Abstract
Catatonia is a serious, common syndrome of motoric and behavioral dysfunction, which carries high morbidity and mortality. Electroconvulsive therapy (ECT) is the definitive treatment for catatonia, but access to ECT for the treatment of catatonia remains inappropriately limited. Catatonia is observable, detectable, and relevant to various medical specialties, but underdiagnosis impedes the delivery of appropriate treatment and heightens risk of serious complications including iatrogenesis. Current understanding of catatonia’s pathophysiology links it to the current understanding of ECT’s mechanism of action. Definitive catatonia care requires recognition of the syndrome, workup to identify and treat the underlying cause, and effective management including appropriate referral for ECT. Even when all of these conditions are met, and despite well-established data on the safety and efficacy of ECT, stigma surrounding ECT and legal restrictions for its use in catatonia are additional critical barriers. Addressing the underdiagnosis of catatonia and barriers to its treatment with ECT is vital to improving outcomes for patients. While no standardized protocols for treatment of catatonia with ECT exist, a large body of research guides evidence-based care and reveals where additional research is warranted. The authors conducted a review of the literature on ECT as a treatment for catatonia. Based on the review, the authors offer strategies and future directions for improving access to ECT for patients with catatonia, and propose an algorithm for the treatment of catatonia with ECT.
Introduction
A man in his late 70s sat inanimate, his eyes staring unfocused at a great distance, and arms stiffly suspended in mid-air. Upon removal of his pillow, his head remained elevated off the mattress. He had become severely constipated and retained his urine. Half an hour after receiving treatment, the return of fluid movement and spontaneous joking bears witness to his apparent revival from this strange, frozen state. The medical students – one of the authors (JJC) included – were astonished. Yet the nurses, anesthesia team, and psychiatry attending were not surprised, having regularly witnessed his recoveries. The catatonic syndrome separating this man from his world and its exquisite responsiveness to electroconvulsive therapy (ECT) had inspired countless cohorts of medical students, and was eventually reported.Citation1
Catatonia and ECT share an interwoven past over the last century. Catatonia is a neuropsychiatric syndrome characterized by behavioral and motor disturbances. Accurately described by Karl Kahlbaum as a distinct clinical phenotype in the late 19th century, catatonia was subsequently subsumed by Emil Kraepelin as a type of dementia praecox, later schizophrenia, where it remained in psychiatric nosology until the 2013 publication of the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5). The phenomenological and epidemiological errors in this categorization of catatonia have been well documented,Citation2,Citation3 and likely contribute to significant misconceptions and underdiagnosis of this psychomotor syndrome.
ECT is a treatment which relies upon the therapeutic benefits of inducing a seizure in the brain, is exquisitely effective for catatonia, and can be lifesaving in severe catatonic states. Even before larger observational studies describing the effectiveness of ECT in treating catatonia, inducing seizures to provide rapid recovery was reported as far back as the late 1930s.Citation4 Moreover, these findings have been further strengthened by studies with increasingly rigorous study designs, and have revealed previously unrecognized causes of catatonia, while delineating catatonia’s varied phenotypes.
In spite of being among the safest and most effective of known neuropsychiatric treatments, ECT remains underutilized. A multitude of factors likely influence underutilization. Principal among them are suboptimal recognition of catatonia by psychiatric and non-psychiatric physicians, mismanagement of catatonia when recognized, ongoing stigmatization of ECT, lack of patient access to ECT, and remaining neuroscience gaps in our understanding of the pathophysiology of catatonia and corresponding mechanisms of therapeutic action of ECT. This confluence of potentially contributing reasons predicts that many patients suffering from catatonia will not be presented with ECT as a treatment option.
Here, we review the evidence to date correlated with our current neuroscience-informed understanding of catatonia and ECT, how we assess and treat catatonia, the tolerance and safety of ECT, and disparities in access to ECT as a treatment. Finally, we offer practical strategies for improving recognition of catatonia, considering ECT early in its treatment course, and present an algorithmic approach to treatment.
Catatonia: The Syndrome
Features
Catatonia is known to occur in the setting of a variety of psychiatric, neurologic, and general medical conditions. The DSM-5 defines catatonia as the presence of three or more of the following 12 psychomotor features: stupor, catalepsy, waxy flexibility, mutism, negativism, posturing, mannerism, stereotypy, agitation, grimacing, echolalia, and echopraxia.Citation5 Of the several rating scales for catatonia, many have been previously reviewedCitation6,Citation7 and are summarized in .Citation8–Citation14
Of these, the Bush–Francis catatonia rating scale (BFCRS) is widely used in both research and clinical settings, was published with standardized exam instructions making it relatively straightforward to teach to trainees, and is the scale used in the authors’ practices. The BFCRS incorporates 23 clinical features to assess symptom severity and measure response to treatment.Citation9 The two clinical states of catatonia most described and represented in this scale are the stuporous state and the excited state. Stuporous catatonia manifests as a paucity of movement with symptoms including immobility, unresponsiveness, mutism, rigidity, staring, and refusal of oral intake. Excited catatonia is characterized by excessive motor activity, combativeness, echophenomena, impulsivity, and repetitive speech and behavior. Alternation between these seemingly opposite catatonic states is not uncommon, and the rapid alternation itself was historically recognized as a catatonic symptom, despite not being recognized in most modern scales.Citation15 With the most notable exception being the Northoff Catatonia Scale, most scales also leave out affective features, such as emotional lability, anxiety, flat affect, and ambivalence, despite Kahlbaum’s original characterization of catatonia as having an affective domain.Citation12
Mechanisms
While the pathophysiology of catatonia remains unclear, symptomatology and response to treatment have provided the beginning for exploration of the underlying pathophysiology. Pharmacological interventions used to manage catatonia involve targeting y-aminobutyric acid (GABA)-A, glutamate, and dopamine, suggesting dysfunction in these neurotransmitter systems may play a role in catatonia.Citation16,Citation17 Some evidence supports the idea that potency of dopamine D2 receptor blockade is directly related to the risk of exacerbating catatonia or provoking malignant features.Citation18 In comparing catatonic subjects to non-catatonic subjects, decreased GABA-A receptor density in the left sensorimotor cortex was visualized in catatonic subjects on functional imaging.Citation19 In addition, a deficiency of cortical GABA has been observed in catatonia and is hypothesized to alter basal ganglia modulation and lead to motor symptomatology.Citation20 This disturbance has been shown to be corrected with the administration of lorazepam, providing the theory that the quick reversal of catatonic symptoms with lorazepam may be related to the normalization of regulatory circuits.Citation19,Citation21 Dopamine hypoactivity and glutamate hyperactivity have also been postulated to have a role in catatonia as dopamine antagonists can induce a catatonic state and N-methyl-D-aspartate (NMDA) antagonists have been shown to treat catatonia. Vagal theories of catatonia, which help explain the autonomic instability, have also been elaborated.Citation22,Citation23
Genetics may also play a role in the pathogenesis of catatonia as a study of multiplex families reported significant heritability estimates for catatonia symptoms, frequency, and severity.Citation24 In addition, studies have identified a locus of interest on chromosome 15q15 in families with inherited vulnerability to periodic catatonia, a psychomotor disturbance during acute psychosis.Citation25 The Prader Willi Syndrome, a genetic syndrome whose behavioral phenotype includes catatonia, compulsive behaviors, stereotypies, psychosis, and excessive somnolence, is also related to an abnormality on chromosome 15, interestingly in a region containing several GABA-A receptor subunit genes.Citation16 The Klein–Levin syndrome of episodic hypersomnolence has been proposed as a model for how diencephalic dysfunction can contribute to the catatonic phenotype.Citation16 Additionally, theories have suggested an intense evolutionary-based fear response can induce catatonia.Citation26 Functional imaging of catatonic subjects during negative emotional states has shown alterations in activation of the orbitofrontal cortex and in the functional connectivity to the premotor cortex,Citation27 which provides a potential link from emotional/limbic signals to motor control circuits. Dysfunction in the posterior parietal cortex has been postulated to contribute to the termination of movements, resulting in motor posturing, while medial prefrontal and anterior cingulate dysfunction, regions typically involved in the initiation of activity, has been hypothesized to contribute to mutism and stupor.Citation20
Much is now known about the effects of ECT at neurochemical, neuroendocrine, synaptic, and cellular levels.Citation28 Recent data have demonstrated regional increases in brain volume post-ECT, which are likely related to neurogenic and synaptogenic effects.Citation29 While a full mechanistic understanding of the relative contributions of each of these components to the therapeutic effects of ECT for catatonia (and other states) remains incomplete, the same is true for many other treatments for neuropsychiatric disease. The current understanding of ECT’s mechanism compares favorably to many psychotropic medications, anticonvulsant medications, and vagus nerve stimulation, none of which suffer the same level of stigmatization.Citation6
Morbidity and Mortality
Though research has estimated the prevalence of catatonia to be 5–18% on inpatient psychiatric units, the syndrome remains under-recognized.Citation30–Citation32 Not identifying catatonia as the appropriate diagnosis and the resulting lack of treatment can lead to dangerous medical complications including pressure ulcers, nutritional deficiencies, electrolyte disturbances, venous thrombosis, pulmonary embolism, urinary tract infections, muscle contractures, and aspiration pneumonia. Without timely treatment, a patient may develop autonomic instability with hyperthermia, intense excitement, rigidity, and delirium. These symptoms indicate a life-threatening state called malignant catatonia, with a mortality rate as high as 20% if untreated.Citation30 The symptoms can be indistinguishable from neuroleptic malignant syndrome (NMS), a condition associated with exposure to dopamine antagonists such as antipsychotic drugs. As there are no identifying clinical or laboratory markers that distinguish the two, and no differential response to treatment, NMS is best considered to be a drug-induced cause of malignant catatonia.Citation18
Differential Diagnosis
The differential diagnosis of catatonia must include numerous conditions which can present similarly or comorbidly.Citation17,Citation33,Citation34 Here we provide several examples of how to consider catatonia and its differential diagnosis. However, this is by no means intended as a comprehensive list of all of the differential considerations, which must be tailored to the history and exam findings of each individual case. Catatonia can worsen with the addition of dopamine antagonists; however, patients can also experience drug-induced parkinsonism with overlapping symptoms of immobility, staring, and rigidity. Akathisia, literally the inability to remain seated, is an internally excited motoric state, commonly mistaken for anxiety. Active catatonic motor features such as maintaining postures anti-gravity, repetitive movements or speech, or echophenomena would not be expected in drug-induced parkinsonism or akathisia alone. Nonconvulsive status epilepticus can lead to odd behavioral automatisms which might look like stereotypies or mannerisms, and indeed epilepsy is a relatively common cause of catatonia, but some features, such as the prolonged holding of induced postures (catalepsy) or echophenomena, would be less commonly expected in epilepsy. Akinetic mutism can lead to a mute and immobile state with a refusal to eat, but should not have active motor features, and is typically associated with a structural brain lesion. Symptoms of stuporous catatonia and hypoactive delirium can overlap, as can excited catatonia and hyperactive delirium. Despite controversy in the DSM-5 criteria, it is clear that delirium and catatonia frequently co-occur, leading to difficulty in diagnosis and appropriate treatment as benzodiazepines can improve catatonia but exacerbate delirium.Citation35,Citation36 Delirium alone should not display active catatonic motor features. The serotonin syndrome is an example of a cause of a hyperkinetic state, where the clinical features will commonly include those of both excited catatonia and hyperactive delirium. A physical motor exam encompassing assessment for catatonia-specific motor findings (eg, the BFCRS) and additional motor components of a traditional neurology exam (eg, tremor, tone, strength, coordination) is needed to rule catatonia in or out.
Prevalence and Etiology
Catatonia’s prevalence varies widely in different neuropsychiatric states. Its prevalence in different psychiatric conditions is summarized in .Citation14,Citation31,Citation37–Citation54
Figure 1 Prevalence of catatonia. Insufficient prevalence data for other conditions frequently cooccurring with catatonia include: obsessive-compulsive disorder, Tourette’s syndrome, alcohol/sedative withdrawal, and selective mutism.
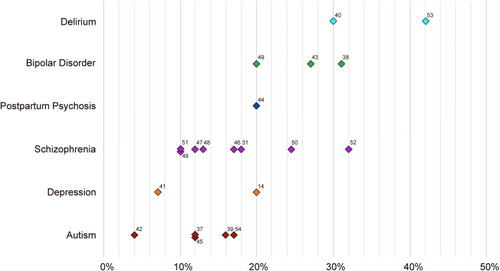
Listed at the bottom of the figure are conditions widely recognized as presenting comorbidly with catatonia (and/or for which catatonia is on the differential diagnosis), yet no systematic data regarding the prevalence of catatonia in these conditions were found. Studies have estimated that catatonia has a medical or neurological etiology in 21–46% of cases,Citation33 though precise and specific prevalence numbers are not available, and in some acute medical and surgical settings the rate may be even higher.Citation55 Potential causes are varied and numerous, and include metabolic derangements (eg, electrolyte disturbances, cardiac, renal, liver dysfunction, and vitamin deficiency), inflammatory states (infectious, autoimmune, or paraneoplastic), neurologic conditions (eg, vascular, structural, seizure, degenerative, and genetic/developmental disorders), toxidromes (eg, use of recreational substances, certain medications), and withdrawal phenomena.
Evaluation and Management
Workup and Supportive Care
Regardless of etiology, catatonia is best treated by addressing both the syndrome itself and the underlying cause.Citation30,Citation56 Once the syndrome is recognized on exam, a thorough workup is warranted.Citation17,Citation36 Depending on the history, presence of comorbid symptoms, and physical exam findings, corresponding serum, urine, and cerebrospinal fluid studies, electroencephalogram, and magnetic resonance imaging of the brain should be considered.
In particular, medication regimens and recent medication changes should be evaluated, specifically the presence of dopamine antagonists, or the recent discontinuation of GABA-ergic or dopaminergic drugs. The risk of worsening catatonia or provoking malignant features is high with dopamine antagonist medications, which should be held when catatonia is recognized.Citation18 Monitoring signs for the development of malignant catatonia such as hyperautonomia and fever is essential, with other less specific markers including elevated creatine phosphokinase, decreased serum iron, and leukocytosis.Citation17,Citation57
Additionally, supportive care to manage medical complications of catatonia is vital. Frequent repositioning in the setting of prolonged immobility can prevent muscle contractures and pressure ulcers.Citation58 Other measures include controlling pyrexia and hyperautonomia, ensuring adequate oxygenation, electrolyte repletion, appropriately hydrating to prevent acute kidney injury and rhabdomyolysis, maintaining adequate nutrition, venous thromboembolism prophylaxis, catheter use in the setting of urinary retention, bowel regimens for constipation, and implementing protocols to reduce aspiration risk.Citation58 Finally, throughout care, a catatonia rating scale score should be tracked regularly to monitor the severity of symptoms and response to treatment.
Treatment
Catatonia responds well to treatment. An established feature of catatonia is its robust response to benzodiazepines. Benzodiazepines have become the mainstay of first-line treatment with remission rates as high as 70–80%.Citation55,Citation59 The administration of 1–2 mg of lorazepam can function as both a treatment and diagnostic tool, known as the lorazepam challenge, with a reduction in catatonic symptoms confirming the diagnosis.Citation57 If the initial dose is ineffective, it can be repeated every 30–60 minutes with serial symptom assessments. Intravenous administration is preferred to ensure adherence and rapid absorption. When catatonia responds partially to lower doses, patients may require rapid titration to doses of 20–30 mg/day of lorazepam to achieve full symptom resolution.Citation60 Monitoring for sedation and respiratory depression is essential when escalating the dose of benzodiazepine; however, sedation typically precedes respiratory depression and thus serves as a signal to slow or halt titration,Citation61 which can often be done on a general medical or psychiatric unit of a general hospital by including “hold for sedation” in the medication order. Furthermore, catatonia is a state where these high doses are typically tolerated without inducing over-sedation.Citation30 However, additional precautions may be needed in situations where medical backup is less robust and in patients with a higher risk of respiratory depression, such as those with known pulmonary disease. Though lorazepam is the most common benzodiazepine used in catatonia, successful use of diazepam, oxazepam, and clonazepam has also been reported.Citation17 Zolpidem, a positive allosteric modulator of GABA-A receptors, has also been shown to be a safe and effective alternative treatment to catatonia. Similar to the lorazepam challenge, a zolpidem challenge has been proposed, using doses from 7.5 mg to 40 mg daily.Citation55,Citation62 After, or in concert with, GABA-ergic medications, ECT is the definitive treatment for catatonia, as will be discussed below. NMDA antagonists, amantadine and memantine, have some evidence to support their use as second or third line agents.Citation63 It is unclear if these agents work through direct glutamate antagonism or indirect effects on GABA and dopamine.Citation64,Citation65 Case reports have also published support for the use of mood stabilizers in the treatment of catatonia, including valproate, topiramate, and carbamazepine, with their effects possibly mediated through GABA-ergic actions.Citation55,Citation66-Citation68 Several case reports describe the effective treatment of catatonia with repetitive transcranial magnetic stimulationCitation69 or second-generation antipsychotic medications,Citation56 but if the latter are given, each dose should be given in combination with a benzodiazepine.Citation63 Algorithmic approaches to these treatments have been published.Citation63,Citation64
ECT
Indication
There is wide consensus that in cases refractory to benzodiazepine treatment or in life-threatening conditions that require rapid resolution, such as malignant catatonia, ECT should be initiated.Citation30,Citation70 Other considerations which may drive ECT’s use earlier in the course of catatonia include situations that may have relative contraindications to benzodiazepine use. These could include catatonia in pregnancy or the peripartum period, comorbid substance use disorders, and psychosocial circumstances which present a concern for safely prescribing high doses of controlled substances, among others. Some have suggested that ECT should be elevated to first-line status for the treatment of catatonia due to the safety profile and rapidity of response.Citation71
Efficacy
Response rates of catatonia to ECT are excellent, with most modern studies reporting response in 80–100% of cases, including in the setting of non-response to benzodiazepine.Citation44,Citation59,Citation70–Citation73 Response rates approached 80% in a literature review of catatonia in children and adolescents with diverse underlying etiologies.Citation74 Lower response rates (59% and 71%) described in two retrospective studiesCitation75,Citation76 may be attributable to study populations with higher rates of underlying psychotic disorder, delay in appropriate treatment, and/or recent use of dopamine antagonists.Citation55 There is little standardization regarding what constitutes benzodiazepine non-response. As noted above, high doses of benzodiazepines are often needed, but are often not trialed adequately in routine clinical care. Zisselman and Jaffe recommend preparing for ECT early: immediately upon non-response to initial lorazepam challenge, even while lorazepam doses are being uptitrated.Citation56 Hatta et al suggest ECT should be the treatment of choice for catatonia.Citation71 Their randomized controlled trial (RCT) of catatonic patients (n=50) had several successive arms pitting ECT against various pharmacotherapies: first lorazepam, then intravenous haloperidol or oral risperidone, followed by chlorpromazine, with ECT offered to patients who failed pharmacotherapy at each step. All patients who received ECT at any step achieved remission (100% response rate). Response rates for all other treatments were considerably less impressive, including just 2% for lorazepam (though the maximum daily dose was limited to just 3 mg per day). On a larger scale, the 2016 updated Canadian Network for Mood and Anxiety Treatments guidelines for the treatment of major depressive disorder recommend ECT as a first-line treatment when catatonic features are present,Citation77 with level 3 evidence.
Of note, this body of evidence includes very few RCTs, and those that do exist are limited in rigor. A 2018 systematic review of 28 studies – the majority observational and including 3 RCTs – on the treatment of 564 catatonic patients with ECT concluded that none of the studies adequately demonstrated efficacy (ECT superior to placebo) or effectiveness (ECT superior to comparable treatment), despite robustly showing that catatonic symptoms improve significantly following ECT.Citation78 However, a sham-controlled trial of ECT for catatonia would be ethically impermissible due to lack of equipoise based on the available evidence, combined with the grave risks of withholding or delaying treatment for catatonia, and thus may be considered similar to the lack of RCT evidence supporting the use of a parachute to prevent death when falling from an airplane.Citation79
There are case reports of ECT effectively treating catatonia caused by myocardial infarction,Citation55 lupus,Citation80 encephalomyelitis,Citation81 multiple sclerosis,Citation82 and other complex medical conditions.Citation83 Safe and effective use of ECT to treat deliriumCitation84 suggests likely benefit when co-occurring with catatonia. Additionally, there are case reports of dramatic improvement in symptoms of chronic catatonia once the syndrome was recognized and ECT administered, regardless of etiology;Citation55 in parallel, a 1999 RCT demonstrated that lorazepam failed to treat chronic catatonia in eighteen patients (though all patients had chronic schizophrenia and the total daily dose of lorazepam was limited to 6 mg/day).Citation85 Limited but compelling evidence also supports the use of ECT for catatonia associated with autism,Citation86–Citation89 anti-NMDA receptor encephalitis,Citation90,Citation91 toxic psychosis,Citation92 obsessive compulsive disorder,Citation93–Citation95 and repetitive self-injury and tics.Citation8
Predictors of Response
Attempts to elucidate predictors of response to ECT trace back to observations made by Laszlo Meduna in 1934, where longer duration of illness predicted poorer response in the first nine catatonic patients to be treated with (chemically induced) convulsive therapy.Citation4,Citation55 Small studies since then support this duration of illness finding, but have otherwise offered few consistent findings of additional predictors of response. A small retrospective study of 27 patients found improvement to be significantly associated with younger age and autonomic dysregulation, especially elevated temperature.Citation75 An observational study of 63 patients with catatonic schizophrenia demonstrated that faster response was associated with a shorter duration of illness, higher initial BFCRS score, and higher rates of waxy flexibility and gegenhalten (a term used to describe a form of hypertonia involving motoric resistance to passive movements); echophenomena predicted slower response.Citation70
Evidence supporting the high response rate of catatonia to ECT is such that, if a suboptimal response is seen, a more extensive workup for an underlying neurologic condition should be considered.Citation96 Swartz et al described four patients with chronic neurologic pathology (Alzheimer disease; cerebellar atrophy with complex partial seizures; spinal cord injury; post-encephalitis cognitive deficits) whose catatonia responded poorly to ECT.Citation97 A study of ECT in 26 patients with catatonia and bipolar disorder demonstrated that patients prescribed anticholinergics or dopamine agonists (suggestive of possible underlying neurologic pathology) were more frequently nonresponders.Citation98 The authors hypothesized that this might represent a “structural” variant of catatonia that is less responsive to ECT. Further evidence supporting this idea is a series showing that catatonia as a sequela of hypoxic brain injury was unresponsive to ECT.Citation99
It is well established that catatonia due to underlying mood disorders has a better response to treatment, including ECT, compared to catatonia due to underlying psychotic disorders,Citation55 leading some to postulate that catatonia in schizophrenia is an altogether different syndrome. Response to ECT in co-occurring catatonia and schizophrenia is likely augmented when combined with clozapine,Citation55 though a 2005 Cochrane Review concluded that ECT for chronic catatonia in schizophrenia is not significantly beneficial;Citation100 the updated Review from 2019 offered no new insight.Citation101
Administration
Lack of rigorous comparison studies has hindered the development of a standardized treatment protocol for catatonia with ECT. Most evidence supports a “robust” approach: bitemporal ECT, standard pulse width (brief pulse), with a substantially suprathreshold stimulus,Citation55 with some arguing that stuporous catatonia in particular should be conceptualized as always potentially life-threatening, and that potential for cognitive side effects should be a distant secondary consideration.Citation102,Citation103 Numerous case reports describe successful treatment of catatonia with alternative electrode placements, including right unilateral (RUL),Citation104–Citation110 left anterior right temporal,Citation111 and biparietotemporal,Citation112 and/or ultrabrief pulse (UB) stimuli,Citation81,Citation106,Citation107,Citation109,Citation110 but it is unclear whether or not there is benefit to choosing them over the traditional “robust” approach to treating catatonia. Reasons for alternate placement/stimulus varied and included lack of consent for bitemporal placement and hopes of inducing fewer cognitive side effects based on extrapolating data from studies on UB-RUL ECT in depression.Citation113 Many of these studies commented on the preservation of patients’ premorbid cognitive function or the lack of ECT-induced cognitive impairments, but most did not systematically evaluate cognition. One used brief bedside cognitive testing (of attention, concentration, short-term recall, and biographical recall), in a single case, and only post-ECT.Citation106 A second compared Mini-Mental State Examination scores in a case, pre (26/30) and post-ECT (28/30).Citation110 A third described a single case in which extensive formal cognitive batteries were completed post-ECT; they showed some deficits but were suspected to be premorbid.109
There is some agreement that if the ECT-induced convulsive response lasts less than 25 seconds, the stimulus dose should be increased.Citation96 Minimal data exist regarding the frequency of treatments for catatonia; the most common protocol is thrice weekly, except in cases of malignant catatonia where daily treatment may be considered until symptoms improve.Citation55 The total number of treatments typically ranges between 12 and 20 in order to achieve meaningful improvement or remission, although rapid response can also be seen.Citation55 The number of treatments should be titrated to clinical response and cannot be accurately predicted ahead of time.Citation102 Recurrence of catatonia is common upon ECT cessation, and maintenance ECT (mECT) has been described as helping to prevent relapse,Citation55,Citation82,Citation88,Citation114–Citation116 with some suggesting this approach may be preferred over polypharmacy.Citation96 While it is well established that mECT is beneficial in both mood disorders and schizophrenia for preventing relapse following successful treatment with acute ECT,Citation117–Citation119 evidence supporting the same for catatonia is based mostly on case reports. In one retrospective study of 42 older patients with a serious mental illness (approximately 25% with catatonia), mECT was highly effective in preventing hospital readmission and reducing readmission length of stay.Citation120 Based on these data and on direct experience, we propose the treatment algorithm in .
Figure 2 Proposed algorithm for ECT treatment of catatonia.Citation81,Citation102-Citation110
Notes: †If malignant features, consider daily BT treatment. ††Consider maintenance ECT for cases of catatonia of unclear etiology, recurrence of catatonic features in setting of continuation-phase ECT, or history of recurrent catatonia with pharmacologic treatment-resistance. *Monitor treatment response and consider seizure optimization throughout. If seizure quality is in question between treatment 1–5, first consider increasing to max UB-RUL, switching to BT, or increasing BT to 2.5× ST prior to treatment #6. Also consider optimizing via hyperventilation, switch to etomidate or ketamine, tapering or d/c anticonvulsants, or adding flumazenil or caffeine. ¥- If lysis or max benefit is lost during CP-ECT, consider further optimization of seizure parameters, increase in stimulus dosing, and return to the previous treatment level where lysis or max benefit is achieved, and proceed according to algorithm.
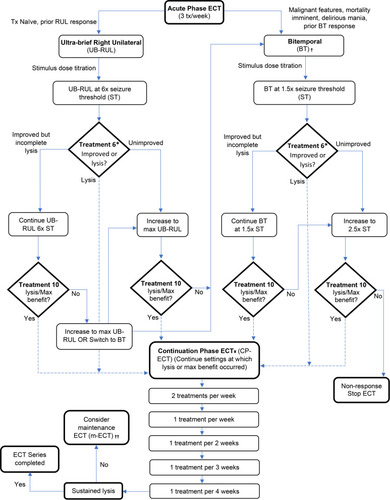
Electrographic markers of seizure quality have been primarily evaluated in patients with depression, not catatonia.Citation121 If there is a concern for inadequate seizure, in addition to increasing stimulus dose, optimizations include ensuring pre-stimulus hyperventilation, changing anesthetic to an agent with less anticonvulsant properties (etomidate or ketamine), adjusting psychotropics (removing anticonvulsants and/or adding medications that lower seizure threshold, such as flumazenil,Citation122 or less commonly, caffeineCitation123). In cases of prolonged immobility, the use of succinylcholine increases the risk of hyperkalemia, and non-depolarizing neuromuscular blockade (now safely reversible with sugammadexCitation124) should be considered.Citation56 Historically, all psychotropics were discontinued prior to starting ECT; however, updated evidence and clinical practice argue against this. Current guidelines recommend carefully considering each patient’s medications and adjusting only if necessary.Citation125,Citation126
In Combination with Benzodiazepine
In practice, ECT is often administered in the setting of ongoing benzodiazepine administration. Previously, recommendations against the practice were common, citing possibly reduced benefit.Citation127,Citation128 A later case series of catatonic patients receiving bitemporal, brief-pulse ECT suggested that combined therapy was superior to monotherapy.Citation129 The authors observed that symptoms improved more dramatically and rapidly once one of the treatments was added to the other, regardless of which was initiated first; they thus alluded to a possible “synergistic” effect between benzodiazepines and ECT.Citation129 An additional case report described a patient with catatonia and OCD whose symptoms resolved only after coadministration of lorazepam and ECT.Citation93 While the literature remains small, the combination is often feasible, though may require benzodiazepine reversal with flumazenil to achieve therapeutic seizures.
Safety and Tolerability
ECT is generally safe. In 2018, the FDA reclassified ECT devices from high risk (class III) to moderate risk (class II) when used for catatonia that is treatment-resistant or requiring a rapid response in patients 13 years old and above.Citation130 A 2009 review determined ECT to be low risk when using American College of Cardiology/American Heart Association guidelines for non-cardiac surgery,Citation131 and a meta-analysis published in 2017 showed a mortality rate of 2.1 per 100,000 treatments, no higher than rates of general anesthesia alone, with only one ECT-related death in 414,747 included treatments published since 2001.Citation132
Due to the immediately life-threatening nature of some ECT-responsive states (eg, malignant catatonia), there are no absolute contraindications to ECT. Any relative contraindications must be weighed against the risks of leaving a likely ECT-responsive catatonic state untreated. ECT can be performed safely in the setting of most neurologicalCitation133 and cardiovascularCitation131 comorbidities. Some conditions present particularly heightened risk (eg, space-occupying brain lesion causing increased intracranial pressure; pheochromocytoma; recent myocardial infarction; cerebrovascular hemorrhage; severe pulmonary disease,Citation131,Citation134 and hypoxic brain injuryCitation99). Prior to ECT, patients should undergo a thorough medical evaluation, and specialty consultation should be obtained, when indicated, to assist with risk stratification and optimization of comorbidities. Guidelines around this have been published.Citation131,Citation134
ECT has been shown to be relatively safe in pregnancy,Citation135–Citation137 in the elderly,Citation138 and in children and adolescents,Citation92,Citation139 though some have challenged that safety data on ECT in children with autism were not systematically collected.Citation116 It has been demonstrated definitively that ECT does not cause structural brain damage,Citation140 even in patients who have received hundreds of lifetime treatments.Citation1 Additionally, cognitive side effects from acute ECT do not worsen with mECT.Citation119 Reports suggest ECT has no long-term impact on cognition,Citation141 even in patients receiving upward of 100 lifetime treatments,Citation142 in patients with preexisting dementia,Citation143,Citation144 or in the still-developing brain.Citation145 While few systematic studies of long-term cognitive outcomes have been reported, two large cohort studies found ECT was not associated with incident dementia in those who received ECT.Citation146,Citation147
Common side effects are headache, myalgias, and memory deficits. Memory impairments, when present, typically return to normal within weeks after an ECT course, and objectively resolve by six months post-treatment.Citation131 Memory effects were improved markedly with the move from sine to square wave ECT,Citation148 and can be reduced further with other techniques including electrode placement and pulse width modifications.Citation113,Citation148 The majority of studies actually show improvement in subjective memory with ECT,Citation149 likely related to symptom improvements, though in some cases, subjective memory impairment may last beyond six months.Citation131 To the authors’ knowledge, there are no studies specifically looking at cognitive side effects in catatonic patients, though it is worth noting that catatonia itself significantly impairs cognition,Citation27,Citation150 and improvement on a brief cognitive measure, such as a clock drawing test, has been reported in catatonia responsive to ECT.Citation80,Citation150,Citation151 Studies looking at depression and other conditions have shown overall improvement of cognition with ECT,Citation152,Citation153 suggesting that benefits of treatment outweigh the impact of side effects; this is likely true for catatonia as well. Risk for post-ECT delirium may be elevated in patients with catatonia compared to other indications for ECT; a study looking at associated factors found that presence of catatonia, longer seizure duration, high stimulus intensity, and bitemporal electrode placement increased risk, but that dexmedetomidine was preventive.Citation154 There is a single report of four cases of catatonia emerging during ECT thought to be attributable to the rapid discontinuation of benzodiazepine pre-procedure.Citation155
Informed Consent
Evaluation for treatment with ECT should be done by clinicians with experience administering ECT, and, specific to catatonia, with experience in assessing catatonic signs. The informed consent process is conceptually no different for ECT than that for any other medical intervention.Citation156 Patients with catatonia are often unable to provide consent, though capacity is dynamic and should be reassessed at appropriate intervals, especially as treatment improves symptoms.Citation157 A 2018 case series reported that in 3 cases of catatonia for which ECT was initially administered involuntarily, each patient regained capacity and consented to additional ECT treatment.Citation158
Access
Legal
Laws regarding ECT vary widely across the United StatesCitation159 and can be quite restrictive, often making it extremely challenging to provide appropriate and timely treatment. This is especially true when decisional capacity is limited, as is often the case in catatonia. In the most restrictive states, court order, guardianship, or advance directive is required when a patient lacks decisional capacity (ie, surrogate decision-makers who would otherwise be legally permitted to consent to other medical interventions are prohibited from consenting to ECT), and/or ECT is entirely prohibited for certain age groups.Citation56,Citation159 Many have argued that these restrictions are arbitraryCitation160 and violate the ethical principle of justice in health care.Citation161 Others have described cases where restrictive consent processes delayed care and thusly contributed to extreme morbidity and increased risk of mortality.Citation56,Citation58,Citation87,Citation162 In one case, it took 19 days to obtain a court order.Citation56 The question has been raised as to whether catatonia, particularly when caused by underlying medical or neurologic disease, should be subject to mental health-specific ECT laws.Citation160,Citation163
Stigma and Myth
Even when treatment is not legally or decisionally limited, the pursuit of ECT is often hindered by stigma and fear. Misinformation in the media, film, on the internet, and spread by anti-ECT groups has perpetuated myths regarding outdated methods and extreme side effects. Health-care providers can additionally be a source of misinformation. Several studies demonstrated that the extent of ECT knowledge positively correlated with a positive view of ECT in both providers and patients.Citation156 Furthermore, two studies of patients who underwent ECT demonstrated that the majority felt ECT was beneficial (79% and 83%) and would undergo ECT again (81% and 98%),Citation156 while in a third study, 80% of patients reported overall satisfaction with ECT, regardless of whether their treatment was voluntary or involuntary.Citation164
Provider Experience
Appropriate recommendation of ECT for catatonia remains limited worldwide by provider comfort with ECT. A few studies show that limited experience with ECT prevents providers from appropriately recommending it.Citation165–Citation167 Use in Germany is relatively low, despite the fact that psychiatrists there generally had positive views of ECT and felt it was underused.Citation168 Even in Norway, where ECT is widely available and viewed very positively, psychiatrists expressed concern about its underuse.Citation169
Under-Recognition of Catatonia
Under-recognition of catatonia is significant, and it follows then that this limits access to appropriate treatment with ECT. In 2005, van der Heijden et al demonstrated significant underdiagnosis of catatonia; clinicians diagnosed catatonia in only 2% of 139 patients, whereas a research team identified it in 18%.Citation31 In one retrospective review, neurology residents failed to identify catatonia on emergency room consultation in all of the 12 patients who eventually received a catatonia diagnosis.Citation170 A three-year retrospective chart review determined that 59% of patients meeting three or more of the DSM-5 criteria for catatonia in a general medical hospital were not diagnosed with catatonia, even in the third of those cases for which psychiatric consult was obtained.Citation32 The precise reasons for this under-recognition are not known, but may include the historic errors in nosology,Citation16 low catatonia knowledge,Citation31,Citation171,Citation172 lack of physical examinations done by psychiatrists,Citation173,Citation174 or confusion regarding the opposing clinical features and variable presentations of catatonia. Unfamiliarity with catatonia has been evidenced in a study showing markedly lower catatonia knowledge in internal medicine residents compared to psychiatry residents.Citation171 At the same time, low catatonia knowledge has also been demonstrated among psychiatrists.Citation31,Citation171,Citation172
Additional Barriers
Additional barriers in the US include required post-procedure transportation with escort,Citation175 a decrease in the number of hospitals providing ECT,Citation176 geographic limitations, and disparities in insurance coverage.Citation177 Disproportionately low ECT use in people of color points to sociodemographic barriers as well.Citation178–Citation180 Globally, use of ECT is heterogeneous,Citation181–Citation187 and geographic limitations, lack of training, lack of anesthesiologists, cost, stigma, health-care inequity, and national laws have been described as obstacles to access.Citation183,Citation188-Citation192 Outdated treatment protocols and heavy use for conditions less responsive to ECT (ie, schizophrenia) are common across Asia, based on a study of 334 institutions in 29 Asian countries.Citation193 In Scandinavia, ECT is widely available and tends to follow updated treatment guidelines, but unequal geographic distribution can limit access.Citation169,Citation194,Citation195
Discussion
Despite catatonia officially being moved out from under the umbrella of schizophrenia with the publication of DSM-5 in 2013, it continues to be misunderstood and artificially siloed, within the already artificially siloed specialty of psychiatry. Fink et al argued for the recognition of catatonia as a systemic medical syndrome.Citation196 Others have argued for catatonia to be viewed as a type of altered mental status, akin to and co-occurring with delirium,Citation36 a syndrome with which general medical practitioners have much greater familiarity. Under-recognition of catatonia among psychiatric and non-psychiatric physicians, along with cumbersome legal restrictions placed on ECT, to which very few other medical interventions are subject (rivaled only by pregnancy termination), leaves catatonia oddly disenfranchised, at significant detriment to patients.
Psychiatry as a clinical neuroscience specialty must be better integrated with the rest of medicine,Citation197,Citation198 and catatonia is a chief case-study in the argument. It is imperative that clinicians across many disciplines improve recognition and diagnosis of catatonia. There is reason to be hopeful, as two small studies demonstrated that catatonia-specific education is correlated with improved knowledge of the syndrome and increased comfort managing catatonia in practice.Citation171,Citation172 Other resources for learning catatonia are available including the Catatonia Information CenterCitation199 <catatonia.org> run by Dr Andrew Francis, and the National Neuroscience Curriculum Initiative, an open-access educational collaborative which has a module on the use of ECT for catatonia in autism,Citation200 and which hosted a live case conference on catatonia as part of their Quarantine Curriculum’s Mind-Body Day.Citation201
Widespread legal restrictions on ECT that place legal systems in between a doctor and patient in the therapeutic relationship, or disallow surrogate decision-makers, impede potentially life-saving or morbidity-reducing treatment in catatonia, and are inappropriate, inequitable, and harmful. Advocacy for changes to these laws will help our patients with catatonia suffer less discrimination in their access to care. In the meantime, psychiatric advance directives should be encouraged whenever possible. A patient with a history of ECT-responsive catatonia, who faces significant difficulty with the prospect of surrogate consent (eg, due to psychosocial or local jurisdiction factors), would be an excellent candidate for long-term planning with psychiatric advance directives providing consent to ECT in future catatonic states.
Access to appropriate diagnosis and care for ECT is a problem worldwide. Possible solutions include training more ECT providers, developing standardized methods for performing catatonia exams via telemedicine, and advocating for hospital inclusion of dedicated space for ECT. Misinformation is also widespread. Correcting this begins with standardized, adequate education of providers, and is then followed by education of our colleagues, patients, and the broader public. An example of how to perform psychoeducation about ECT using modern neuroscience-informed language has been published.Citation202
There is currently no standardized, evidence-based approach to the ECT treatment of patients with catatonia. Based on direct experience with treating catatonia in two separate academic medical centers and this review of the literature, we suggest a practical, flexible ECT algorithm for the treatment of catatonia []. Influenced by the best available published evidence, our proposed algorithm also leverages over a decade of direct experience with its implementation for treating catatonia at the University of Texas at Austin.Citation203 While not specifically in use at the University of Illinois at Chicago, the algorithm is overall consistent with ECT practice there as well. The suggested algorithm provides a general decision-making framework for ECT practitioners treating patients with catatonic syndromes across a spectrum of phenotypes and severity. Our algorithm prioritizes a balance of safety, tolerability, and relative effectiveness. Others have recently proposed pharmacologic algorithms for the treatment of catatonia utilizing similar approaches.Citation63,Citation64 The proposed algorithm may also be considered as a rational tool with the potential to inform RCTs and high-powered studies that compare various ECT practices in the treatment of catatonia. Finally, a standardized approach to ECT treatment of catatonia could improve communication and coordination of clinical care for patients receiving treatments across several different medical centers. Our suggested algorithm is not a substitute for the application of clinical expertise, nor does it subvert individualized decision-making. All ECT treatment decisions should weigh the possible consequences of under-treating a catatonic syndrome in any given clinical context.
The limitations of our review center on the lack of high-quality evidence for ECT in catatonia. While this is partially mitigated by the high quantity of such evidence, we must recognize the need to improve our evidence base for treating catatonia with ECT. Designing ethically permissible controlled studies of ECT for catatonia is challenging but not impossible. As described, case reports and one of the authors’ practices (JLK) suggest that nontraditional (ie, other than bitemporal) electrode placements are effective to some degree for catatonia. Bitemporal placement could be compared to other techniques (such as UB-RUL ECT), to examine efficacy and tolerability, with strict clinical parameters for abandoning the less-efficacious treatment. These, or other, techniques would have to be chosen because of a theoretical advantage, such as improved cognitive outcomes, and if one arm shows less efficacy, the study can be interpreted as having been an unintentional partial “sham” (as the clinical interaction, anesthesia, and stimulus-induced seizure would all be similar). These data would further solidify the evidence base for the superior treatment arm. An intentional “sham” ECT study in catatonia is not ethically permissible due to the risks of undertreatment or delayed treatment of catatonia. Our proposed algorithm provides multiple possible decision-points which could be compared in future clinical trials.
In summary, there are several crucial elements to the effective treatment of catatonia, which are highlighted in . Through catatonia education, measurement-based care (BFCRS), access to medical workups from psychiatric settings, and social and legal advocacy for ECT, we can address these barriers, and improve outcomes in catatonia.
Table 1 Features of Rating Scales for CatatoniaCitation8–Citation14
Table 2 Catatonia Clinical Points
Disclosure
The authors report no conflicts of interest in this work.
References
- Anderson D, Wollmann R, Dinwiddie SH. Neuropathological evaluation of an 84-year-old man after 422 ECT treatments. J ECT. 2014;30(3):248–250. doi:10.1097/YCT.000000000000006224755716
- Taylor MA, Fink M. Catatonia in psychiatric classification: a home of its own. Am J Psychiatry. 2003;160(7):1233–1241. doi:10.1176/appi.ajp.160.7.123312832234
- Fink M, Shorter E, Taylor MA. Catatonia is not schizophrenia: kraepelin’s error and the need to recognize catatonia as an independent syndrome in medical nomenclature. Schizophr Bull. 2010;36(2):314–320. doi:10.1093/schbul/sbp05919586994
- Gazdag G, Bitter I, Ungvari GS, Baran B, Fink M. Laszlo Meduna’s pilot studies with camphor inductions of seizures: the first 11 patients. J ECT. 2009;25(1):3–11. doi:10.1097/YCT.0b013e31819359fc19209069
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). American Psychiatric Pub; 2013.
- McCall WV, Andrade C, Sienaert P. Searching for the mechanism(s) of ECT’s therapeutic effect. J ECT. 2014;30(2):87–89. doi:10.1097/YCT.000000000000012124755719
- Sienaert P, Rooseleer J, De Fruyt J. Measuring catatonia: a systematic review of rating scales. J Affect Disord. 2011;135(1–3):1–9. doi:10.1016/j.jad.2011.02.01221420736
- Braunig P, Kruger S, Shugar G, Hoffler J, Borner I. The catatonia rating scale: development, reliability, and use. Compr Psychiatry. 2000;41(2):147–158. doi:10.1016/S0010-440X(00)90148-210741894
- Bush G, Fink M, Petrides G, Dowling F, Francis A. Catatonia I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93(2):129–136. doi:10.1111/j.1600-0447.1996.tb09814.x8686483
- Carroll BT, Kirkhart R, Ahuja N, et al. Katatonia: a new conceptual understanding of catatonia and a new rating scale. Psychiatry (Edgmont). 2008;5(12):42–50.
- Lund CE, Mortimer AM, Rogers D, McKenna PJ. Motor, volitional and behavioural disorders in schizophrenia. 1: assessment using the modified Rogers scale. Br J Psychiatry. 1991;158(333–326):323–327. doi:10.1192/bjp.158.3.3232036529
- Northoff G, Koch A, Wenke J, et al. Catatonia as a psychomotor syndrome: a rating scale and extrapyramidal motor symptoms. Mov Disord. 1999;14(3):404–416. doi:10.1002/1531-8257(199905)14:3<404::AID-MDS1004>3.0.CO;2-510348462
- Rogers D. The motor disorders of severe psychiatric illness: a conflict of paradigms. Br J Psychiatry. 1985;147:221–232. doi:10.1192/bjp.147.3.2212866007
- Starkstein SE, Petracca G, Teson A, et al. Catatonia in depression: prevalence, clinical correlates, and validation of a scale. J Neurol Neurosurg Psychiatry. 1996;60(3):326–332. doi:10.1136/jnnp.60.3.3268609512
- Shorter E, Fink M. The Madness of Fear: A History of Catatonia. Oxford University Press; 2018.
- Dhossche DM, Stoppelbein L, Rout UK. Etiopathogenesis of catatonia: generalizations and working hypotheses. J ECT. 2010;26(4):253–258. doi:10.1097/YCT.0b013e3181fbf96d21076339
- Daniels J. Catatonia: clinical aspects and neurobiological correlates. J Neuropsychiatry Clin Neurosci. 2009;21(4):371–380. doi:10.1176/jnp.2009.21.4.37119996245
- Lee JW. Neuroleptic-induced catatonia: clinical presentation, response to benzodiazepines, and relationship to neuroleptic malignant syndrome. J Clin Psychopharmacol. 2010;30(1):3–10. doi:10.1097/JCP.0b013e3181c9bfe620075641
- Northoff G, Steinke R, Czcervenka C, et al. Decreased density of GABA-A receptors in the left sensorimotor cortex in akinetic catatonia: investigation of in vivo benzodiazepine receptor binding. J Neurol Neurosurg Psychiatry. 1999;67(4):445–450. doi:10.1136/jnnp.67.4.44510486389
- Northoff G. What catatonia can tell us about “top-down modulation”: a neuropsychiatric hypothesis. Behav Brain Sci. 2002;25(5):555–577. doi:10.1017/S0140525X0200010912958742
- Richter A, Grimm S, Northoff G. Lorazepam modulates orbitofrontal signal changes during emotional processing in catatonia. Hum Psychopharmacol. 2010;25(1):55–62. doi:10.1002/hup.108420041475
- Dhossche DM. Vagal intimations for catatonia and electroconvulsive therapy. J ECT. 2014;30(2):111–115. doi:10.1097/YCT.000000000000013424800686
- Porges SW. The polyvagal perspective. Biol Psychol. 2007;74(2):116–143. doi:10.1016/j.biopsycho.2006.06.00917049418
- Peralta V, Goldberg X, Ribeiro M, Sanchez-Torres AM, Fananas L, Cuesta MJ. Familiality of psychotic disorders: a polynosologic study in multiplex families. Schizophr Bull. 2016;42(4):975–983. doi:10.1093/schbul/sbv19226707865
- Stober G, Saar K, Ruschendorf F, et al. Splitting schizophrenia: periodic catatonia-susceptibility locus on chromosome 15q15. Am J Hum Genet. 2000;67(5):1201–1207. doi:10.1086/32118311001582
- Caroff S, Mann S, Francis A, Fricchione G. Catatonia: From Psychopathology to Neurobiology. APPI; 2004.
- Northoff G, Nagel D, Danos P, Leschinger A, Lerche J, Bogerts B. Impairment in visual-spatial function in catatonia: a neuropsychological investigation. Schizophr Res. 1999;37(2):133–147. doi:10.1016/S0920-9964(98)00150-910374649
- Jiang J, Wang J, Li C. Potential mechanisms underlying the therapeutic effects of electroconvulsive therapy. Neurosci Bull. 2017;33(3):339–347. doi:10.1007/s12264-016-0094-x28032314
- Gbyl K, Videbech P. Electroconvulsive therapy increases brain volume in major depression: a systematic review and meta-analysis. Acta Psychiatr Scand. 2018;138(3):180–195. doi:10.1111/acps.1288429707778
- Fink M, Taylor M. Catatonia: A Clinician’s Guide to Diagnosis and Treatment. Cambridge University Press; 2006.
- van der Heijden FM, Tuinier S, Arts NJ, Hoogendoorn ML, Kahn RS, Verhoeven WM. Catatonia: disappeared or under-diagnosed? Psychopathology. 2005;38(1):3–8. doi:10.1159/00008396415714008
- Llesuy JR, Medina M, Jacobson KC, Cooper JJ. Catatonia under-diagnosis in the general hospital. J Neuropsychiatry Clin Neurosci. 2018;30(2):145–151. doi:10.1176/appi.neuropsych.1706012329325478
- Llesuy JR, Coffey MJ, Jacobson KC, Cooper JJ. Suspected delirium predicts the thoroughness of catatonia evaluation. J Neuropsychiatry Clin Neurosci. 2017;29(2):148–154. doi:10.1176/appi.neuropsych.1509023027899050
- Smith JH, Smith VD, Philbrick KL, Kumar N. Catatonic disorder due to a general medical or psychiatric condition. J Neuropsychiatry Clin Neurosci. 2012;24(2):198–207. doi:10.1176/appi.neuropsych.1106012022772668
- Saddawi-Konefka D, Berg SM, Nejad SH, Bittner EA. Catatonia in the ICU: an important and underdiagnosed cause of altered mental status. A case series and review of the literature. Crit Care Med. 2014;42(3):234–241. doi:10.1097/CCM.0000000000000053
- Oldham MA, Lee HB. Catatonia vis-a-vis delirium: the significance of recognizing catatonia in altered mental status. Gen Hosp Psychiatry. 2015;37(6):554–559. doi:10.1016/j.genhosppsych.2015.06.01126162545
- Billstedt E, Gillberg IC, Gillberg C. Autism after adolescence: population-based 13- to 22-year follow-up study of 120 individuals with autism diagnosed in childhood. J Autism Dev Disord. 2005;35(3):351–360. doi:10.1007/s10803-005-3302-516119476
- Braunig P, Kruger S, Shugar G. Prevalence and clinical significance of catatonic symptoms in mania. Compr Psychiatry. 1998;39(1):35–46. doi:10.1016/S0010-440X(98)90030-X9472454
- Ghaziuddin N, Dhossche D, Marcotte K. Retrospective chart review of catatonia in child and adolescent psychiatric patients. Acta Psychiatr Scand. 2012;125(1):33–38. doi:10.1111/j.1600-0447.2011.01778.x22040029
- Grover S, Ghosh A, Ghormode D. Do patients of delirium have catatonic features? an exploratory study. Psychiatry Clin Neurosci. 2014;68(8):644–651. doi:10.1111/pcn.1216824521083
- Grover S, Chakrabarti S, Ghormode D, Agarwal M, Sharma A, Avasthi A. Catatonia in inpatients with psychiatric disorders: a comparison of schizophrenia and mood disorders. Psychiatry Res. 2015;229(3):919–925. doi:10.1016/j.psychres.2015.07.02026260564
- Hutton J, Goode S, Murphy M, Le Couteur A, Rutter M. New-onset psychiatric disorders in individuals with autism. Autism. 2008;12(4):373–390. doi:10.1177/136236130809165018579645
- Kruger S, Cooke RG, Spegg CC, Braunig P. Relevance of the catatonic syndrome to the mixed manic episode. J Affect Disord. 2003;74(3):279–285. doi:10.1016/S0165-0327(02)00088-512738047
- Nahar A, Kondapuram N, Desai G, Chandra PS. Catatonia among women with postpartum psychosis in a mother-baby inpatient psychiatry unit. Gen Hosp Psychiatry. 2017;45:40–43. doi:10.1016/j.genhosppsych.2016.12.01028274337
- Ohta M, Kano Y, Nagai Y. Catatonia in individuals with autism spectrum disorders in adolescence and early adulthood: a long-term prospective study. Int Rev Neurobiol. 2006;72:41–54.16697290
- Peralta V, Cuesta MJ. Motor features in psychotic disorders. II. Development of diagnostic criteria for catatonia. Schizophr Res. 2001;47(2–3):117–126. doi:10.1016/S0920-9964(00)00035-911278128
- Peralta V, Campos MS, de Jalon EG, Cuesta MJ. DSM-IV catatonia signs and criteria in first-episode, drug-naive, psychotic patients: psychometric validity and response to antipsychotic medication. Schizophr Res. 2010;118(1–3):168–175. doi:10.1016/j.schres.2009.12.02320071147
- Peralta V, Campos MS, De Jalon EG, Cuesta MJ. Motor behavior abnormalities in drug-naive patients with schizophrenia spectrum disorders. Mov Disord. 2010;25(8):1068–1076. doi:10.1002/mds.2305020222137
- Solmi M, Pigato GG, Roiter B, et al. Prevalence of catatonia and its moderators in clinical samples: results from a meta-analysis and meta-regression analysis. Schizophr Bull. 2018;44(5):1133–1150. doi:10.1093/schbul/sbx15729140521
- Stober G. Genetic predisposition and environmental causes in periodic and systematic catatonia. Eur Arch Psychiatry Clin Neurosci. 2001;251(Suppl 1):I21–24. doi:10.1007/PL0001419611776267
- Stompe T, Ortwein-Swoboda G, Ritter K, Marquart B, Schanda H. The impact of diagnostic criteria on the prevalence of schizophrenic subtypes. Compr Psychiatry. 2005;46(6):433–439. doi:10.1016/j.comppsych.2005.03.00316275210
- Ungvari GS, Leung SK, Ng FS, Cheung HK, Leung T. Schizophrenia with prominent catatonic features (‘catatonic schizophrenia’): demographic and clinical correlates in the chronic phase. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(1):27–38. doi:10.1016/j.pnpbp.2004.08.00715610942
- Wilson JE, Carlson R, Duggan MC, et al. Delirium and catatonia in critically ill patients: the delirium and catatonia prospective cohort investigation. Crit Care Med. 2017;45(11):1837–1844. doi:10.1097/CCM.000000000000264228841632
- Wing L, Shah A. Catatonia in autistic spectrum disorders. Br J Psychiatry. 2000;176:357–362. doi:10.1192/bjp.176.4.35710827884
- Sienaert P, Dhossche DM, Vancampfort D, De Hert M, Gazdag G. A clinical review of the treatment of catatonia. Front Psychiatry. 2015;37:181. doi:10.3389/fpsyt.2014.00181
- Zisselman MH, Jaffe RL. ECT in the treatment of a patient with catatonia: consent and complications. Am J Psychiatry. 2010;167(2):127–132. doi:10.1176/appi.ajp.2009.0905070320123920
- Rasmussen SA, Mazurek MF, Rosebush PI. Catatonia: our current understanding of its diagnosis, treatment and pathophysiology. World J Psychiatry. 2016;6(4):391–398. doi:10.5498/wjp.v6.i4.39128078203
- Clinebell K, Azzam PN, Gopalan P, Haskett R. Guidelines for preventing common medical complications of catatonia: case report and literature review. J Clin Psychiatry. 2014;75(6):644–651. doi:10.4088/JCP.13r0887025004188
- Unal A, Altindag A, Demir B, Aksoy I. The use of lorazepam and electroconvulsive therapy in the treatment of catatonia: treatment characteristics and outcomes in 60 patients. J ECT. 2017;33(4):290–293. doi:10.1097/YCT.000000000000043328640169
- Fink M, Taylor MA. The catatonia syndrome: forgotten but not gone. Arch Gen Psychiatry. 2009;66(11):1173–1177. doi:10.1001/archgenpsychiatry.2009.14119884605
- Kang M, Galuska MA, Ghassemzadeh S. Benzodiazepine toxicity In: StatPearls. Treasure Island (FL): StatPearls Publishing LLC; 2020 Available from: https://www.ncbi.nlm.nih.gov/books/NBK482238/.
- Thomas P, Cottencin O, Rascle C, Vaiva G, Goudemand M, Bieder J. Catatonia in French psychiatry: implications of the zolpidem challenge test. Psychiatr Ann. 2007;37:45–54. doi:10.3928/00485713-20070101-02
- Beach SR, Gomez-Bernal F, Huffman JC, Fricchione GL. Alternative treatment strategies for catatonia: a systematic review. Gen Hosp Psychiatry. 2017;48:1–19. doi:10.1016/j.genhosppsych.2017.06.01128917389
- Carroll BT, Goforth HW, Thomas C, et al. Review of adjunctive glutamate antagonist therapy in the treatment of catatonic syndromes. J Neuropsychiatry Clin Neurosci. 2007;19(4):406–412. doi:10.1176/jnp.2007.19.4.40618070843
- Carpenter SS, Hatchett AD, Fuller MA. Catatonic schizophrenia and the use of memantine. Ann Pharmacother. 2006;40(2):344–346. doi:10.1345/aph.1G29716380435
- Kruger S, Braunig P. Intravenous valproic acid in the treatment of severe catatonia. J Neuropsychiatry Clin Neurosci. 2001;13(2):303–304. doi:10.1176/jnp.13.2.30311449040
- Kritzinger PR, Jordaan GP. Catatonia: an open prospective series with carbamazepine. Int J Neuropsychopharmacol. 2001;4(3):251–257. doi:10.1017/S146114570100248611602030
- McDaniel WW, Spiegel DR, Sahota AK. Topiramate effect in catatonia: a case series. J Neuropsychiatry Clin Neurosci. 2006;18(2):234–238. doi:10.1176/jnp.2006.18.2.23416720802
- Walther S, Stegmayer K, Wilson JE, Heckers S. Structure and neural mechanisms of catatonia. Lancet Psychiatry. 2019;6(7):610–619. doi:10.1016/S2215-0366(18)30474-731196794
- Raveendranathan D, Narayanaswamy JC, Reddi SV. Response rate of catatonia to electroconvulsive therapy and its clinical correlates. Eur Arch Psychiatry Clin Neurosci. 2012;262(5):425–430. doi:10.1007/s00406-011-0285-422207031
- Hatta K, Miyakawa K, Ota T, Usui C, Nakamura H, Arai H. Maximal response to electroconvulsive therapy for the treatment of catatonic symptoms. J ECT. 2007;23(4):233–235. doi:10.1097/yct.0b013e318158794918090694
- Perugi G, Medda P, Toni C, Mariani MG, Socci C, Mauri M. The role of electroconvulsive therapy (ECT) in bipolar disorder: effectiveness in 522 patients with bipolar depression, mixed-state, mania and catatonic features. Curr Neuropharmacol. 2017;15(3):359–371. doi:10.2174/1570159X1466616101723364228503107
- Girish K, Gill NS. Electroconvulsive therapy in Lorazepam non-responsive catatonia. Indian J Psychiatry. 2003;45(1):21–25.21206808
- Consoli A, Benmiloud M, Wachtel L, Dhossche D, Cohen D, Bonnot O. Electroconvulsive therapy in adolescents with the catatonia syndrome: efficacy and ethics. J ECT. 2010;26(4):259–265. doi:10.1097/YCT.0b013e3181fb392421099377
- van Waarde JA, Tuerlings JH, Verwey B, van der Mast RC. Electroconvulsive therapy for catatonia: treatment characteristics and outcomes in 27 patients. J ECT. 2010;26(4):248–252. doi:10.1097/YCT.0b013e3181c18a1319935090
- Morrison JR. Catatonia: prediction of outcome. Compr Psychiatry. 1974;15(4):317–324. doi:10.1016/0010-440X(74)90053-44412345
- Milev RV, Giacobbe P, Kennedy SH, et al. Canadian network for mood and anxiety treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 4. neurostimulation treatments. Can J Psychiatry. 2016;61(9):561–575. doi:10.1177/070674371666003327486154
- Leroy A, Naudet F, Vaiva G, Francis A, Thomas P, Amad A. Is electroconvulsive therapy an evidence-based treatment for catatonia? a systematic review and meta-analysis. Eur Arch Psychiatry Clin Neurosci. 2018;268(7):675–687. doi:10.1007/s00406-017-0819-528639007
- Smith GC, Pell JP. Parachute use to prevent death and major trauma related to gravitational challenge: systematic review of randomised controlled trials. BMJ. 2003;327(7429):1459–1461. doi:10.1136/bmj.327.7429.145914684649
- Boeke A, Pullen B, Coppes L, Medina M, Cooper JJ. Catatonia associated with systemic lupus erythematosus (SLE): a report of two cases and a review of the literature. Psychosomatics. 2018;59(6):523–530. doi:10.1016/j.psym.2018.06.00730270156
- Omi T, Matsunaga H, Kanai K, Shiraishi N, Miyazato Y, Sumikura H. Treatment of possible PERM underlying malignant catatonia and accompanying psychotic symptoms with modified electroconvulsive therapy: a case report. J ECT. 2019;35(1):3–4. doi:10.1097/YCT.000000000000053629877963
- Pontikes TK, Dinwiddie SH. Electroconvulsive therapy in a patient with multiple sclerosis and recurrent catatonia. J ECT. 2010;26(4):270–271. doi:10.1097/YCT.0b013e3181d039e621155152
- Romanowicz M, Sola CL. Electroconvulsive therapy-responsive catatonia in a medically complicated patient. J ECT. 2010;26(3):234–237. doi:10.1097/YCT.0b013e3181c18a8c19935089
- Coffey MJ, Cooper JJ. Therapeutic uses of seizures in neuropsychiatry. Focus (Am Psychiatr Publ). 2019;17(1):13–17. doi:10.1176/appi.focus.2018002331975954
- Ungvari GS, Chiu HF, Chow LY, Lau BS, Tang WK. Lorazepam for chronic catatonia: a randomized, double-blind, placebo-controlled cross-over study. Psychopharmacology (Berl). 1999;142(4):393–398. doi:10.1007/s00213005090410229064
- Wachtel LE, Hermida A, Dhossche DM. Maintenance electroconvulsive therapy in autistic catatonia: a case series review. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(4):581–587. doi:10.1016/j.pnpbp.2010.03.01220298732
- Wachtel LE, Dhossche DM, Kellner CH. When is electroconvulsive therapy appropriate for children and adolescents? Med Hypotheses. 2011;76(3):395–399. doi:10.1016/j.mehy.2010.11.00121129852
- Wachtel LE. Treatment of catatonia in autism spectrum disorders. Acta Psychiatr Scand. 2019;139(1):46–55. doi:10.1111/acps.1298030506668
- Withane N, Dhossche DM. Electroconvulsive treatment for catatonia in autism spectrum disorders. Child Adolesc Psychiatr Clin N Am. 2019;28(1):101–110. doi:10.1016/j.chc.2018.07.00630389070
- Coffey MJ, Cooper JJ. Electroconvulsive therapy in anti-n-methyl-d-aspartate receptor encephalitis: a case report and review of the literature. J ECT. 2016;32(4):225–229. doi:10.1097/YCT.000000000000033427295461
- Warren N, Grote V, O’Gorman C, Siskind D. Electroconvulsive therapy for anti-n-methyl-d-aspartate (NMDA) receptor encephalitis: a systematic review of cases. Brain Stimul. 2019;12(2):329–334. doi:10.1016/j.brs.2018.11.01630528383
- Dhossche DM, Withane N. Electroconvulsive therapy for catatonia in children and adolescents. Child Adolesc Psychiatr Clin N Am. 2019;28(1):111–120. doi:10.1016/j.chc.2018.07.00730389071
- Makhinson M, Furst BA, Shuff MK, Kwon GE. Successful treatment of co-occurring catatonia and obsessive-compulsive disorder with concurrent electroconvulsive therapy and benzodiazepine administration. J ECT. 2012;28(3):35–36. doi:10.1097/YCT.0b013e318254c2ea
- D’Urso G, Mantovani A, Barbarulo AM, Labruna L, Muscettola G. Brain-behavior relationship in a case of successful ECT for drug refractory catatonic OCD. J ECT. 2012;28(3):190–193. doi:10.1097/YCT.0b013e318254264922569374
- Duarte-Batista P, Coelho M, Quintas S, et al. Anterior limb of internal capsule and bed nucleus of stria terminalis stimulation for Gilles de la Tourette syndrome with obsessive-compulsive disorder in adolescence: a case of success. Stereotact Funct Neurosurg. 2020;1–9.
- Luchini F, Medda P, Mariani MG, Mauri M, Toni C, Perugi G. Electroconvulsive therapy in catatonic patients: efficacy and predictors of response. World J Psychiatry. 2015;5(2):182–192. doi:10.5498/wjp.v5.i2.18226110120
- Swartz CM, Acosta D, Bashir A. Diminished ECT response in catatonia due to chronic neurologic condition. J ECT. 2003;19(2):110–114. doi:10.1097/00124509-200306000-0001012792461
- Medda P, Toni C, Luchini F, Giorgi Mariani M, Mauri M, Perugi G. Catatonia in 26 patients with bipolar disorder: clinical features and response to electroconvulsive therapy. Bipolar Disord. 2015;17(8):892–901. doi:10.1111/bdi.1234826643014
- Quinn DK, Abbott CC. Catatonia after cerebral hypoxia: do the usual treatments apply? Psychosomatics. 2014;55(6):525–535. doi:10.1016/j.psym.2014.03.01025262046
- Tharyan P, Adams CE. Electroconvulsive therapy for schizophrenia. Cochrane Database Syst Rev. 2005;2.
- Sinclair DJ, Zhao S, Qi F, Nyakyoma K, Kwong JS, Adams CE. Electroconvulsive therapy for treatment-resistant schizophrenia. Cochrane Database Syst Rev. 2019;3.
- Fink M, Kellner CH, McCall WV. Optimizing ECT technique in treating catatonia. J ECT. 2016;32(3):149–150. doi:10.1097/YCT.000000000000034527428478
- Kellner CH, Popeo DM, Aloysi AS. Electroconvulsive therapy for catatonia. Am J Psychiatry. 2010;167:1127–1128. doi:10.1176/appi.ajp.2010.10020261
- Cristancho P, Jewkes D, Mon T, Conway C. Successful use of right unilateral ECT for catatonia: a case series. J ECT. 2014;30(1):69–72. doi:10.1097/YCT.0b013e31829a01d323859978
- Baker AS, Suh E, Prudic J. Malignant catatonia: role of right unilateral electroconvulsive therapy. J ECT. 2008;24(2):168–170. doi:10.1097/YCT.0b013e318151414418580565
- Rhoads JC, Votolato NA, Young JL, Gilchrist RH. The successful use of right unilateral ultra-brief pulse electroconvulsive therapy in an adolescent with catatonia. Brain Stimul. 2010;3(1):51–53. doi:10.1016/j.brs.2009.07.00320633430
- Kugler JL, Hauptman AJ, Collier SJ, et al. Treatment of catatonia with ultrabrief right unilateral electroconvulsive therapy: a case series. J ECT. 2015;31(3):192–196. doi:10.1097/YCT.000000000000018525243751
- Little JD, Munday J, Atkins M. Right unilateral ECT at 6x seizure threshold: is it effective in the psychoses? J ECT. 2003;19(3):158–163. doi:10.1097/00124509-200309000-0000812972986
- Mon T, L’Ecuyer S, Farber NB, et al. The use of electroconvulsive therapy in a patient with juvenile systemic lupus erythematosus and catatonia. Lupus. 2012;21(14):1575–1581. doi:10.1177/096120331246480323161578
- Cupina D, Patil S, Loo C. Chronic catatonic schizophrenia treated successfully with right unilateral ultrabrief pulse electroconvulsive therapy: case report. J ECT. 2013;29(2):134–136. doi:10.1097/YCT.0b013e31827659e423303422
- Pinna M, Manchia M, Pillai G, Salis P, Minnai GP. Efficacy and safety of electroconvulsive therapy in the first trimester of pregnancy: a case of severe manic catatonia. Bipolar Disord. 2015;17(5):567–571. doi:10.1111/bdi.1229725854818
- Suzuki K, Shindo T, Katsura M, et al. Resolution of catatonia by successful seizure induction via electroconvulsive therapy with electrodes applied bilaterally to the parietotemporal region. J ECT. 2007;23(2):103–105. doi:10.1097/yct.0b013e31803025f617548981
- Sackeim HA, Prudic J, Nobler MS, et al. Effects of pulse width and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. Brain Stimul. 2008;1(2):71–83. doi:10.1016/j.brs.2008.03.00119756236
- Sajith SG, Liew SF, Tor PC. Response to electroconvulsive therapy in patients with autism spectrum disorder and intractable challenging behaviors associated with symptoms of catatonia. J ECT. 2017;33(1):63–67. doi:10.1097/YCT.000000000000033827428481
- Torr J, D’Abrera JC. Maintenance electroconvulsive therapy for depression with catatonia in a young woman with Down syndrome. J ECT. 2014;30(4):332–336. doi:10.1097/YCT.000000000000011624755717
- DeJong H, Bunton P, Hare DJ. A systematic review of interventions used to treat catatonic symptoms in people with autistic spectrum disorders. J Autism Dev Disord. 2014;44(9):2127–2136. doi:10.1007/s10803-014-2085-y24643578
- Santos Pina L, Bouckaert F, Obbels J, et al. Maintenance electroconvulsive therapy in severe bipolar disorder: a retrospective chart review. J ECT. 2016;32(1):23–28. doi:10.1097/YCT.000000000000025326172058
- Fox HA. Continuation and maintenance electroconvulsive therapy–a conceptual framework? J ECT. 2015;31(2):27–28. doi:10.1097/YCT.0000000000000224
- Rabheru K. Maintenance electroconvulsive therapy (M-ECT) after acute response: examining the evidence for who, what, when, and how? J ECT. 2012;28(1):39–47. doi:10.1097/YCT.0b013e318245575822330700
- Shelef A, Mazeh D, Berger U, Baruch Y, Barak Y. Acute electroconvulsive therapy followed by maintenance electroconvulsive therapy decreases hospital re-admission rates of older patients with severe mental illness. J ECT. 2015;31(2):125–128. doi:10.1097/YCT.000000000000019725373561
- Minelli A, Abate M, Zampieri E, et al. Seizure adequacy markers and the prediction of electroconvulsive therapy response. J ECT. 2016;32(2):88–92. doi:10.1097/YCT.000000000000027426397151
- Krystal AD, Watts BV, Weiner RD, Moore S, Steffens DC, Lindahl V. The use of flumazenil in the anxious and benzodiazepine-dependent ECT patient. J ECT. 1998;14(1):5–14. doi:10.1097/00124509-199803000-000029661088
- Bozymski KM, Potter TG, Venkatachalam V, Pandurangi AK, Crouse EL. Caffeine sodium benzoate for electroconvulsive therapy augmentation. J ECT. 2018;34(4):233–239. doi:10.1097/YCT.000000000000050329768288
- Hoshi H, Kadoi Y, Kamiyama J, et al. Use of rocuronium-sugammadex, an alternative to succinylcholine, as a muscle relaxant during electroconvulsive therapy. J Anesth. 2011;25(2):286–290. doi:10.1007/s00540-011-1095-621293886
- Merk W, Kucia K. Combined use of ECT and psychotropic drugs. Psychiatr Pol. 2015;49(6):1241–1253. doi:10.12740/PP/3746226909399
- American Psychiatric Association. The Practice of Electroconvulsive Therapy: Recommendations for Treatment, Training, and Privileging (a Task Force Report of the American Psychiatric Association). American Psychiatric Publication; 2008.
- Stromgren LS, Dahl J, Fjeldborg N, Thomsen A. Factors influencing seizure duration and number of seizures applied in unilateral electroconvulsive therapy. Anaesthetics and benzodiazepines. Acta Psychiatr Scand. 1980;62(2):158–165. doi:10.1111/j.1600-0447.1980.tb00603.x6110313
- Pettinati HM, Stephens SM, Willis KM, Robin SE. Evidence for less improvement in depression in patients taking benzodiazepines during unilateral ECT. Am J Psychiatry. 1990;147(8):1029–1035.2375437
- Petrides G, Divadeenam KM, Bush G, Francis A. Synergism of lorazepam and electroconvulsive therapy in the treatment of catatonia. Biol Psychiatry. 1997;42(5):375–381. doi:10.1016/S0006-3223(96)00378-29276078
- Food and Drug Administration. Neurological devices; reclassification of electroconvulsive therapy devices; effective date of requirement for premarket approval for electroconvulsive therapy devices for certain specified intended uses. Fed Regist. 2018;83(246):66103–66124.30596410
- Tess AV, Smetana GW. Medical evaluation of patients undergoing electroconvulsive therapy. N Engl J Med. 2009;360(14):1437–1444. doi:10.1056/NEJMra070775519339723
- Torring N, Sanghani SN, Petrides G, Kellner CH, Ostergaard SD. The mortality rate of electroconvulsive therapy: a systematic review and pooled analysis. Acta Psychiatr Scand. 2017;135(5):388–397. doi:10.1111/acps.1272128332236
- Ducharme S, Murray ED, Seiner SJ, Tayeb H, Legesse B, Price BH. Retrospective analysis of the short-term safety of ECT in patients with neurological comorbidities: a guide for pre-ECT neurological evaluations. J Neuropsychiatry Clin Neurosci. 2015;27(4):311–321. doi:10.1176/appi.neuropsych.1408019525658682
- Sundsted KK, Burton MC, Shah R, Lapid MI. Preanesthesia medical evaluation for electroconvulsive therapy: a review of the literature. J ECT. 2014;30(1):35–42. doi:10.1097/YCT.0b013e3182a3546f24091900
- Ward HB, Fromson JA, Cooper JJ, De Oliveira G, Almeida M. Recommendations for the use of ECT in pregnancy: literature review and proposed clinical protocol. Arch Womens Ment Health. 2018;21(6):715–722. doi:10.1007/s00737-018-0851-029796968
- Sinha P, Goyal P, Andrade C. A meta-review of the safety of electroconvulsive therapy in pregnancy. J ECT. 2017;33(2):81–88. doi:10.1097/YCT.000000000000036228009621
- Coshal S, Jones K, Coverdale J, Livingston R. An overview of reviews on the safety of electroconvulsive therapy administered during pregnancy. J Psychiatr Pract. 2019;25(1):2–6. doi:10.1097/PRA.000000000000035930633726
- Kellner CH, Husain MM, Knapp RG, et al. Right unilateral ultrabrief pulse ECT in geriatric depression: Phase 1 of the PRIDE study. Am J Psychiatry. 2016;173(11):1101–1109. doi:10.1176/appi.ajp.2016.1508110127418379
- Puffer CC, Wall CA, Huxsahl JE, Frye MA. A 20 year practice review of electroconvulsive therapy for adolescents. J Child Adolesc Psychopharmacol. 2016;26(7):632–636. doi:10.1089/cap.2015.013926784386
- Devanand DP, Dwork AJ, Hutchinson ER, Bolwig TG, Sackeim HA. Does ECT alter brain structure? Am J Psychiatry. 1994;151(7):957–970.8010381
- Elias A, Chathanchirayil SJ, Bhat R, Prudic J. Maintenance electroconvulsive therapy up to 12 years. J Affect Disord. 2014;156:228–231. doi:10.1016/j.jad.2013.11.00524355648
- Devanand DP, Verma AK, Tirumalasetti F, Sackeim HA. Absence of cognitive impairment after more than 100 lifetime ECT treatments. Am J Psychiatry. 1991;148(7):929–932.2053635
- Acharya D, Harper DG, Achtyes ED, et al. Safety and utility of acute electroconvulsive therapy for agitation and aggression in dementia. Int J Geriatr Psychiatry. 2015;30(3):265–273. doi:10.1002/gps.413724838521
- Hermida AP, Tang YL, Glass O, Janjua AU, McDonald WM. Efficacy and safety of ECT for behavioral and psychological symptoms of dementia (BPSD): a retrospective chart review. Am J Geriatr Psychiatry. 2020;28(2):157–163. doi:10.1016/j.jagp.2019.09.00831668364
- Wachtel LE, Dhossche DM, Reti IM, Hughes-Wheatland R. Stability of intellectual functioning during maintenance electroconvulsive therapy. Pediatr Neurol. 2012;47(3):219–221. doi:10.1016/j.pediatrneurol.2012.05.01222883291
- Osler M, Rozing MP, Christensen GT, Andersen PK, Jorgensen MB. Electroconvulsive therapy and risk of dementia in patients with affective disorders: a cohort study. Lancet Psychiatry. 2018;5(4):348–356. doi:10.1016/S2215-0366(18)30056-729523431
- Chu CW, Chien WC, Chung CH, et al. Electroconvulsive therapy and risk of dementia: a nationwide cohort study in Taiwan. Front Psychiatry. 2018;9:397. doi:10.3389/fpsyt.2018.0039730245639
- Sackeim HA, Prudic J, Fuller R, Keilp J, Lavori PW, Olfson M. The cognitive effects of electroconvulsive therapy in community settings. Neuropsychopharmacology. 2007;32(1):244–254. doi:10.1038/sj.npp.130118016936712
- Vann Jones S, McCollum R. Subjective memory complaints after electroconvulsive therapy: systematic review. BJ Psych Bull. 2019;43(2):73–80. doi:10.1192/bjb.2018.45
- Medina M, Cooper JJ. Utility of the clock drawing test in the assessment of catatonia. J Neuropsychiatry Clin Neurosci. 2019;31(1):89–91. doi:10.1176/appi.neuropsych.1809021030404532
- Moussa T, Afzal K, Cooper J, Rosenberger R, Gerstle K, Wagner-Weiner L. Pediatric anti-NMDA receptor encephalitis with catatonia: treatment with electroconvulsive therapy. Pediatr Rheumatol Online J. 2019;17(1):8. doi:10.1186/s12969-019-0310-030777097
- Kalisova L, Kubinova M, Michalec J, Albrecht J, Madlova K, Raboch J. Cognitive functioning in patients treated with electroconvulsive therapy. Neuropsychiatr Dis Treat. 2018;14:3025–3031. doi:10.2147/NDT.S18242330510424
- Vasavada MM, Leaver AM, Njau S, et al. Short- and long-term cognitive outcomes in patients with major depression treated with electroconvulsive therapy. J ECT. 2017;33(4):278–285. doi:10.1097/YCT.000000000000042628617690
- Tsujii T, Uchida T, Suzuki T, Mimura M, Hirano J, Uchida H. Factors associated with delirium following electroconvulsive therapy: a systematic review. J ECT. 2019;35(4):279–287. doi:10.1097/YCT.000000000000060631764452
- Malur C, Francis A. Emergence of catatonia during ECT. J ECT. 2001;17(3):201–204. doi:10.1097/00124509-200109000-0001111528313
- Payne NA, Prudic J. Electroconvulsive therapy: part II: a biopsychosocial perspective. J Psychiatr Pract. 2009;15(5):369–390. doi:10.1097/01.pra.0000361278.73092.8519820554
- Bostwick JM, Chozinski JP. Temporal competency in catatonia. J Am Acad Psychiatry Law. 2002;30(3):371–376.12380416
- Methfessel I, Sartorius A, Zilles D. Electroconvulsive therapy against the patients’ will: a case series. World J Biol Psychiatry. 2018;19(3):236–242. doi:10.1080/15622975.2017.129329628299981
- Livingston R, Wu C, Mu K, Coffey MJ. Regulation of electroconvulsive therapy: a systematic review of US state laws. J ECT. 2018;34(1):60–68. doi:10.1097/YCT.000000000000046028991068
- Cooper JJ, Brakel SJ, Dinwiddie SH. Limiting legal intrusions in ECT practice, a commentary on “regulation of electroconvulsive therapy: a systematic review of US state laws”. J ECT. 2018;34(1):69. doi:10.1097/YCT.000000000000047629329153
- Ottosson J Is ECT an ethical treatment? Psychiatric Times; 2004 Available from https://www.psychiatrictimes.com/electroconvulsive-therapy/ect-ethical-treatment. Accessed 129, 2019..
- Wachtel LE, Griffin MM, Dhossche DM, Reti IM. Brief report: electroconvulsive therapy for malignant catatonia in an autistic adolescent. Autism. 2010;14(4):349–358. doi:10.1177/136236130935013520591959
- Cooper JJ, Afzal KI. Safety of electroconvulsive therapy in 2 very young pediatric patients with catatonia related to anti-n-methyl-d-aspartate receptor encephalitis. J ECT. 2019;35(3):216–217. doi:10.1097/YCT.000000000000057530720552
- Takamiya A, Sawada K, Mimura M, Kishimoto T. Attitudes toward electroconvulsive therapy among involuntary and voluntary patients. J ECT. 2019;35(3):165–169. doi:10.1097/YCT.000000000000057130694874
- Bilginer C, Karadeniz S. Knowledge, attitudes, and experience of child and adolescent psychiatrists in Turkey concerning pediatric electroconvulsive therapy. Asian J Psychiatr. 2019;46:74–78. doi:10.1016/j.ajp.2019.09.03531639553
- De Meulenaere M, De Meulenaere J, Ghaziuddin N, Sienaert P. Experience, knowledge, and attitudes of child and adolescent psychiatrists in Belgium toward pediatric electroconvulsive therapy. J ECT. 2018;34(4):247–252. doi:10.1097/YCT.000000000000048929465501
- van der Wurff FB, Stek ML, Hoogendijk WJ, Beekman AT. Discrepancy between opinion and attitude on the practice of ECT by psychiatrists specializing in old age in the Netherlands. J ECT. 2004;20(1):37–41. doi:10.1097/00124509-200403000-0000815087995
- Vocke S, Bergmann F, Chikere Y, Loh N, Grozinger M. Electroconvulsive therapy as viewed by German psychiatrists: a comparison of 3 subgroups. J ECT. 2015;31(2):110–113. doi:10.1097/YCT.000000000000020825621540
- Schweder LJ, Lydersen S, Wahlund B, Bergsholm P, Linaker OM. Electroconvulsive therapy in Norway: rates of use, clinical characteristics, diagnoses, and attitude. J ECT. 2011;27(4):292–295. doi:10.1097/YCT.0b013e318208e24b21983754
- Anand S, Kumar Paliwal V, Singh LS, Uniyal R. Why do neurologists miss catatonia in neurology emergency? a case series and brief literature review. Clin Neurol Neurosurg. 2019;184:105375. doi:10.1016/j.clineuro.2019.10537531147176
- Cooper JJ, Roig Llesuy J. Catatonia education: needs assessment and brief online intervention. Acad Psychiatry. 2017;41(3):360–363. doi:10.1007/s40596-016-0632-x27837452
- Majnoni d’Intignano L, Granon O, Fovet T, Thomas P, Bonin B, Amad A. Catatonia education in France: a cross-sectional study. Encephale. 2019;45(5):391–396. doi:10.1016/j.encep.2019.03.00431227209
- Azzam PN, Gopalan P, Brown JR, Aquino PR. Physical examination for the academic psychiatrist: primer and common clinical scenarios. Acad Psychiatry. 2016;40(2):321–327. doi:10.1007/s40596-015-0334-925894730
- Medina M, Garza DM, Cooper JJ. Physical examination skills among chief residents in psychiatry: practices, attitudes, and self-perceived knowledge. Acad Psychiatry. 2020;44(1):68–72. doi:10.1007/s40596-019-01124-931659714
- Edelen CG. Access to outpatient electroconvulsive therapy at VA medical centers. J ECT. 2018;34(2):133–134. doi:10.1097/YCT.0000000000000493
- Case BG, Bertollo DN, Laska EM, et al. Declining use of electroconvulsive therapy in United States general hospitals. Biol Psychiatry. 2013;73(2):119–126. doi:10.1016/j.biopsych.2012.09.00523059049
- Patel RS, Sreeram V, Thakur T, Bachu R, Youssef NA. A national study for regional variation of inpatient ECT utilization from 4411 hospitals across the United States. Ann Clin Psychiatry. 2019;31(3):200–208.31369658
- Williams J, Chiu L, Livingston R. Electroconvulsive therapy (ECT) and race: a report of ECT use and sociodemographic trends in Texas. J ECT. 2017;33(2):111–116. doi:10.1097/YCT.000000000000037928009623
- Ona CM, Onoye JM, Goebert D, et al. Sociodemographic characterization of ECT utilization in Hawaii. J ECT. 2014;30(1):43–46. doi:10.1097/YCT.000000000000007524080537
- Euba R. Electroconvulsive therapy and ethnicity. J ECT. 2012;28(1):24–26. doi:10.1097/YCT.0b013e318223834e21983756
- Kalisova L, Madlova K, Albrecht J, Michalec J, Kubinova M, Raboch J. Electroconvulsive therapy in the Czech Republic. J ECT. 2018;34(2):108–112. doi:10.1097/YCT.000000000000046629166317
- Buley N, Copland E, Hodge S, Chaplin R. A further decrease in the rates of administration of electroconvulsive therapy in England. J ECT. 2017;33(3):198–202. doi:10.1097/YCT.000000000000037427930427
- Gazdag G, Dragasek J, Takacs R, et al. Use of electroconvulsive therapy in central-eastern European countries: an overview. Psychiatr Danub. 2017;29(2):136–140. doi:10.24869/psyd.2017.13628636570
- Ma Y, Rosenheck R, Fan N, He H. Rates and patient characteristics of electroconvulsive therapy in China and comparisons with the United States. J ECT. 2019;35(4):251–257. doi:10.1097/YCT.000000000000058931764448
- Benson-Martin JJ, Milligan PD. A survey of the practice of electroconvulsive therapy in South Africa. J ECT. 2015;31(4):253–257. doi:10.1097/YCT.000000000000023925856021
- Sanz-Fuentenebro J, Vera I, Verdura E, et al. Pattern of electroconvulsive therapy use in Spain: proposals for an optimal practice and equitable access. Rev Psiquiatr Salud Ment. 2017;10(2):87–95. doi:10.1016/j.rpsm.2015.12.00326907892
- Takebayashi M. The development of electroconvulsive therapy in Japan. J ECT. 2010;26(1):14–15. doi:10.1097/YCT.0b013e3181c18a7920190595
- Delva NJ, Graf P, Patry S, et al. Access to electroconvulsive therapy services in Canada. J ECT. 2011;27(4):300–309.21983755
- Kaliora SC, Braga RJ, Petrides G, Chatzimanolis J, Papadimitriou GN, Zervas IM. The practice of electroconvulsive therapy in Greece. J ECT. 2013;29(3):219–224. doi:10.1097/YCT.0b013e31827e0d4923296395
- Buccelli C, Di Lorenzo P, Paternoster M, D’Urso G, Graziano V, Niola M. Electroconvulsive therapy in Italy: will public controversies ever stop? J ECT. 2016;32(3):207–211. doi:10.1097/YCT.000000000000030126841302
- James BO, Inogbo CF. Implementing modified electroconvulsive therapy in Nigeria: current status and psychiatrists’ attitudes. J ECT. 2013;29(2):e25–26. doi:10.1097/YCT.0b013e3182801cce23609516
- Ribeiro RB, Melzer-Ribeiro DL, Rigonatti SP, Cordeiro Q. Electroconvulsive therapy in Brazil after the “psychiatric reform”: a public health problem–example from a university service. J ECT. 2012;28(3):170–173. doi:10.1097/YCT.0b013e31824d288922551774
- Chanpattana W, Kramer BA, Kunigiri G, Gangadhar BN, Kitphati R, Andrade C. A survey of the practice of electroconvulsive therapy in Asia. J ECT. 2010;26(1):5–10. doi:10.1097/YCT.0b013e3181a7436819444137
- Nordanskog P, Hulten M, Landen M, Lundberg J, von Knorring L, Nordenskjold A. Electroconvulsive therapy in Sweden 2013: data from the national quality register for ECT. J ECT. 2015;31(4):263–267. doi:10.1097/YCT.000000000000024325973769
- Bjornshauge D, Hjerrild S, Videbech P. Electroconvulsive therapy practice in the Kingdom of Denmark: a nationwide register and questionnaire-based study. J ECT. 2019;35(4):258–263. doi:10.1097/YCT.000000000000058631764449
- Fink M, Fricchione G, Rummans T, Shorter E. Catatonia is a systemic medical syndrome. Acta Psychiatr Scand. 2016;133(3):250–251. doi:10.1111/acps.1251026426740
- Schildkrout B, Benjamin S, Lauterbach MD. Integrating neuroscience knowledge and neuropsychiatric skills into psychiatry: the way forward. Acad Med. 2016;91(5):650–656. doi:10.1097/ACM.000000000000100326630604
- Arbuckle MR, Travis MJ, Ross DA. Integrating a neuroscience perspective into clinical psychiatry today. JAMA Psychiatry. 2017;74(4):313–314. doi:10.1001/jamapsychiatry.2016.384928273288
- Francis A, Fink M. Catatonia. Catatonia Information Center; 2017 Available from https://sites.psu.edu/catatonia/about-us. Accessed 23, 2020.
- Cooper JJ, Lichtor S Electroconvulsive therapy, autism, and catatonia. National Neuroscience Curriculum Initiative; 2017 Available from https://www.nncionline.org/course/electroconvulsive-therapy-autism-and-catatonia/. Accessed 423, 2020.
- The NNCI “Quarantine Curriculum” Committee. NNCI quarantine curriculum. National Neuroscience Curriculum Initiative; 2020 Available from https://www.nncionline.org/nnci-quarantine-curriculum. Accessed 423, 2020..
- Cooper JJ, Korb AS, Akil M. Bringing neuroscience to the bedside. Focus (Am Psychiatr Publ). 2019;17(1):2–7. doi:10.1176/appi.focus.2018003331975952
- Stone J, Collier S. Moving Toward an Evidence-Based Protocol for Electroconvulsive Therapy. Poster presented at: Annual International Society for ECT and Neurostimulation (ISEN) Conference; May 6, 2018; New York City, NY
