behavior of sick animalsNeurosci Biobehav Rev19881221231373050629
- DantzerRCytokine-induced sickness behavior: mechanisms and implicationsAnn N Y Acad Sci200193322223412000023
- KentSBlutheRMKelleyKWDantzerRSickness behavior as a new target for drug developmentTrends Pharmacol Sci199213124281542935
- SmithRSThe macrophage theory of depressionMed Hypotheses19913542983061943879
- YirmiyaREndotoxin produces a depressive-like episode in ratsBrain Res1996711121631748680850
- KentSRodriguezFKelleyKWDantzerRReduction in food and water intake induced by microinjection of interleukin-1 beta in the ventromedial hypothalamus of the ratPhysiol Behav1994565103110367824567
- FaheyBHickeyBKelleherDO’DwyerAMO’MaraSMThe widely-used anti-viral drug interferon-alpha induces depressive- and anxiogenic-like effects in healthy ratsBehav Brain Res20071821808717588681
- FrenoisFMoreauMO’ConnorJLipopolysaccharide induces delayed FosB/DeltaFosB immunostaining within the mouse extended amygdala, hippocampus and hypothalamus, that parallel the expression of depressive-like behaviorPsychoneuroendocrinology200732551653117482371
- AnismanHPoulterMOGandhiRMeraliZHayleySInterferon-alpha effects are exaggerated when administered on a psychosocial stressor backdrop: cytokine, corticosterone and brain monoamine variationsJ Neuroimmunol2007186124553
- Righetti-VeltemaMConne-PerreardEBousquetAManzanoJRisk factors and predictive signs of postpartum depressionJ Affect Disord19984931671809629946
- Spath-SchwalbeEHansenKSchmidtFAcute effects of recombinant human interleukin-6 on endocrine and central nervous sleep functions in healthy menJ Clin Endocrinol Metab1998835157315799589658
- BrydonLHarrisonNAWalkerCSteptoeACritchleyHDPeripheral inflammation is associated with altered substantia nigra activity and psychomotor slowing in humansBiol Psychiatry200863111022102918242584
- EisenbergerNIInagakiTKRamesonLTMashalNMIrwinMRAn fMRI study of cytokine-induced depressed mood and social pain: the role of sex differencesNeuroimage200947388189019376240
- SchwarczRPellicciariRManipulation of brain kynurenines: glial targets, neuronal effects, and clinical opportunitiesJ Pharmacol Exp Ther2002303111012235226
- BenderDABiochemistry of tryptophan in health and diseaseMol Aspects Med1983621011976371429
- WernerERFuchsDHausenATryptophan degradation in patients infected by human immunodeficiency virusBiol Chem Hoppe Seyler198836953373403166737
- FuchsDForsmanAHagbergLImmune activation and decreased tryptophan in patients with HIV-1 infectionJ Interferon Res19901065996032128302
- FuchsDMollerAAReibneggerGStockleEWernerERWachterHDecreased serum tryptophan in patients with HIV-1 infection correlates with increased serum neopterin and with neurologic/psychiatric symptomsJ Acquir Immune Defic Syndr19903998738762166783
- DaleWEDangYBrownORTryptophan metabolism through the kynurenine pathway in rat brain and liver slicesFree Radic Biol Med200029219119810980407
- KonanKVTaylorMWImportance of the two interferon-stimulated response element (ISRE) sequences in the regulation of the human indoleamine 2,3-dioxygenase geneJ Biol Chem19962713219140191458702590
- ChonSYHassanainHHPineRGuptaSLInvolvement of two regulatory elements in interferon-gamma-regulated expression of human indoleamine 2,3-dioxygenase geneJ Interferon Cytokine Res19951565175267553221
- HeyesMPSaitoKMarkeySPHuman macrophages convert L-tryptophan into the neurotoxin quinolinic acidBiochem J1992283Pt 36336351534219
- Aberati-GianiDCesuraAMExpression of the kynurenine enzymes in macrophages and microglial cells: regulation by immune modulatorsAmino Acids19981413251255
- FujigakiHSaitoKFujigakiSThe signal transducer and activator of transcription 1alpha and interferon regulatory factor 1 are not essential for the induction of indoleamine 2,3-dioxygenase by lipopolysaccharide: involvement of p38 mitogen-activated protein kinase and nuclear factor-kappaB pathways, and synergistic effect of several proinflammatory cytokinesJ Biochem2006139465566216672265
- SchrocksnadelKWirleitnerBWinklerCFuchsDMonitoring tryptophan metabolism in chronic immune activationClin Chim Acta200636412829016153626
- O’ConnorJCLawsonMAAndreCInduction of IDO by bacille Calmette-Guerin is responsible for development of murine depressive-like behaviorJ Immunol200918253202321219234218
- O’ConnorJCAndreCWangYInterferon-gamma and tumor necrosis factor-alpha mediate the upregulation of indoleamine 2,3- dioxygenase and the induction of depressive-like behavior in mice in response to bacillus Calmette-GuerinJ Neurosci200929134200420919339614
- O’ConnorJCLawsonMAAndreCLipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in miceMol Psychiatry200914551152218195714
- AlterMJEpidemiology of hepatitis CHepatology1997263 Suppl 162S65S9305666
- ShepardCWFinelliLAlterMJGlobal epidemiology of hepatitis C virus infectionLancet Infect Dis20055955856716122679
- LauerGMWalkerBDHepatitis C virus infectionN Engl J Med20013451415211439948
- DieperinkEHoSBThurasPWillenbringMLA prospective study of neuropsychiatric symptoms associated with interferon-alpha-2b and ribavirin therapy for patients with chronic hepatitis CPsychosomatics200344210411212618532
- HorikawaNYamazakiTIzumiNUchiharaMIncidence and clinical course of major depression in patients with chronic hepatitis type C undergoing interferon-alpha therapy: a prospective studyGen Hosp Psychiatry2003251343812583926
- HauserPKhoslaJAuroraHA prospective study of the incidence and open-label treatment of interferon-induced major depressive disorder in patients with hepatitis CMol Psychiatry20027994294712399946
- KrausMRSchaferAFallerHCsefHScheurlenMPsychiatric symptoms in patients with chronic hepatitis C receiving interferon alfa-2b therapyJ Clin Psychiatry200364670871412823087
- SchaferAWittchenHUSeufertJKrausMRMethodological approaches in the assessment of interferon-alfa-induced depression in patients with chronic hepatitis C – a critical reviewInt J Methods Psychiatr Res200716418620118188838
- BonaccorsoSPuzellaAMarinoVImmunotherapy with interferon-alpha in patients affected by chronic hepatitis C induces an intercorrelated stimulation of the cytokine network and an increase in depressive and anxiety symptomsPsychiatry Res200110512455511740970
- WichersMCKenisGKoekGHRobaeysGNicolsonNAMaesMInterferon-alpha-induced depressive symptoms are related to changes in the cytokine network but not to cortisolJ Psychosom Res200762220721417270579
- WichersMCKoekGHRobaeysGVerkerkRScharpeSMaesMIDO and interferon-alpha-induced depressive symptoms: a shift in hypothesis from tryptophan depletion to neurotoxicityMol Psychiatry200510653854415494706
- RaisonCLDantzerRKelleyKWCSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: relationship to CNS immune responses and depressionMol Psychiatry201015439340319918244
- RoseWCHainesWJWarnerDTThe amino acid requirement of man. V. The role of lysine, arginine and tryptophanJournal of Biological Chemistry1950206142143013130563
- SchaechterJDWurtmanRJSerotonin release varies with brain tryptophan levelsBrain Res1990532122032102178030
- AndersonIMFerrierINBaldwinRCEvidence-based guidelines for treating depressive disorders with antidepressants: a revision of the 2000 British Association for Psychopharmacology guidelinesJ Psychopharmacol200822434339618413657
- DelgadoPLCharneyDSPriceLHAghajanianGKLandisHHeningerGRSerotonin function and the mechanism of antidepressant action. Reversal of antidepressant-induced remission by rapid depletion of plasma tryptophanArch Gen Psychiatry19904754114182184795
- HoodSDBellCJNuttDJAcute tryptophan depletion. Part I: rationale and methodologyAust N Z J Psychiatry200539755856415996136
- MaesMMeltzerHYScharpeSRelationships between lower plasma L-tryptophan levels and immune-inflammatory variables in depressionPsychiatry Res19934921511657908745
- ReimoldMBatraAKnobelAAnxiety is associated with reduced central serotonin transporter availability in unmedicated patients with unipolar major depression: a [11C]DASB PET studyMol Psychiatry200813660661355718268503
- JoensuuMTolmunenTSaarinenPIReduced midbrain serotonin transporter availability in drug-naive patients with depression measured by SERT-specific [(123)I] nor-beta-CIT SPECT imagingPsychiatry Res2007154212513117289353
- SargentPAKjaerKHBenchCJBrain serotonin1 A receptor binding measured by positron emission tomography with [11C] WAY–100635: effects of depression and antidepressant treatmentArch Gen Psychiatry200057217418010665620
- DrevetsWCThaseMEMoses-KolkoELSerotonin-1 A receptor imaging in recurrent depression: replication and literature reviewNucl Med Biol200734786587717921037
- YathamLNLiddlePFShiahISBrain serotonin2 receptors in major depression: a positron emission tomography studyArch Gen Psychiatry200057985085810986548
- DoggrellSAThe role of 5-HT on the cardiovascular and renal systems and the clinical potential of 5-HT modulationExpert Opin Investig Drugs2003125805823
- BonaccorsoSMarinoVPuzellaAIncreased depressive ratings in patients with hepatitis C receiving interferon-alpha-based immunotherapy are related to interferon-alpha-induced changes in the serotonergic systemJ Clin Psychopharmacol2002221869011799348
- KrausMRSchaferAFallerHCsefHScheurlenMParoxetine for the treatment of interferon-alpha-induced depression in chronic hepatitis CAliment Pharmacol Ther20021661091109912030950
- MusselmanDLLawsonDHGumnickJFParoxetine for the prevention of depression induced by high-dose interferon alfaN Engl J Med20013441396196611274622
- McMenamyRHBinding of indole analogues to human serum albumin. Effects of fatty acidsJ Biol Chem196524011423542435845825
- OldendorfWHSzaboJAmino acid assignment to one of three blood-brain barrier amino acid carriersAm J Physiol1976230194981251917
- SalomonRMKennedyJSJohnsonBWAssociation of a critical CSF tryptophan threshold level with depressive relapseNeuropsychopharmacology200328595696012736634
- FernstromJDDiet-induced changes in plasma amino acid pattern: effects on the brain uptake of large neutral amino acids, and on brain serotonin synthesisJ Neural Transm Suppl1979155567290763
- RaisonCLBorisovASMajerMActivation of central nervous system inflammatory pathways by interferon-alpha: relationship to monoamines and depressionBiol Psychiatry200965429630318801471
- JouvetMSleep and serotonin: an unfinished storyNeuropsychopharmacology1999212 Suppl24S27S10432485
- CapuronLMillerAHCytokines and psychopathology: lessons from interferon-alphaBiol Psychiatry2004561181982415576057
- BhattiTGillinJCSeifritzEEffects of a tryptophan-free amino acid drink challenge on normal human sleep electroencephalogram and moodBiol Psychiatry199843152599442344
- MoorePGillinCBhattiTRapid tryptophan depletion, sleep electroencephalogram, and mood in men with remitted depression on serotonin reuptake inhibitorsArch Gen Psychiatry19985565345399633672
- Carhart-HarrisRLNuttDJMunafoMRChristmasDMWilsonSJEquivalent effects of acute tryptophan depletion on REM sleep in ecstasy users and controlsPsychopharmacology (Berl)2009206218719619585107
- WilsonSJBaileyJERichASAdroverMPotokarJNuttDJUsing sleep to evaluate comparative serotonergic effects of paroxetine and citalopramEur Neuropsychopharmacol2004141536737215336297
- RaisonCLRyeDBWoolwineBJChronic interferon-alpha administration disrupts sleep continuity and depth in patients with hepatitis c: association with fatigue, motor slowing, and increased evening cortisolBiol Psychiatry2010681094294920537611
- BoissardRGervasoniDSchmidtMHBarbagliBFortPLuppiPHThe rat ponto-medullary network responsible for paradoxical sleep onset and maintenance: a combined microinjection and functional neuroanatomical studyEur J Neurosci200216101959197312453060
- FullerPMSaperCBLuJThe pontine REM switch: past and presentJ Physiol2007584Pt 373574117884926
- KnoxWEMehlerAHThe conversion of tryptophan to kynurenine in liver. I. The coupled tryptophan peroxidase-oxidase system forming formylkynurenineJ Biol Chem1950187141943014794727
- SilvaNMRodriguesCVSantoroMMReisLFAlvarez-LeiteJIGazzinelliRTExpression of indoleamine 2,3-dioxygenase, tryptophan degradation, and kynurenine formation during in vivo infection with Toxoplasma gondii: induction by endogenous gamma interferon and requirement of interferon regulatory factor 1Infect Immun200270285986811796621
- OkudaSNishiyamaNSaitoHKatsukiH3-Hydroxykynurenine, an endogenous oxidative stress generator, causes neuronal cell death with apoptotic features and region selectivityJ Neurochem19987012993079422375
- StoneTWEndogenous neurotoxins from tryptophanToxicon2001391617310936623
- SantamariaAGalvan-ArzateSLisyVQuinolinic acid induces oxidative stress in rat brain synaptosomesNeuroreport200112487187411277599
- BehanWMMcDonaldMDarlingtonLGStoneTWOxidative stress as a mechanism for quinolinic acid-induced hippocampal damage: protection by melatonin and deprenylBr J Pharmacol199912881754176010588931
- StoneTWAddaeJIThe pharmacological manipulation of glutamate receptors and neuroprotectionEur J Pharmacol200244723285296
- SaitoKCrowleyJSMarkeySPHeyesMPA mechanism for increased quinolinic acid formation following acute systemic immune stimulationJ Biol Chem19932682115496155038340378
- WuHQGuidettiPGoodmanJHKynurenergic manipulations influence excitatory synaptic function and excitotoxic vulnerability in the rat hippocampus in vivoNeuroscience200097224325110799756
- MyintAMKimYKVerkerkRScharpeSSteinbuschHLeonardBKynurenine pathway in major depression: evidence of impaired neuroprotectionJ Affect Disord2007981214315116963126
- MullerNSchwarzMJDehningSThe cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetineMol Psychiatry200611768068416491133
- MendlewiczJKriwinPOswaldPSoueryDAlboniSBrunelloNShortened onset of action of antidepressants in major depression using acetylsalicylic acid augmentation: a pilot open-label studyInt Clin Psychopharmacol200621422723116687994
- LokeYKTrivediANSinghSMeta-analysis: gastrointestinal bleeding due to interaction between selective serotonin uptake inhibitors and non-steroidal anti-inflammatory drugsAliment Pharmacol Ther2008271314017919277
- KerrSJSayerGPWhickerSDRowettDSSaltmanDCMantAAll-cause mortality of elderly Australian veterans using COX-2 selective or non-selective NSAIDs: a longitudinal studyBr J Clin Pharmacol201171693694221276041
- AbrahamNSEl-SeragHBHartmanCRichardsonPDeswalACyclooxygenase-2 selectivity of non-steroidal anti-inflammatory drugs and the risk of myocardial infarction and cerebrovascular accidentAliment Pharmacol Ther200725891392417402995
- LobSKonigsrainerARammenseeHGOpelzGTernessPInhibitors of indoleamine-2,3-dioxygenase for cancer therapy: can we see the wood for the trees?Nat Rev Cancer20099644545219461669
- BerginkVvan MegenHJWestenbergHGGlutamate and anxietyEur Neuropsychopharmacol200414317518315056476
- SwansonCJBuresMJohnsonMPLindenAMMonnJASchoeppDDMetabotropic glutamate receptors as novel targets for anxiety and stress disordersNat Rev Drug Discov20054213114415665858
- Aan het RotMCollinsKAMurroughJWSafety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depressionBiol Psychiatry201067213914519897179
- BermanRMCappielloAAnandAAntidepressant effects of ketamine in depressed patientsBiol Psychiatry200047435135410686270
- MathewSJMurroughJWaan het RotMCollinsKAReichDLCharneyDSRiluzole for relapse prevention following intravenous ketamine in treatment-resistant depression: a pilot randomized, placeb-controlled continuation trialInt J Neuropsychopharmacol2010131718219288975
- Machado-VieiraRYuanPBrutscheNBrain-derived neurotrophic factor and initial antidepressant response to an N-methyl- D-aspartate antagonistJ Clin Psychiatry200970121662166619744406
- ZarateCAJrSinghJBCarlsonPJA randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depressionArch Gen Psychiatry200663885686416894061