Abstract
Sleep apnea is an entity characterized by repetitive upper airway obstruction resulting in nocturnal hypoxia and sleep fragmentation. It is estimated that 2%–4% of the middle-aged population has sleep apnea with a predilection in men relative to women. Risk factors of sleep apnea include obesity, gender, age, menopause, familial factors, craniofacial abnormalities, and alcohol. Sleep apnea has been increasingly recognized as a major health burden associated with hypertension and increased risk of cardiovascular disease and death. Increased airway collapsibility and derangement in ventilatory control responses are the major pathological features of this disorder. Polysomnography (PSG) is the gold-standard method for diagnosis of sleep apnea and assessment of sleep apnea severity; however, portable sleep monitoring has a diagnostic role in the setting of high pretest probability sleep apnea in the absence of significant comorbidity. Positive pressure therapy is the mainstay therapy of sleep apnea. Other treatment modalities, such as upper airway surgery or oral appliances, may be used for the treatment of sleep apnea in select cases. In this review, we focus on describing the sleep apnea definition, risk factor profile, underlying pathophysiologic mechanisms, associated adverse consequences, diagnostic modalities, and treatment strategies.
Introduction
Obstructive sleep apnea (OSA) is a condition characterized by repetitive episodes of complete or partial collapse of the upper airway during sleep resulting in complete cessation (apnea) or reduction (hypopnea) of airflow leading to arousal and hypoxia.Citation1 Apnea is defined as complete cessation of oronasal airflow for at least 10 seconds. Alternatively, the definition of hypopnea requires (1) a drop of ≥ 30% of oronasal airflow from baseline associated with ≥ 4% decrease in oxyhemoglobin saturation, (2) a drop of ≥ 50% of oronasal airflow from baseline and ≥ 3% decrease of oxyhemoglobin saturation, (3) a reduction in airflow as above along with an associated electroencephalographic arousal. In sleep study monitoring, the frequency of apneas and hypopneas per hour of sleep (apnea-hypopnea index [AHI]) is the key measure to define and stratify the severity of OSA, although inherent limitations to this metric include not taking into consideration degree of accompanying hypoxia, length of respiratory events, etc. AHI levels of 5, 15, and 30 have been used as cutpoints to define mild moderate and severe OSA, respectively.Citation1 Apnea can be distinguished as obstructive vs central based upon presence or absence of thoracoabdominal effort.Citation2
Symptoms of OSA may include daytime sleepiness, impaired concentration and mood morning headaches, snoring, and witnessed breathing pauses during sleep observed by the bed partner. There are varying sensitivities and specificities for these specific clinical symptoms, and clinical prediction rules incorporating these symptoms have been shown to be poor predictors in identifying OSA and assessing OSA severityCitation3
Many studies have revealed an association between OSA severity and other common causes of increased mortality such as hypertension,Citation4 stroke,Citation5 coronary artery disease,Citation6 and occupational,Citation7 as well as automobile accidents.Citation8 For this reason, OSA has been increasingly recognized as a major public health issue imposing great economic burden, thereby mandating early recognition and treatment.
Prevalence of OSA
Although OSA was described in the middle of the last century, data describing the prevalence of this disease were not available until 1993 when the results from the Wisconsin Sleep Cohort Study were reported. This study involved 602 participants who were 30–60 years of age and evaluated using overnight polysomnography. The prevalence of OSA (defined as AHI ≥ 5) in this study was 24% in men and 9% in women, and the prevalence of OSA syndrome (OSAS), ie, OSA with associated symptoms (defined as AHI ≥ 5 and daytime sleepiness) was 4% in men and 2% in women.Citation9 The prevalence of OSA was estimated in Southern Pennsylvania households, 1,741 participants between the ages 20 and 100 years were evaluated using overnight PSG. The prevalence of OSA in this cross-sectional study was similar to the Wisconsin Sleep Cohort Study: prevalence of OSA (AHI ≥ 10) was 17% in men and 5% in women and the prevalence of OSAS with concomitant symptoms was 3.3% in men and 1.2% in women.Citation10,Citation11
OSA prevalence studies have been performed in various countries involving individuals of various ethnicities. For example, in an Australian study, the prevalence of OSA was investigated in 485 male participants between the ages 40 and 65 years using a portable, sleep monitoring system. The prevalence of OSA and symptomatic OSAS were 25.9% and 3.1%, respectivelyCitation12 In Europe, 560 patients were evaluated between the ages 30 and 70 years using overnight PSG. Twenty-six percent of men and 28% of women had OSA (AHI ≥ 5), and 3.4% of men and 3% of women had OSAS (AHI ≥ 5 and daytime sleepiness).Citation13 One of the first Asian epidemiological studies estimated the prevalence of OSA in 259 patients in Hong Kong. The prevalence of OSA (AHI = 5) was 8.8% in men and 3.7% in women, and the prevalence of OSAS (AHI = 5 and daytime sleepiness) was 4.1% in men and 2.1% in women.Citation14,Citation15
It is interesting to note the comparable OSA prevalence estimates in this study involving Asians with a lower body mass index (BMI) compared with the participants of the Wisconsin Study, who have a notably higher BMI suggesting a risk factor profile contributing to OSA in Asians involving factors other than overweight/obesity such as genetic or craniofacial anatomical factors. Epidemiological studies from other Asian countries including Korea and India, have shown similar findings.Citation16–Citation18
In summary, the prevalence of OSA (defined as AHI ≥ 5) were 17%–27% in men and 3%–28% in women. This disparity in the prevalence among these studies, particularly the Spanish study in which 26% of men and 28% of women have OSA,Citation13 may be attributed to methodological differences including varying population age, health status of participants, ethnicity, methods of participant enrollment, use of different definitions of hypopnea, use of different techniques in measuring airflow, and using portable home monitoring such as in the Australian studyCitation12
Clinical presentation of OSA
Snoring
Snoring is caused by the vibration of the structures in the oral cavity, and oropharynx is considered one of the most common symptoms for which patients or partners seek medical attention. Habitual snoring is common in the general population; in one report, 40% of women and 60% of men are habitual snorers.Citation9 When considering snoring as a symptom of OSA, approximately 70%–80% of patients who snore have OSACitation19–Citation21 and 95% of patients who have OSA snore.Citation22
Excessive daytime sleepiness
Daytime sleepiness is the most common daytime symptom in patients with OSA. Because there are many causes of sleepiness, such as insufficient sleep, mood disorders, medication side effects, etc; sleepiness is poorly correlated with the severity of OSA,Citation3 and daytime somnolence is not a specific marker for OSA. Nevertheless, daytime sleepiness is a very useful screening tool to evaluate response to therapy in patients with OSA.Citation23 Various scales used to subjectively assess the degree of sleepiness include Profile of Mood States,Citation24 Stanford Sleepiness Scale,Citation25 and the Epworth Sleepiness Scale.Citation26 Of those scales, the Epworth Sleepiness Scale is widely used because it rates the chance of dozing in different everyday situations within the last month rather than reflecting a momentary mood state. Objective ways of measuring sleepiness include multiple sleep latency testing and the maintenance of wakefulness test.
Other symptoms
Other symptoms of OSA include, but are not restricted to, witnessed apneas, nocturnal choking, unrefreshed sleep, morning headaches, sleep maintenance insomnia, and fatigue. Although clinical symptoms have a poor correlation with the severity of OSA, several prediction models have been developed to provide an OSA screening tool. Most of those models depend on clinical symptoms, anthropometric measurements, and an upper airway anatomy evaluation. Despite the high sensitivity, these prediction rules have minimal clinical utility given the low specificity, and also are of limited use in the pediatric population.Citation27–Citation34
Risk factors of OSA
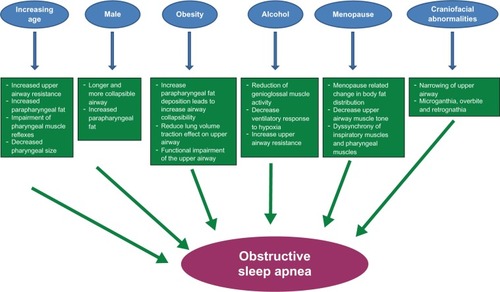
Some of the major risk factors of OSA and their respective pathophysiologic mechanisms are summarized in .
Figure 1 Schematic representation of different risk factors and proposed mechanisms by which they result in obstructive sleep apnea.
Aging
The Sleep Heart Health Study shows a simple, positive linear correlation between age and OSA until the age of approximately 65 years at which point there is a plateau in the prevalence.Citation35 Studies published regarding the prevalence of OSA in the elderly report ranges of 5.6%–70% in referral-based samples and in some population-based studies.Citation36–Citation40 In a review of OSA and the elderly, it has been found that the prevalence of OSA in men is 28%–62% and in women is 19.5%–60%.Citation40 In the elderly, it is thought that OSA may be an entirely different entity altogether and associated with a different constellation of symptoms compared with middle-aged adults. Specifically, the consequences of OSA in the elderly may be more related to behavioral morbidity than cardiovascular outcomes.Citation41 For instance, evidence has shown stronger relationships with cognitive impairments, such as dementia,Citation42 and mood changes, such as depression, and weaker relationships with reporting of snoring in older adults,Citation43 as well as with BMI.Citation44 Also, our analysis of the MrOS Sleep Study, a cohort of elderly men, has identified similar OSA correlates as described in middle-aged adults; however, the strength of the association of risk factors, such as obesity, and symptoms, such as snoring, were comparatively mitigated.Citation45
In an effort to explain the relationship between OSA and age, different hypotheses have been proposed and tested. Function and structure of the upper airway have been a focus of interest in various reports investigating increasing age and upper airway pathophysiology. For example, aging is associated with increased upper airway resistance, increased parapharyngeal fat, decreased pharyngeal size, and impairment of pharyngeal muscle reflexes that are important to maintain upper airway patency, the latter of which is independent of BMI and gender.Citation46–Citation51
Gender
OSA is more common in men, with a male-to-female ratio of 2–4:1 in community-based studiesCitation9,Citation10,Citation13,Citation15,Citation16 and approximately 10:1 in sleep clinic referral samples.Citation52 The disparity between the gender-based community and the clinic prevalence of OSA may be explained by fact that women often do not have the classic symptomatology of OSA. Women may be more likely to report morning headaches, difficulty initiating sleep, and fatigue related to OSA compared with reports of restless sleep and witnessed apneas.Citation53 Furthermore, women with OSA are more likely to be treated for depression, to have insomnia, and to have hypothyroidism than men with the same degree of OSA.Citation54
Another factor that may explain this phenomenon is that women are less likely to be referred to sleep centers because of the notion that OSA is a disease of men. Indeed, OSA was first described in men, and earlier studies exclusively enrolled men. Although it is likely that women with OSA are underdiagnosed, there are other gender-based factors at hand. The sex-based disparity of OSA occurrence is likely attributable to a variety of factors, including hormonal influences, and sex-based phenotypes, including physical features such as craniofacial morphology and fat deposition. The structure and the function of the upper airway show gender-based differences, including a shorter and smaller upper airway observed in women compared with men.Citation55,Citation56 Although this upper airway anatomical difference may appear to predispose women rather than men to upper airway collapse, men have been found to have longer airways compared with women, thereby resulting in increased vulnerability to collapse. Data also indicate that women have a more stable upper airway,Citation57 as supported by pharyngeal critical closing pressures that are higher compared with BMI-matched sleep apneic men.Citation58
Another possible explanation of gender-based differences in OSA risk is the variation in the distribution of adipose tissue between men and women. Men tend to have more fat in the upper body including the neck (android), thereby predisposing to upper airway collapse compared with women who tend to have lower body fat (gynoid).Citation59 Consistent with this observation is that the measurement of neck circumference and waist circumference is better correlated with the severity of OSA than BMI.Citation35 An imaging study, magnetic resonance imaging, confirmed that men also have more predominant pharyngeal fat and soft tissue compared with women.Citation60
In summary, it appears that there are important differential structural and functional upper airway differences between men and women, which place men at higher risk for OSA. However, women with OSA often have an atypical symptomatic presentation of OSA symptoms that warrant vigilant screening and recognition.
Obesity
OSA is highly prevalent in the obese and overweight population. Several cross-sectional studies revealed a monotonic relationship between OSA and weight, BMI, neck circumference, waist-to-hip ratio, and other measures of body habitus.Citation9–Citation11,Citation14,Citation15,Citation35 Furthermore, fluctuations in weight have been demonstrated to influence severity of OSA. In the Wisconsin Sleep Cohort, a 10% weight gain in subjects with no OSA (AHI < 5) was associated with a 6-fold increase in the odds of developing moderate to severe OSA (AHI ≥ 15) after consideration of relevant confounding factors, including age and gender.Citation61 Also, a 10% weight gain was associated with an approximate 30% worsening in the degree of OSA, and alternatively, a 10% weight loss was associated with an approximate 30% improvement in the degree of OSA. Similar results were shown in the Sleep Heart Health Study, in which an increase in weight by 10 kg was observed to increase the odds of having an AHI (> 15 events per hour) by 5.2-fold in men and 2.5-fold in women over a 5-year period.Citation62
These results indicate that women were less vulnerable to the effect of weight gain-related influence on OSA risk, and this could be explained by gender-dependent differences in fat distribution patterns given men tend to have more truncal obesity, and potentially also due to hormonal factors. The impact of weight on OSA appears to be influenced by age as well. In the Cleveland Family Study, the effect of adiposity measured as BMI on OSA was noted to diminish after the age of 60 years.Citation63 Similar findings have been reported in the Sleep Heart Health Study in which OSA in individuals older than 70 years was weakly related to BMI and other measures of body habitus.Citation35
The effect of weight loss on OSA has been studied in morbidly obese patients with moderate to severe OSA, who underwent bariatric surgery. Dramatic weight loss after surgery was associated with a similar reduction in the degree of OSA and nocturnal hypoxia improvement; however, the majority of these patients still had residual OSA requiring treatment, potentially attributable to persisting excess weight as the average postsurgical BMI was 37.7 kg/m2.Citation64
Genetics
Earlier reports, describing a high prevalence of OSA between family members, suggested that OSA has a familial component apart from the influence of obesityCitation65,Citation66 Several small family-based studies have focused on defining the genetic basis of OSA. In a case series, non-overweight relatives of patients with OSA were found to have more daytime sleepiness, snoring, apneas, and arousals compared with matched controls.Citation67
In the Cleveland Family Study, which is the largest, assembled familial aggregation study investigating OSA, sleep apnea was identified to be more prevalent in relatives of index probands of OSA (21%) compared with neighborhood control subjects (12%).Citation66 In another study, relatives of patients with OSA reported more sleepiness, tiredness, and fatigue.Citation68 Data from twin studies showed that the concordance of snoring, a symptom of OSA, is higher among monozygotic twins than dizygotic.Citation69
The heritability of OSA is partially explained by intermediate, genetically-determined risk factors. Obesity, which is a known risk factor of OSA, is partially under genetic control. A twin study showed that 77% of the variability of the BMI is explained by intrafamilial factors.Citation70 Genome-wide association studies have revealed at least 15 genetic loci linked to obesity-related phenotypes.Citation71 However, there is strong evidence that both OSA and obesity have both shared and unshared genetic determinants as demonstrated in the Cleveland Family study. Different loci linked to apnea have been identified with an intermediate logarithmic odds (LOD) score, a measure of the likelihood of 2 genetic loci being within a measurable distance of each other. After adjustment for BMI, the AHI linkage to one of the loci was markedly attenuated. On the other hand, a locus identified on chromosome 19p demonstrated an LOD of 1.45 after BMI adjustment suggesting that this locus could be of potential interest in complex, multifactorial disease.Citation72
Other risk factors which predispose to OSA that are genetically determined include craniofacial morphologic characteristics and ventilatory control mechanisms. Craniofacial familial features have been identified to be a strong indicator of risk for the development of OSA.Citation68 In addition, craniofacial abnormalities are parts of different genetic disorders in which OSA is highly prevalent, such as Down syndrome and Marfan’s syndrome.Citation73 In animal studies, deficiencies in endothelin 1, retonoic acid receptor aZ, and transforming growth factor X2 have been associated with craniofacial abnormalities. These characteristics were also associated with increased respiratory failure suggesting the possible role of the upper airway and sleep apnea given that phenotypic craniofacial factors are a known risk.
Menopause
Different cross-sectional studies have identified menopause as a risk factor for OSA. A prevalence of OSA in postmenopausal women has been described as 2.7% compared with 0.6% in premenopausal women.Citation10 Consistent with these results, another study estimated the odds of having AHI ≥ 15 in menopausal women by 3.49 compared with 1.07 in premenopausal women after controlling for potential confounders, including age and BMI.Citation77 The effects of hormone replacement therapy in postmenopausal women with OSA in the Sleep Heart Health Study revealed an inverse relationship between OSA severity and hormone replacement therapy was such that in multivariable adjusted models, the odds of having OSA in those taking hormone replacement therapy was ~55% lower than those not taking therapyCitation78
Different mechanisms have been postulated to explain the associative relationship between OSA and the hypoestrogenic state in menopausal women. Menopause-related change in body fat distribution, such as predominant central obesity and increased adiposity around the upper airway, increases the risk for upper airway collapse.Citation59,Citation79 Also, the tone of the muscles such as the geniglossus, a primary pharyngeal dilator, is lower during wakefulness in postmenopausal women compared with premenopausal.Citation80 In addition, estrogen and progestin have a role in regulating the ventilatory drive, which may cause imbalance of forces in favor of increased collapsibility of the upper airway. Finally, low estrogen and progestin levels in menopause may lead to dyssynchrony of the inspiratory muscles and pharyngeal muscles that may result in upper airway obstruction.Citation81
Ethnicity
The current cross-sectional data evaluating the prevalence of OSA in different ethnic groups have shown compatible estimates. Earlier population-based studies that included predominantly Caucasians, however, showed no difference in OSA prevalence between Caucasians and African Americans.Citation35 However, African Americans have been observed to have more severe OSA relative to Caucasians in groups younger than 25 years and older than 65 years.Citation36,Citation82 One study demonstrated that approximately 50% of this OSA risk was attributable to neighborhood disadvantage,Citation83 after adjusting for common OSA risk factors including obesity. The authors postulate that this association between OSA and neighborhood-level conditions may be explained by the environmental and social stressors that are often encountered in low socioeconomic neighborhoods.Citation84 Race-based differences in chemoreceptor and baroreceptor responses also may predispose African Americans to development of OSA.Citation85
Despite similar prevalence rate between different ethnic groups, there were some noteworthy findings; Asian population have lower BMI compared with western populations, yet they have the same prevalence rate of OSA. Furthermore, Asians tend to have more severe OSA than Caucasians after adjustment for other risk factors.Citation86,Citation87 In an elderly US cohort of men, the highest prevalence of OSA was among the Asian-American sample, who, after considering the effects of obesity, had a 2-fold higher OSA prevalence compared with Caucasians.Citation45 As this increased risk in Asians was noted in a US-based Asian sample, these findings support an intrinsic ethnic/genetic susceptibility of OSA risk rather than environmental influence. Data describing OSA in Hispanics are scant. An earlier report from the Sleep Heart Health Study showed that frequent snoring is more common in Hispanics compared with their counterparts after adjusting for BMI.Citation63 Recently, a cross-sectional study conducted in South America in 4 Latin communities, 1.9%–6.4% of the population reported daytime sleepiness, snoring, and witnessed apnea. In a subset of the study population, unattended, simplified respiratory polygraphy was performed demonstrating an OSA prevalence of 10%.Citation88 Variation in OSA ethnicity-based risk may be a result of genetic, environmental, and cultural factors. Also, compared with Caucasians, many minority groups have lower socioeconomic statusCitation89 and subsequently higher prevalence of obesity, less health care access, and lower level of health awareness; all of which represent risk factors for OSA. Finally, most epidemiological studies identify ethnicity based on self-report, which is subject to some degree of error, as racial admixture is often not completely taken into account.
Nasal obstruction
Nasal passages represent the gateway of ambient air to the body. Nasal obstruction causes limitation in airflow, an effect that is more pronounced during sleep and that can exacerbate apneas and nocturnal desaturation associated with OSA. Different mechanical factors can cause nasal obstruction including anatomical anomalies, like septal deviation, and inflammatory disease causing mucosal edema, namely rhinitis.
Several reports have examined the relationship between the nose and OSA. Studies with small sample sizes have evaluated the response of intermittent experimental nasal occlusion in healthy subjects. All subjects demonstrated frequent apneas, frequent arousals, and changes in sleep architecture.Citation90–Citation92 Clinical studies investigating the relationship of OSA and nasal obstruction, expressed as nasal resistance measured by rhinometry, have shown inconsistent results. Some studies showed that increased nasal resistance is not a determinant of OSA severityCitation93–Citation95 and treatment of nasal obstruction did not result in substantive changes in severity measures of OSA.Citation96 On the other hand, in a study measuring nasal resistance in more than 500 individuals with symptoms of OSA before performing PSG, daytime nasal obstruction was identified as an independent risk factor for OSA and contributed to 2.3% of the variance.Citation97 In a larger population-based community study, self-reported nasal congestion was identified as an independent risk factor for habitual snoring, including snoring without frank apneas.Citation98
In addition, addressing nasal congestion symptoms is certainly an important component to the approach to enhancing likelihood of positive airway pressure (PAP) adherence. Approaches often involve the use of heated humidification, nasal saline, nasal corticosteroids, and antihistamines. Isolated treatment of increasing nasal passage patency as a treatment for OSA are unlikely to be effective as therapies, such as a nasal strips aimed to increase the caliber of the nasal passages, have failed to demonstrate substantive improvement in OSA degree.Citation99
Craniofacial anatomy
Different craniofacial characteristics have been associated with the development of OSA by causing narrowing of the upper airway and increased upper airway collapsibility including inferiorly positioned hyoid bone, posterior placement of maxilla and mandible, enlarged tongue and soft palate, and smaller velopharyngeal cross-sectional area.Citation100–Citation105 Race-based differences in craniofacial features likely confer varied reasons for increased OSA risk. For example, in the Cleveland Family Study, brachycephaly, decreased middle cranial fossa, and intermaxillary length were associated with increased OSA risk in Caucasians while increased tongue area and soft palate length were more of an OSA risk in African Americans.Citation82 Also, Hispanic patients with OSA have lower maxillary and mandibular positioning.Citation106 Clinical craniofacial morphologic features that are OSA risk factors include micrognathia, retrognathia, or crossbite.
Smoking
Smoking causes difficulty initiating sleep, sleep fragmentation, and daytime sleepiness.Citation107,Citation108 Data are inconsistent as far as identifying smoking as a risk factor for OSA. In the Wisconsin Sleep Cohort Study, a higher risk of snoring and OSA was noted in smokers compared with never-smokers or former smokers with an odds ratio of 2.29 and 4.44, respectively.Citation109 This finding was confirmed by another study reporting that current smokers were 2.5 times more likely to have OSA than former and nonsmokers combined and 2.8 times more likely to have OSA than former smokers alone.Citation110 This effect is noticeable on passive smokers who have a high risk for habitual snoring.Citation111 Several hypotheses have been proposed about the mechanism(s) by which smoking can increase OSA risk. The inflammatory effect of smoking on airway and the changes in lung volumes may predispose to increase upper airway collapsibility, and the effect of nicotine on sleep stability and ventilatory drive may also play a role. Whether smoking is a true risk factor for OSA or a public health problem that is prevalent in a population that is at high risk for OSA remains unclear.
Alcohol
Alcohol causes decreased sleep latency and, in larger quantity, increase the occurence of slow wave sleep.Citation112 Short-term effects of alcohol on OSA were studied in healthy subjects and in subjects with OSA after introducing a certain amount of alcohol or placebo, and the findings of those studies showed that alcohol resulted in increased frequency of apnea, longer apneas, and more frequent hypoxic episodes.Citation113–Citation116 However, 2 other studies failed to replicate those results.Citation117,Citation118 The long-term effects of alcohol on sleep were studied in the Wisconsin Sleep Cohort Study that showed an increase in usual alcohol consumption in men which was associated with increased risk of mild or worse OSA.Citation119 The results of this study were consistent with other cross-sectional studies in different populations.Citation17,Citation43,Citation120
Alcohol likely exacerbates OSA via different mechanisms including selective reduction of genioglossal muscle activity,Citation121 decreased ventilatory responses to hypercapnia and hypoxia,Citation122,Citation123 increased upper airway resistance, and increased tendency of an unstable upper airway to collapse.Citation115,Citation124
Pathophysiology of OSA
Breathing is a function of centrally located breathing centers that control respiratory muscles to allow airflow through the airways to ensure gas exchange in the lungs. Any dysfunction at the level of the respiratory centers (unstable ventilation), upper airway (obstruction), or combination of both can lead to abnormal breathing patterns more prominent during sleep, causing derangements in gas exchange and frequent arousals.
Upper airway patency
The upper airway in humans is a collapsible tube with a predominance of soft tissue and little bony or rigid support. In the normal human, the upper airway is patent during wakefulness and sleep as the net forces tend to keep the upper airway open and requires at least −5 cm H2O to collapse under passive conditions.Citation125 However, this is not the case in obese subjects in which case during sleep, the airway pressure required to collapse the airway, ie, critical closing pressure,Citation126,Citation127 is close to atmospheric pressure and even positive.Citation125 The airway is most at risk for complete collapse at the end of expiration where tissue pressure is higher than intraluminal pressure.Citation125,Citation128 Anatomical factors also increase OSA risk as occurs in obese individuals who have increased parapharyngeal fat.Citation129,Citation130 Certain craniofacial features such as retrognathia may be associated with increased risk of OSA due to a smaller caliber and more crowded upper airway.Citation131
Individual posture may influence upper airway size as the supine position is associated with prolapse of the tongue and palatal structure posteriorly, and that explains the reason why OSA is typically worse in the supine position.Citation132,Citation133 Other forces counteract factors that tend to collapse upper airway including activation of the pharyngeal muscles. More than 20 pharyngeal muscles act in a complex and coordinated matter to maintain upper airway patency. The most extensively studied is the genioglossus muscle that has 3 main neuronal controls: (1) a reflex-mediated activation of the genioglossus through laryngeal mechanoreceptors in response to negative luminal pressure,Citation134–Citation136 (2) respiratory neurons in the medulla, which activate the genioglossus muscle 50–100 ms earlier than the diaphragm to maintain a patent airway just before inspiration,Citation137 and (3) the hypoglossal motor neuron that has a constant excitatory input during a wake state from upper airway serotonergic and adrenergic neurons.Citation138,Citation139
Finally, lung volume affects upper airway patency, ie, as the lungs are connected to the upper airway, it applies caudal traction that stiffens pharyngeal walls and makes it less likely for the upper airway to collapse.Citation140–Citation142 Any decrease in lung volumes such as in obesity and change in posture from upright to supine result in less tension on the pharyngeal walls and a more collapsible airway.Citation143
Ventilatory control
Central respiratory centers in the brain stem tightly regulate oxygen and carbon dioxide levels in the blood via many feedback loops that involve different chemoreceptors and mechanoreceptors, resulting in changes in the pattern and depth of ventilation to maintain blood gases within narrow limits. Such a complex system can become unstable. Instability of the system is best explained by the loop gain principal,Citation144–Citation146 which is an engineering concept. Loop gain is the ratio of a corrective response (ventilation) to the disturbance itself. A high-gain system responds quickly and vigorously to a disturbance, whereas a low-gain system responds slowly and weakly.Citation147 The 2 primary variables influencing loop gain are known as controller gain and plant gain, and both are important in ventilatory stability. The controller gain represents chemoresponsiveness or the hypoxic and hypercapnic ventilatory responses. A high controller gain is, therefore, generally due to rapid hypercapnic responsiveness.Citation148 Plant gain reflects the effectiveness of a given level of ventilation to eliminate CO2. High loop gain destabilizes ventilation in wake and sleep states, although it is less obvious during awake because breathing patterns during wakefulness are highly influenced by behaviors, such as talking and eating. It is believed that high loop gain plays a role in the pathophysiology of OSA in which breathing centers respond quickly and vigorously (high controller) to minor changes in CO2, which results in a drop in CO2 below the apneic threshold, resulting in pauses in breathing leading to CO2 retention and so forth.Citation149–Citation152
Pathogenesis of OSA
Patients with OSA have smaller and more collapsible airways than normal. In the awake state, the airway is patent because of pharyngeal dilator muscle activation. However, in the sleep state, the activity of those muscles is markedly diminishedCitation153 and lung volume decreases in the supine position causing substantial restriction in airflow resulting in hypopnea or apnea.Citation143 This mechanical load and CO2 retention activate the pharyngeal muscles in order to restore upper airway patency.Citation154,Citation155 Recruitment of pharyngeal dilator muscles can be achieved and upper airway patency is restored without a cortical arousal in some individuals, whereas in others, the arousal is required for effective muscle recruitment; this phenomenon has been described as compensatory effectiveness.Citation156
Ventilatory instability plays a role in OSA as supported by work describing that loop gain is higher in patient with severe OSA during non-REM sleep than those with mild OSA.Citation144,Citation157 Indeed, after each apnea, patients with OSA hyperventilate, CO2 drops to the apneic threshold, expiratory time becomes prolonged, and the upper airway may collapse again.
Moreover, several reports have described decreased responsiveness of the pharyngeal muscles to negative upper airway pressure due to damage to the sensory nerves in the upper airway or to the muscle itself. This damage may be due to inflammation caused by vibration, snoring, or trauma.Citation158–Citation161 Data also support the notion of ventilatory variability at sleep–wake transitions as a predictor of OSA severity.Citation162 In addition, humoral and inflammatory mediators released from the visceral adipose tissue may play a role in ventilatory regulation. The most studied is leptin, which binds to its receptors in the hypothalamus, causing reduced satiety and increased ventilation.Citation163 Leptin stimulates respiratory drive, as shown in leptin-deficient or resistant mice that exhibited features of central hypoventilation and obesity.Citation164
Overall, there are a variety of ventilatory control mechanisms that may predispose to an unstable upper airway, including alterations in loop gain, impairments in upper airway motor and neural control, and responsiveness, as well as humoral mechanisms.
OSA and adverse clinical outcomes
OSA, likely via mechanisms of intermittent hypoxia, sympathetic nervous system activation, and alterations in intrathoracic pressures, has been associated with metabolic dysregulation and cardioavascular sequelae, such as diabetes mellitus, hypertension, congestive heart failure, and stroke.
Inflammation, endothelial dysfunction and atherosclerosis
Repetitive episodes of oxygen desaturation and reoxygenation, which is a hallmark feature of OSA, may represent a risk for increased oxidative stress.Citation165 Increase in oxygen free radical productionCitation166 results in lipid peroxidation,Citation167 protein oxidation, DNA damage, and alteration in cellular hemostasis, leading to upregulation of certain proinflammatory genes controlled by nuclear factor κB.Citation168 Indeed, compared to healthy control, patients with OSA have elevated tumor necrosis factor α,Citation169,Citation170 interleukin 6,Citation171,Citation172 interleukin 8,Citation173,Citation174 monocyte chemoattractant protein 1,Citation175 and cellular adhesion molecules including intercellular adhesion molecule 1,Citation175 L-selectin,Citation174 and vascular adhesion molecule 1.Citation174,Citation176 This combination of an enhanced systemic inflammatory state along with increased oxidative stress and elevated sympathetic nervous system activation may result in endothelial dysfunction and increased arterial stiffness,Citation177,Citation178 factors that play a central role in the initiation and acceleration of atherosclerosis.Citation178 Atherosclerosis has been identified by recent research as a consequence of an augmented inflammatory state causing endothelial injury that progresses to atheroma formation.Citation179 Furthermore, treatment of OSA with PAP is associated with a reduction in inflammatory markers levelsCitation171,Citation180 and improvement in endothelial function measured by pulse wave velocity (ascertained by arterial applanation tonometry) and endothelial-dependent vasodilation.Citation177,Citation181
Hypertension
The primary mechanism considered to link OSA to the development of hypertension is sympathetic nervous system overactivity. It has been postulated that intermittent hypoxia and negative intrathoracic pressure lead to chemoreceptor activation and increased sympathetic outflow, and subsequently endothelial dysfunction that predisposes to increased arterial stiffness and this in turn leads to development of hypertension. This theory was examined in animals when intermittent hypoxia caused by repetitive airway obstruction caused transient elevation blood pressure, and this effect lasted after exposure.Citation182,Citation183 Those findings were substantiated by studies conducted on humans. Using plasma and urinary catecholamines as a method of measuring sympathetic activity, it was shown that urinary catecholamine in morbidly obese hypertensive patients with OSA was higher compared with normotensive, obese individuals without OSA.Citation184 Other studies have reported consistent findings such that catecholamine levels were higher in the mornings and at baseline compared with controls. Moreover, decreased level of catecholamines after successful termination of apnea by tracheostomy or PAP has been described.Citation185–Citation189
Cross-sectional data from the Pennsylvania cohort and the Sleep Heart Health Study showed a high prevalence of hypertension in participants with OSA.Citation190,Citation191 A causal association of OSA and hypertension was supported by longitudinal data from the Wisconsin Cohort Study demonstrating a dose-response relationship between the severity of OSA measured by AHI and incident hypertension with a 2.89 increased odds of developing hypertension in subjects with AHI ≥ 15 during a 4-year follow-up period.Citation192 Recent findings from the Sleep Heart Health Study also demonstrated a significant longitudinal relationship between OSA and the development of hypertension among individuals who were normotensive at baseline examination; however, these relationships were attenuated to borderline significance after taking into account obesityCitation193
There are several factors that may account for the difference between the results of the Wisconsin Cohort Study and the Sleep Heart Health Study, including the different definitions used in the assessment of the AHI as a marker of OSA, differences in the ascertainment of blood pressure measurement (ie, measuring blood pressure at home vs clinic), and using different cutpoints in defining hypertension (140/90 vs 135/85). The estimated effect in the Sleep Heart Health Study, however, was similar to the cross-sectional findings, and the reduction in power due to restricting the sample to those who were normotensive at baseline may have accounted for these marginal findings. The strength of the OSA-hypertension association has also been noted to decreases with age, which may be secondary to competing risk factors or survivorship bias.Citation190,Citation194
Several interventional trials have investigated the relationship between hypertension and elimination of apneas by PAP. Several of these studies have demonstrated that PAP has a blood pressure lowering effect,Citation195–Citation200 whereas others did not.Citation201,Citation202 Inconsistency in the results of those trials can be explained by different methodology, enrolling individuals who are normotesive at baseline, treatment for short duration, or the differential interindividual responsiveness to treatment. Of note, OSA has been clearly recognized as a secondary cause of hypertension in the seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure.Citation203
Coronary artery disease
It has been shown that OSA is associated with activation of thrombotic pathways and leads to a state of enhanced inflammation that serves as intermediate mechanisms leading to cardiovascular disease.Citation204 Furthermore, repetitive episodes of hypoxia, intrathoracic pressure swings, and increased afterload create a state of demand-supply mismatch causing cardiac ischemia as demonstrated electrocardiographically by ST depression, accompanying episodes of hypoxia and apnea.Citation205–Citation207 There is a linear relationship between AHI and coronary artery calcification score after taking into account potential confounding cardiovascular factors.Citation208 A positive association between AHI and coronary atherosclerotic plaque volume measured by intravascular ultrasound has also been noted.Citation209 These findings are further supported by several small case-control studies, which showed increased OSA in individuals with documented coronary artery disease.Citation5,Citation210–Citation213 The prevalence of OSA in the setting of acute coronary syndrome has been reported to be quite high. Specifically, of 104 patients admitted with acute coronary syndrome, 66.4% had mild to moderate OSA (AHI ≥ 10) and 26.0% had moderate to severe OSA (AHI ≥ 30), with the predominant apnea pattern being obstructive: 72.1%.Citation214
Furthermore, elimination of apnea and hypoxia with PAP is associated with reduction in all-cause mortality and cardiovascular-specific mortality.Citation211,Citation215–Citation218 It has also been reported that patients with untreated severe OSA had a higher incidence of fatal cardiovascular events (1.06 per 100 person-years) and nonfatal cardiovascular events (2.13 per 100 person-years) than that of patients treated with continuous positive airway pressure (CPAP) (0.35, P = 0.0008 and 0.64, P = 0.0001).Citation215
Stroke
Several case–control studies have demonstrated an increased prevalence of OSA in individuals with cerebrovascular disease.Citation219–Citation223 OSA as a preexisting condition or a risk factor for stroke has been investigated in several longitudinal studies. In a population-based study, 1,475 subjects with OSA followed for an average of 4 years, an increased odds of developing stroke in subjects with AHI (odds = 20) compared with controls (OR = 4.48; P = 0.02) was reported.Citation224 In a cohort derived from a sleep referral center, a significant increased risk of stroke, transient ischemic attack, or death from any cause in OSA compared with controls (Hazard ratio = 1.97; P = 0.01) after adjusting for confounding factors including atrial fibrillation was noted. There is also a trend of increased risk of cerebrovascular disease and severity of OSA.Citation225
These results are consistent with findings of OSA and stroke in other observational studies.Citation5,Citation226,Citation227 Studies assessing the effect of OSA treatment on the incidence of stroke are limited. Less vascular problems were observed, including stroke, in subjects with OSA treated by weight loss or tracheostomy compared with no treatment.Citation228,Citation229 If left untreated, OSA was associated with poor outcomes in stroke patients.Citation230,Citation231 Worse functional impairments and a longer period of hospitalization and rehabilitation have been observed in stroke patients with OSA.Citation230 Moreover, poststroke patients with AHI of 15 or greater have been shown to have a higher risk of death.Citation231 Recent findings from The Sleep Heart Health Study cohort have shown an increased risk of stroke in men with mild to moderate OSA, i.e. 6% increased stroke risk for each unit increase in AHI (95% CI: 0.02–0.10).Citation232
Congestive heart failure
There is likely a bidirectional relationship between congestive heart failure and OSA. Heart failure may contribute to OSA development by accumulation of fluid or edema of the upper airway, thereby enhancing collapsibility. Sleep-related reductions in pulmonary function residual capacity may contribute to periodic breathing by reducing oxygen stores.Citation146,Citation233,Citation234 Obstructive apneas and hypopneas can occur during nadirs of neuromuscular output when the relative innervations of the upper airway muscles are insufficient to maintain airway patency.
Conversely OSA may adversely affect cardiac function through mechanisms, including adverse effects of chronic hypertension, nondipping blood pressure, atherosclerosis and ischemic injury, hypoxemia-related and catecholamine-related myocyte injury, and cardiac remodeling. Of 450 patients with a mean left ventricular ejection fraction of 27%, 38% were observed to have OSA and 72% central sleep apnea,Citation235 which is much higher than the reported 5%–10% prevalence of OSA in the healthy middle-aged population.Citation9 Another study of 81 patients with systolic dysfunction found a high prevalence of central sleep apnea, occurring in 40% of the sample with another 11% with OSA.Citation236 Cross-sectional results from the Sleep Heart Health Study demonstrated that modest levels of OSA (ie, AHI ≥ 11) were associated with a 2.38 higher odds of self-reported heart failure, independent of other risk factors.Citation5 Prospective data from the Sleep Heart Health Study have reported that middle-aged men with an AHI ≥ 30 had an approximately 60% increased 8-year incidence of heart failure compared with men with an AHI <5 (Gottlieb, unpublished data).
Cardiac arrhythmias
There is biologic plausibility that OSA-associated hypoxia, fluctuating intrathoracic pressure, and imbalance in autonomic nervous system function may predispose to cardiac arrhythmogenesis.Citation237–Citation239 In the Sleep Heart Health Study cohort, an increased prevalence of complex ventricular ectopy and atrial fibrillation in patients with an AHI ≥ 30 was observed.Citation240 A causal association between sleep apnea and arrhythmias is supported by the findings from a case-cross over study performed in the Sleep Heart Health Study, which examined the temporal distributions of arrhythmias in association with the occurrence of apneas and hypopneas on PSG, demonstrating that the occurrence of discrete paroxysmal atrial fibrillation or nonsustained ventricular tachycardia was 17-fold higher in 90 seconds immediately following an apnea or hypopnea compared with a time period following nonobstructed breathing.Citation241 In a multicenter cohort of older community-dwelling males, there was a dose-response relationship between the severity of sleep apnea and the prevalence of arrhythmias in elderly men. In this study, complex ventricular ectopy was associated most strongly with OSA and hypoxia, whereas atrial fibrillation was most strongly associated with central sleep apnea.Citation242
Untreated sleep apnea has been identified as a risk factor of recurrent atrial fibrillation after treatment by cardioversion.Citation243 Furthermore, the future development of atrial fibrillation in individuals with sleep apnea was retrospectively investigated; nocturnal hypoxia was identified as a future predictor of arrhythmia development.Citation244
Metabolic consequences
Several plausible biological mechanisms have been investigated linking OSA and insulin resistance/diabetes mellitus. Intermittent hypoxia associated with increased sympathetic activity directly can alter glucose metabolism Citation245,Citation246 or indirectly increase other counter-regulatory hormones like cortisol and, possibly, growth hormone that increases insulin resistance.Citation247,Citation248 Moreover, increased oxidative stress associated with intermittent hypoxia generates oxygen free radicals Citation166,Citation249 and produces a state of inflammatory response that has been shown to increase insulin resistance.Citation250 Both OSA and insulin resistance are in large part a product of the worldwide epidemic of obesity.Citation251 However, it is unclear whether increased insulin resistance in patients with OSA occurs through pathways of oxidative stress or, alternatively, through a state of increased systemic inflammatory response.Citation252 A number of reports have investigated the complex relationship between OSA and insulin resistance. Using various methods to measure insulin resistance, several population-based, cross-sectional studies reported an independent association between insulin resistance, glucose intolerance, and diabetes with markers of OSA severity, namely AHI and hypoxia.Citation253–Citation258 These findings were supported by several smaller clinic-based studies.Citation259–Citation264
However, longitudinal data confirming this relationship is sparse. Using snoring as surrogate for OSA, habitual snoring was identified as an independent risk factor for the incidence of self-reported diabetes.Citation265 Similarly, a 2-fold increased risk of laboratory-confirmed diabetes was noted in relation to regular snoring.Citation266
When assessing OSA via PSG, disparate results have been described. Longitudinally, moderate to severe OSA was a significant independent risk factor for incident diabetes in an Australian cohort (fully adjusted OR = 13.45; 95% confidence interval [CI] = 1.59–114.11) with CIs that were notably wide, suggesting lack of measurement precision. Alternatively, an association between OSA and future development of diabetes after a 4-year follow-up period was not found in the Wisconsin Cohort Study. Specifically, the odds ratio for developing diabetes mellitus within 4 years in those with an AHI of 15 or more compared with those with an AHI < 5 was not statistically significant when adjusting for age, sex, and body habitus.Citation267 The discrepancy in these results may be explained by short follow-up periods, small sample sizes, or inconsistencies in measuring markers of insulin resistance, obesity, and OSA. In one study, improvement of insulin sensitivity in patients with OSA after 2 days of treatment remained stable for 3 months, an effect that was more pronounced in individuals with a BMI less than 30 kg/m2.Citation268 These findings were countered by one randomized study. After 3 months of treatment, no difference was noted in insulin sensitivity between intervention and placebo group.Citation269
A series of other studies with a host of limitations, including small sample sizes, etc, have also not identified an improvement in insulin resistance with OSA treatment.Citation270–Citation274 Results from a randomized, crossover, controlled trial did not provide evidence, that impaired glucose tolerance normalizes after 2 months of therapeutic CPAP treatment.Citation275 However, insulin resistance was measured by the oral glucose tolerance test, and insulin glucose product improved in those with severe sleep apnea, suggesting beneficial metabolic effects of CPAP in those with severe disease.Citation276
Another metabolic derangement associated with OSA is dyslipidemia. Mice models of chronic intermittent hypoxia, a hallmark feature of OSA, showed increase in triglyceride and cholesterol biosynthesis and secretion through upregulation of certain liver enzymes that increase availability of fatty acids and lipoprotein secretion.Citation277,Citation278 Similar results were seen in patients with OSA with increased plasma triglycerides and low-density lipoprotein noted to correlate with nocturnal hypoxia.Citation279 Furthermore, treatment of OSA led to a reduction in total cholesterol in adultsCitation280,Citation281 and pediatricCitation282 populations. In one study, however, abnormalities in the lipid profile were noted to be attributable to obesity more than OSA, and treatment of OSA by PAP had no effect on total cholesterol and triglyceride levels.Citation283
Cognitive function and motor vehicle accidents
Patients with OSA are often unaware of their nocturnal symptoms. However, daytime symptoms have a great impact on cognitive function and quality of life. Cognitive function is commonly classified in clinical and research studies into three broad categories: attention, memory and learning, and executive performance.Citation284 Patients with OSA commonly experience difficulties in working, memory problems, and concentration, and these symptoms have been best correlated with a degree of hypoxia at night.Citation285 On the other hand, treatment of OSA is linearly associated with improvement of cognitive dysfunction, and patients with severe disease tend to benefit the most.Citation286–Citation288
As high vigilance is required for safe driving, it is no surprise that patients with OSA are at higher risk for motor vehicle accidents. Indeed, patients with AHI > 15 were significantly more likely to have multiple accidents in 5 years (OR = 7.3).Citation289 Improvement in cognitive and motor function with treatment of OSA is associated with a reduction in collision rate.Citation8,Citation290,Citation291
Quality of life
Patients with OSA often report poor quality of lifeCitation292,Citation293 and poor general health status.Citation294 Different factors can contribute to this perception: excessive daytime sleepiness,Citation295 insomnia, social dysfunction, impaired work performance,Citation296 and higher prevalence of psychiatric problems such as depression.Citation297
To evaluate the degree of impairment in quality of life, different methods have been used in clinical practice and research, which are divided into generic instruments that allow comparison between populations or studies, eg, the Medical Outcomes Study Short Form-36,Citation298 and alternatively, specific instruments that are designed to detect changes among patients with specific disorders, eg, Calgary sleep apnea quality of life index.Citation299 Both of these methods have been used to quantify the degree of impairment and the improvement of quality of life after treatment of OSA.Citation300 Several clinical trials showed improvement of quality of life with consistent usage of PAP after variable periods of follow-up from 2 weeks to 4 months. Those studies showed that the degree of improvement depends mainly on the degree of impairment at baseline.Citation286,Citation301–Citation306
Diagnosis of OSA
The diagnosis of OSA is based upon clinical presentation and physical findings suggestive of the disease in conjunction with objective data obtained from sleep study monitoring. OSA is a highly prevalent disease and, if left untreated, may result in considerable social, economic, and health sequelae. Bearing this in mind, early recognition by the health care provider is of paramount importance.
Polysomnography
PSG is the gold-standard method to diagnose OSA and provides a method to determine the PAP level required for treatment. During PSG, detailed information is obtained by using electroencephalogram, electromyogram, electro-oculogram, electrocardiogram, snore microphone, body position and leg movement, oronasal airflow, chest wall effort, and oxyhemoglobin saturation, as well as video recording.Citation307 Full sleep study monitoring is performed during usual sleep hours with 6 hours of recording optimally needed to establish the diagnosis. Usually, the patient returns to the laboratory for a follow-up sleep study to establish adequate pressure required to eliminate respiratory events. In order to enhance efficiency, split night studies may be performed, which involves an initial portion of sleep recording required to establish the diagnosis followed by PAP titration. Split night studies have inherent drawbacks due to short recording time for the diagnostic portion, representing a less than ideal diagnostic method for those patients with a low pretest probability (as longer sleep time are often needed to establish the diagnosis), and given that the therapeutic part of the split night study may not provide sufficient time to establish the optimal therapeutic PAP setting.
Many factors can affect the accuracy of PSG in ascertaining the diagnosis of OSA. PSG is a highly specific and sensitive test for patients with a high pretest probability. However, this is not the case for patients with low-test probability of OSA. Considering an AHI cutoff of 15 results in a false negative rate of approximately 20%.Citation308–Citation311 Factors that may result in night-to-night variability are body position and use of drugs that affect the percentage of REM sleep (ie, apnea tend to occur in supine position and in REM sleep).Citation312,Citation313
Home portable monitoring
With an increasing number of patients who are referred for the evaluation for OSA and by following current guidelines for the diagnosis of OSA, it has been estimated that 600 sleep studies per 100,000 individuals per year would be required.Citation314 This has placed great pressure on the limited number of sleep laboratories in the country and may contribute to delay in OSA diagnosis and treatment. For this reason, portable home sleep monitoring emerged as a potential alternative for the diagnosis of OSA.
Per the American Academy of Sleep Medicine guidelines, there are different levels of sleep monitoring with levels II–IV categorized as unattended monitoring involving a minimum of 7 channels (Level II), 4 channels (Level III), or 1 channel (Level IV).Citation315 Although portable sleep monitoring is less costly and more convenient for the patient, there are several disadvantages. Absence of direct supervision by a sleep technician increases the likelihood of data loss or obtaining poor quality or uninterruptable sleep studies with some reports of equipment failure or data loss between 8%–16%.Citation316,Citation317
In the Sleep Heart Health Study, ~60 participants underwent both unattended portable monitoring and in-laboratory monitoring within 2 weeks of one another. The intraclass correlation coefficients of the respiratory variables demonstrated good reliability with values of ~0.7–0.8.Citation316 In another study, portable sleep monitoring and PSG were noted to have the best performance in patients with AHI < 5 or AHI > 30.Citation317 Data from a randomized controlled trial comparing PSG and portable sleep monitoring showed that outcomes of quality of life and sleepiness were similar in the 2 groups.Citation318
On the basis of current literature, the American Academy of Sleep Medicine released clinical guidelines regarding the use of portable sleep monitoring. In laboratory, PSG remains the gold-standard method for OSA diagnosis; however, portable sleep monitoring is a diagnostic option in patients with high pretest probability of moderate to severe OSA without significant comorbidity or concern for concomitant sleep disorders. Portable sleep monitoring should not be used for OSA screening, diagnosing other sleep disorders, or in the absence of a comprehensive sleep evaluation by a board certified or board eligible sleep professional.Citation319
Treatment of OSA
Treatment of OSA is focused on reversal of underlying pathology. PAP has been used to act as pneumatic splint in order to keep pharyngeal pressures above pharyngeal critical pressure and, hence, prevent recurrent airway obstruction. However, certain subgroups of patients with craniofacial abnormalities as a primary OSA risk factor may benefit from upper airway surgical intervention or oral appliance to restore normal upper airway anatomy. Furthermore, patient counseling about sleep posture (ie, discouraging sleep in the supine position), sleep hygiene (maintaining regular sleep–wake times and discouraging the use of sedatives, hypnotics, and alcohol particularly given their ability to reduce upper airway muscle tone and worsen OSA), and weight loss is an integral part of the management of OSA.
Positive airway pressure
PAP was first introduced as a treatment of OSA in 1983.Citation320 Since then, and with advances in technology, different ways in delivering PAP have evolved with a large number of clinical trials investigating the efficacy in improving different clinical outcomes. Continuous PAP, known as CPAP, is commonly used to treat OSA by delivering a constant pressure throughout inspiration and expiration to maintain upper airway patency during sleep. It consists of a flow generator that delivers airflow at a constant pressure to the patient through a mask via a tubing system. CPAP was evaluated in clinical trials and was compared to sham CPAP, pills, or no treatment, using parallel or crossover designs. The results were in favor of CPAP comparatively reducing AHI and improving daytime sleepiness assessed subjectively by Epworth sleeping scaleCitation196,Citation286,Citation305,Citation321–Citation331 and objectively by using multiple sleep latency testing.Citation286,Citation305,Citation322–Citation324,Citation326,Citation328,Citation332 Although the effect of CPAP on other outcomes is less established, several reports noted improvement in sleep quality (increased stages 3 and 4)Citation333 and quality of life.Citation328,Citation332,Citation334,Citation335
The effect of treatment of OSA on cardiovascular disease, metabolic disorders, cognitive function, and road traffic accidents has already been discussed earlier in this review. However, most of these studies enrolled patients with moderate to severe OSA; therefore, whether these outcomes are improved in milder degrees of OSA is not as substantiated. In one study, a beneficial effect of CPAP over conservative therapy in individuals with mild OSA in terms of mood, energy level, and general health, as assessed by the Medical Outcome Survey Short Form-36, was observed, and it was most evident among individuals with hypertension or diabetes.Citation336 In another study, those with an AHI in the range 5–10 noted an improvement in cognitive function, psychological well-being, and quality of life with CPAP use.Citation286
Another mode of PAP is bi-level PAP. Bi-level PAP delivers 2 different set pressures: one during inspiration and a different lower pressure during expiration, called inspiratory PAP (IPAP) and expiratory PAP (EPAP), respectively. It has the same indication as CPAP; however, bi-level PAP allows exhalation at a lower pressure, which theoretically makes it more tolerable than CPAP, especially if pressure needs are high.Citation337 Another advantage of bi-level PAP is the pressure differential increase in ventilation via pressure support, which may be important in patients with concomitant hypoventilation, lung restriction, or increased dead space.
Auto-PAP allows unattended in-home titration of PAP by adjusting the pressure to the minimum required. Compared to CPAP, auto-PAP is similar in ability to eliminate respiratory events and to improve subjective sleepiness.Citation338 Adaptive servoventilation (ASV) is one of the new versions of PAP that may be helpful in the treatment of complex sleep apnea (treatment-emergent central sleep apnea) and periodic breathing.Citation339,Citation340 It essentially delivers a constant EPAP, but the inspiratory pressure is autoadjustable to meet a certain percentage of the recent average ventilation or flow depending upon the machine type. The difference between the IPAP and EPAP, in essence, fluctuates to create a tidal volume that is 90% of the average sensed ventilation. ASV has a backup rate that is activated by central apnea. PAP is a chronic treatment for a long-lasting disease. Several factors play a role in the patient’s compliance that is conventionally defined as regular use of the device for more than 70% of nights for more than 4 hours each night.Citation341,Citation342 Several observational studies have estimated the PAP discontinuation rate between 5% and 50%, and most of these cases occurred during the first 3 months of use.Citation342 Factors that predict long-term adherence to therapy are early adherence to therapy, increased OSA severity, and excessive daytime sleepiness.Citation331,Citation343,Citation344 The majority of patients discontinue treatment due to side effects that commonly relate to mask interface problems, such as air leak, skin breakdown, claustrophobia, and mouth dryness. These side effects can be addressed by changing the mask type accordingly (nasal mask vs nasal pillows vs full face-mask) and by ensuring proper mask fit. Other complications are related to nasal symptoms, such as congestion, dryness, and epistaxis. Symptomatic relief can be achieved by using heated humidification and nasal saline solution.Citation345 Pressure intolerance can also sometimes negatively impact patient compliance with CPAP. In these situations, the ramp feature can be used, which gradually increases the pressure from a lower setting to the goal pressure over a specified period of time to allow acclimatization. Other methods to address pressure intolerance include use of expiratory pressure relief or bi-level PAP.
Oral appliances
As PAP has emerged as an effective treatment of OSA, oral appliances have been identified as an alternative treatment modality. Per the American Academy of Sleep Medicine guidelines, oral appliances are indicated for treatment of mild and/or positional OSA. Oral appliances are broadly divided into devices that protrude the mandible forward known as mandibular advancement devices and tongue-retaining devices. The goal of these devices is to maintain upper airway patency during sleep by changing upper airway geometry and by increasing the pharyngeal occlusion pressure.Citation346,Citation347 Most clinical trials have used mandibular advancement devices as a prototype of oral appliances. Relative contraindications for the use of oral appliances include temporomandibular joint disease. Potential side effects include myofascial pain, excessive salivation, temporomandibular joint pain, tooth pain, dry mouth, and gum irritation.Citation348
Studies comparing PAP to oral appliances showed significant differences in favor of PAP. PAP was more efficacious in improving AHI compared to oral appliances. However, the difference in symptomatic improvement was less clear.Citation324,Citation326,Citation349–Citation351
Surgery
Surgical treatment of OSA aims to increase cross-sectional area of the upper airway by removal of redundant tissue, and hence eliminating the obstructive episodes during sleep. Different procedures have been described including uvulopalatopharyngoplasty (UPPP), which was first introduced in 1981,Citation352 and consists of removal of the tonsils, uvula, and part of the soft palate. Overall, UPPP effectively addresses OSA in only 50% of cases treated with this modality.Citation353 Potential complications of this procedure include velopharyngeal insufficiency and postoperative pain. With advances in technology, various other techniques have been used, such as multilevel, temperature-controlled, radiofrequency tissue ablation, laser-assisted uvulopalatoplasty, and maxillomandibular osteotomy. Compared with no treatment, no difference was noted in daytime sleepiness and quality of life in patients who underwent laser-assisted uvulopalatoplasty.Citation354,Citation355 Similar results were reported using temperature-controlled, radiofrequency tissue ablation or radiofrequency surgery of soft palate.Citation356–Citation359 Nevertheless, certain patients with OSA might benefit from surgery. Using anatomical staging system to stratify patients with OSA was more effective in predicting outcome than did the severity-based staging.Citation360
Conclusion
For the past 3–4 decades, OSA has been increasingly recognized as a highly prevalent disorder and source of adverse health consequences, resulting in major population health burden. In the United States, approximately 12 million people, 30–60 years of age, have OSA,Citation9 and 38,000 die each year from cardiovascular disease attributed to OSA.Citation361 OSA is associated with a host of signs and symptoms adversely affecting quality of life, including snoring, daytime somnolence, neurocognitive deficits, and irritability. Substantial morbidity and economic costs are associated with untreated OSA, including those related to daytime sleepiness and hypertension, and cardiovascular comorbidity. Despite the notion that OSA is a prevalent and treatable condition associated with negative health consequences, at least 75% of severe OSA cases are undiagnosed.Citation362,Citation363 Therefore, there is a crucial need to increase recognition of this disorder and address the public health impact of this common condition, including the extent to which treatment of OSA may modify the course of other chronic health conditions, such as insulin resistance, cardiac arrhythmogenesis, heart failure, and cardiovascular disease.
Acknowledgments
Supported by NIH (National Heart Lung Blood Institute) and K23 HL079114, NIH M01 RR00080, American Heart Association National Scientist Development Award 0530188N, Central Society of Clinical Research Award, and NCI 1U54CA116867. The project described was also supported by UL1 RR024989 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Disclosure
The authors report no conflicts of interest in this work.
References
- Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task ForceSleep1999Aug 122566768910450601
- KushidaCALittnerMRMorgenthalerTPractice parameters for the indications for polysomnography and related procedures: an update for 2005Sleep200528449952116171294
- GottliebDJWhitneyCWBonekatWHRelation of sleepiness to respiratory disturbance index: the Sleep Heart Health StudyAm J Respir Crit Care Med199915925025079927364
- YoungTPeppardPPaltaMPopulation-based study of sleep-disordered breathing as a risk factor for hypertensionArch Intern Med199715715174617529250236
- ShaharEWhitneyCWRedlineSSleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health StudyAm J Respir Crit Care Med20011631192511208620
- MeisingerCHeierMLowelHSchneiderADoringASleep duration and sleep complaints and risk of myocardial infarction in middle-aged men and women from the general population: the MONICA/KORA Augsburg cohort studySleep20073091121112717910384
- LindbergECarterNGislasonTJansonCRole of snoring and daytime sleepiness in occupational accidentsAm J Respir Crit Care Med2001164112031203511739131
- Teran-SantosJJimenez-GomezACordero-GuevaraJThe association between sleep apnea and the risk of traffic accidents. Cooperative Group Burgos-SantanderN Engl J Med19993401184785110080847
- YoungTPaltaMDempseyJSkatrudJWeberSBadrSThe occurrence of sleep-disordered breathing among middle-aged adultsN Engl J Med199332817123012358464434
- BixlerEOVgontzasANLinHMPrevalence of sleep-disordered breathing in women: effects of genderAm J Respir Crit Care Med20011633 Pt 160861311254512
- BixlerEOVgontzasANTen HaveTTysonKKalesAEffects of age on sleep apnea in men: I. Prevalence and severityAm J Respir Crit Care Med199815711441489445292
- BearparkHElliottLGrunsteinRSnoring and sleep apnea. A population study in Australian menAm J Respir Crit Care Med19951515145914657735600
- DuranJEsnaolaSRubioRIztuetaAObstructive sleep apneahypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yrAm J Respir Crit Care Med20011633 Pt 168568911254524
- IpMSLamBTangLCLauderIJIpTYLamWKA community study of sleep-disordered breathing in middle-aged Chinese women in Hong Kong: prevalence and gender differencesChest2004125112713414718431
- IpMSLamBLauderIJA community study of sleep-disordered breathing in middle-aged Chinese men in Hong KongChest20011191626911157585
- KimJInKKimJPrevalence of sleep-disordered breathing in middle-aged Korean men and womenAm J Respir Crit Care Med2004170101108111315347562
- UdwadiaZFDoshiAVLonkarSGSinghCIPrevalence of sleep-disordered breathing and sleep apnea in middle-aged urban Indian menAm J Respir Crit Care Med2004169216817314604837
- SharmaSKKumpawatSBangaAGoelAPrevalence and risk factors of obstructive sleep apnea syndrome in a population of Delhi, IndiaChest2006130114915616840395
- PuvanendranKGohKLFrom snoring to sleep apnea in a Singapore populationSleep Res Online199921111411382877
- TamiTADuncanHJPflegerMIdentification of obstructive sleep apnea in patients who snoreLaryngoscope19981084 Pt 15085139546261
- VaidyaAMPetruzzelliGJWalkerRPMcGeeDGopalsamiCIdentifying obstructive sleep apnea in patients presenting for laser-assisted uvulopalatoplastyLaryngoscope199610644314378614217
- GottliebDJYaoQRedlineSAliTMahowaldMWDoes snoring predict sleepiness independently of apnea and hypopnea frequency?Am J Respir Crit Care Med20001624 Pt 11512151711029370
- DouglasNJSystematic review of the efficacy of nasal CPAPThorax19985354144159708236
- BeutlerLEWareJCKaracanIThornbyJIDifferentiating psychological characteristics of patients with sleep apnea and narcolepsySleep19814139477232970
- KribbsNBPackAIKlineLREffects of one night without nasal CPAP treatment on sleep and sleepiness in patients with obstructive sleep apneaAm Rev Respir Dis19931475116211688484626
- JohnsMWDaytime sleepiness, snoring, and obstructive sleep apnea. The Epworth Sleepiness ScaleChest1993103130368417909
- VinerSSzalaiJPHoffsteinVAre history and physical examination a good screening test for sleep apnea?Ann Intern Med199111553563591863025
- MaislinGPackAIKribbsNBA survey screen for prediction of apneaSleep19951831581667610311
- NetzerNCStoohsRANetzerCMClarkKStrohlKPUsing the Berlin Questionnaire to identify patients at risk for the sleep apnea syndromeAnn Intern Med1999131748549110507956
- KirbySDEngPDanterWNeural network prediction of obstructive sleep apnea from clinical criteriaChest1999116240941510453870
- DixonJBSchachterLMO’BrienPEPredicting sleep apnea and excessive day sleepiness in the severely obese: indicators for polysomnographyChest200312341134114112684304
- KushidaCAEfronBGuilleminaultCA predictive morphometric model for the obstructive sleep apnea syndromeAnn Intern Med19971278 Pt 15815879341055
- TsaiWHRemmersJEBrantRFlemonsWWDaviesJMacarthurCA decision rule for diagnostic testing in obstructive sleep apneaAm J Respir Crit Care Med2003167101427143212738600
- RowleyJAAboussouanLSBadrMSThe use of clinical prediction formulas in the evaluation of obstructive sleep apneaSleep200023792993811083602
- YoungTShaharENietoFJPredictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health StudyArch Intern Med2002162889390011966340
- Ancoli-IsraelSKlauberMRStepnowskyCEstlineEChinnAFellRSleep-disordered breathing in African-American elderlyAm J Respir Crit Care Med19951526 Pt 1194619498520760
- Ancoli-IsraelSKripkeDFKlauberMRMorbidity, mortality and sleep-disordered breathing in community dwelling elderlySleep19961942772828776783
- Ancoli-IsraelSKripkeDFKlauberMRMasonWJFellRKaplanOSleep-disordered breathing in community-dwelling elderlySleep19911464864951798880
- Ancoli-IsraelSKripkeDFKlauberMRNatural history of sleep disordered breathing in community dwelling elderlySleep199316Suppl 8S25S298178017
- Ancoli-IsraelSEpidemiology of sleep disordersPhiladelphia, PAWB. Saunders1989
- YoungTSleep-disordered breathing in older adults: is it a condition distinct from that in middle-aged adults?Sleep19961975295308899930
- MantASaundersNAEylandAEPondCDChancellorAHWebsterIWSleep-related respiratory disturbance and dementia in elderly femalesJ Gerontol1988435M140M1443418035
- EnrightPLNewmanABWahlPWManolioTAHaponikEFBoylePJPrevalence and correlates of snoring and observed apneas in 5,201 older adultsSleep19961975315388899931
- Ancoli-IsraelSGehrmanPKripkeDFLong-term follow-up of sleep disordered breathing in older adultsSleep Med20012651151614592266
- MehraRStoneKLBlackwellTPrevalence and correlates of sleep-disordered breathing in older men: osteoporotic fractures in men sleep studyJ Am Geriatr Soc20075591356136417767677
- EikermannMJordanASChamberlinNLThe influence of aging on pharyngeal collapsibility during sleepChest200713161702170917413053
- MalhotraAHuangYFogelRAging influences on pharyngeal anatomy and physiology: the predisposition to pharyngeal collapseAm J Med2006119172e9e1416431197
- ShochatTLoredoJAncoli-IsraelSSleep Disorders in the ElderlyCurr Treat Options Neurol200131193611123856
- MortimoreILBennettSPDouglasNJTongue protrusion strength and fatiguability: relationship to apnoea/hypopnoea index and ageJ Sleep Res20009438939311386206
- MartinSEMathurRMarshallIDouglasNJThe effect of age, sex, obesity and posture on upper airway sizeEur Respir J1997109208720909311508
- PatilSPSchneiderHMarxJJGladmonESchwartzARSmithPLNeuromechanical control of upper airway patency during sleepJ Appl Physiol2007102254755617008440
- KapsimalisFKrygerMHGender and obstructive sleep apnea syndrome, part 1: clinical featuresSleep15200225441241912071542
- AmbrogettiAOlsonLGSaundersNADifferences in the symptoms of men and women with obstructive sleep apnoeaAust N Z J Med19912168638661818545
- ShepertyckyMRBannoKKrygerMHDifferences between men and women in the clinical presentation of patients diagnosed with obstructive sleep apnea syndromeSleep200528330931416173651
- MohseninVGender differences in the expression of sleep-disordered breathing: role of upper airway dimensionsChest200112051442144711713117
- MalhotraAHuangYFogelRBThe male predisposition to pharyngeal collapse: importance of airway lengthAm J Respir Crit Care Med2002166101388139512421747
- MohseninVEffects of gender on upper airway collapsibility and severity of obstructive sleep apneaSleep Med20034652352914607346
- JordanASMcEvoyRDEdwardsJKThe influence of gender and upper airway resistance on the ventilatory response to arousal in obstructive sleep apnoea in humansJ Physiol2004558Pt 3993100415218069
- MillmanRPCarlisleCCMcGarveySTEveloffSELevinsonPDBody fat distribution and sleep apnea severity in womenChest199510723623667842762
- WhittleATMarshallIMortimoreILWraithPKSellarRJDouglasNJNeck soft tissue and fat distribution: comparison between normal men and women by magnetic resonance imagingThorax199954432332810092693
- PeppardPEYoungTPaltaMDempseyJSkatrudJLongitudinal study of moderate weight change and sleep-disordered breathingJAMA2000284233015302111122588
- NewmanABFosterGGivelberRNietoFJRedlineSYoungTProgression and regression of sleep-disordered breathing with changes in weight: the Sleep Heart Health StudyArch Intern Med2005165202408241316287771
- O’ConnorGTLindBKLeeETVariation in symptoms of sleep-disordered breathing with race and ethnicity: the Sleep Heart Health StudySleep2003261747912627736
- GreenburgDLLettieriCJEliassonAHEffects of surgical weight loss on measures of obstructive sleep apnea: a meta-analysisAm J Med2009122653554219486716
- StrohlKPSaundersNAFeldmanNTHallettMObstructive sleep apnea in family membersN Engl J Med197829918969973211413
- RedlineSTishlerPVTostesonTDThe familial aggregation of obstructive sleep apneaAm J Respir Crit Care Med19951513 Pt 16826877881656
- MathurRDouglasNJFamily studies in patients with the sleep apnea-hypopnea syndromeAnn Intern Med199512231741787810934
- GuilleminaultCPartinenMHollmanKPowellNStoohsRFamilial aggregates in obstructive sleep apnea syndromeChest19951076154515517781344
- Ferini-StrambiLCaloriGOldaniASnoring in twinsRespir Med19958953373407638367
- StunkardAJFochTTHrubecZA twin study of human obesityJAMA1986256151543712713
- LoosRJRecent progress in the genetics of common obesityBr J Clin Pharmacol200968681182920002076
- PalmerLJBuxbaumSGLarkinEKWhole genome scan for obstructive sleep apnea and obesity in African-American familiesAm J Respir Crit Care Med2004169121314132115070816
- HollisterDWGodfreyMSakaiLYPyeritzREImmunohistologic abnormalities of the microfibrillar-fiber system in the Marfan syndromeN Engl J Med199032331521592194127
- KuriharaYKuriharaHSuzukiHElevated blood pressure and craniofacial abnormalities in mice deficient in endothelin-1Nature199436864737037108152482
- LohnesDMarkMMendelsohnCFunction of the retinoic acid receptors (RARs) during development (I). Craniofacial and skeletal abnormalities in RAR double mutantsDevelopment199412010272327487607067
- SanfordLPOrmsbyIGittenberger-de GrootACTGF-beta2 knockout mice have multiple developmental defects that are non-overlapping with other TGFbeta knockout phenotypesDevelopment199712413265926709217007
- YoungTFinnLAustinDPetersonAMenopausal status and sleep-disordered breathing in the Wisconsin Sleep Cohort StudyAm J Respir Crit Care Med200316791181118512615621
- ShaharERedlineSYoungTHormone replacement therapy and sleep-disordered breathingAm J Respir Crit Care Med200316791186119212531779
- DeeganPCMcNicholasWTPredictive value of clinical features for the obstructive sleep apnoea syndromeEur Respir J1996911171248834344
- PopovicRMWhiteDPUpper airway muscle activity in normal women: influence of hormonal statusJ Appl Physiol1998843105510629480969
- HudgelDWSPThe Human Airway During SleepSaundersNASCSleep and BreathingNew YorkMarcel Dekker1994191208
- RedlineSTishlerPVHansMGTostesonTDStrohlKPSpryKRacial differences in sleep-disordered breathing in African-Americans and CaucasiansAm J Respir Crit Care Med199715511861929001310
- SpilsburyJCStorfer-IsserAKirchnerHLNeighborhood disadvantage as a risk factor for pediatric obstructive sleep apneaJ Pediatr2006149334234716939744
- ChenEMatthewsKABoyceWTSocioeconomic differences in children’s health: how and why do these relationships change with age?Psychol Bull2002128229532911931521
- CrisostomoIZayyadACarleyDWChemo- and baroresponses differ in African-Americans and Caucasians in sleepJ Appl Physiol1998854141314209760335
- OngKCClerkAAComparison of the severity of sleep-disordered breathing in Asian and Caucasian patients seen at a sleep disorders centerRespir Med19989268438489850368
- LiKKKushidaCPowellNBRileyRWGuilleminaultCObstructive sleep apnea syndrome: a comparison between Far-East Asian and white menLaryngoscope200011010 Pt 11689169311037826
- BouscouletLTVazquez-GarciaJCMuinoAPrevalence of sleep related symptoms in four Latin American citiesJ Clin Sleep Med1520084657958519110888
- TarasiukAGreenberg-DotanSSimonTTalAOksenbergAReuveniHLow socioeconomic status is a risk factor for cardiovascular disease among adult obstructive sleep apnea syndrome patients requiring treatmentChest2006130376677316963673
- OlsenKDKernEBWestbrookPRSleep and breathing disturbance secondary to nasal obstructionOtolaryngol Head Neck Surg19818958048106799913
- LaviePFischelNZomerJEliascharIThe effects of partial and complete mechanical occlusion of the nasal passages on sleep structure and breathing in sleepActa Otolaryngol1983951–21611666829297
- ZwillichCWPickettCHansonFNWeilJVDisturbed sleep and prolonged apnea during nasal obstruction in normal menAm Rev Respir Dis198112421581607258828
- BlakleyBWMahowaldMWNasal resistance and sleep apneaLaryngoscope19879767527543586822
- AtkinsMTaskarVClaytonNStonePWoodcockANasal resistance in obstructive sleep apneaChest19941054113311358162738
- MiljeteigHHoffsteinVColePThe effect of unilateral and bilateral nasal obstruction on snoring and sleep apneaLaryngoscope199210210115011521405965
- KerrPMillarTBucklePKrygerMThe importance of nasal resistance in obstructive sleep apnea syndromeJ Otolaryngol19922131891951404570
- LofasoFCosteAd’OrthoMPNasal obstruction as a risk factor for sleep apnoea syndromeEur Respir J200016463964311106205
- YoungTFinnLPaltaMChronic nasal congestion at night is a risk factor for snoring in a population-based cohort studyArch Intern Med252001161121514151911427099
- WenzelMSchonhoferBSiemonKKohlerDNasal strips without effect on obstructive sleep apnea and snoringPneumologie19975112110811109487771
- JamiesonAGuilleminaultCPartinenMQuera-SalvaMAObstructive sleep apneic patients have craniomandibular abnormalitiesSleep1986944694773809860
- LoweAASantamariaJDFleethamJAPriceCFacial morphology and obstructive sleep apneaAm J Orthod Dentofacial Orthop19869064844913098087
- LoweAAOnoTFergusonKAPaeEKRyanCFFleethamJACephalometric comparisons of craniofacial and upper airway structure by skeletal subtype and gender in patients with obstructive sleep apneaAm J Orthod Dentofacial Orthop199611066536648972813
- MaltaisFCarrierGCormierYSeriesFCephalometric measurements in snorers, non-snorers, and patients with sleep apnoeaThorax19914664194231858079
- ZucconiMFerini-StrambiLPalazziSOrenaCZontaSSmirneSHabitual snoring with and without obstructive sleep apnoea: the importance of cephalometric variablesThorax19924731571611519191
- SchwabRJUpper airway imagingClin Chest Med199819133549554216
- WillMJEsterMSRamirezSGTinerBDMcAnearJTEpsteinLComparison of cephalometric analysis with ethnicity in obstructive sleep apnea syndromeSleep199518108738758746394
- WetterDWYoungTBThe relation between cigarette smoking and sleep disturbancePrev Med19942333283348078854
- PhillipsBADannerFJCigarette smoking and sleep disturbanceArch Intern Med199515577347377695462
- WetterDWYoungTBBidwellTRBadrMSPaltaMSmoking as a risk factor for sleep-disordered breathingArch Intern Med199415419221922247944843
- KashyapRHockLMBowmanTJHigher prevalence of smoking in patients diagnosed as having obstructive sleep apneaSleep Breath20015416717211868156
- FranklinKAGislasonTOmenaasEThe influence of active and passive smoking on habitual snoringAm J Respir Crit Care Med2004170779980315242843
- YulesRBLippmanMEFreedmanDXAlcohol administration prior to sleep. The effect on EEG sleep stagesArch Gen Psychiatry196716194976015940
- ScanlanMFRoebuckTLittlePJRedmanJRNaughtonMTEffect of moderate alcohol upon obstructive sleep apnoeaEur Respir J200016590991311153591
- TaasanVCBlockAJBoysenPGWynneJWAlcohol increases sleep apnea and oxygen desaturation in asymptomatic menAm J Med19817122402457258218
- DawsonABigbyBGPocetaJSMitlerMMEffect of bedtime alcohol on inspiratory resistance and respiratory drive in snoring and nonsnoring menAlcohol Clin Exp Res19972121831909113250
- TsutsumiWMiyazakiSItasakaYTogawaKInfluence of alcohol on respiratory disturbance during sleepPsychiatry Clin Neurosci200054333233311186100
- BerryRBDesaMMLightRWEffect of ethanol on the efficacy of nasal continuous positive airway pressure as a treatment for obstructive sleep apneaChest19919923393431989792
- ScrimaLHartmanPGHillerFCEffect of three alcohol doses on breathing during sleep in 30–49 year old nonobese snorers and nonsnorersAlcohol Clin Exp Res19891334204272665559
- PeppardPEAustinDBrownRLAssociation of alcohol consumption and sleep disordered breathing in men and womenJ Clin Sleep Med20073326527017561593
- TanigawaTTachibanaNYamagishiKUsual alcohol consumption and arterial oxygen desaturation during sleepJAMA2004292892392515328323
- KrolRCKnuthSLBartlettDJrSelective reduction of genioglossal muscle activity by alcohol in normal human subjectsAm Rev Respir Dis198412922472506421210
- JohnstoneREReierCEAcute respiratory effects of ethanol in manClin Pharmacol Ther19731445015084723257
- SahnSALakshminarayanSPiersonDJWeilJVEffect of ethanol on the ventilatory responses to oxygen and carbon dioxide in manClin Sci Mol Med197549133381149393
- MitlerMMDawsonAHenriksenSJSobersMBloomFEBedtime ethanol increases resistance of upper airways and produces sleep apneas in asymptomatic snorersAlcohol Clin Exp Res19881268018053064641
- IsonoSRemmersJETanakaAShoYSatoJNishinoTAnatomy of pharynx in patients with obstructive sleep apnea and in normal subjectsJ Appl Physiol1997824131913269104871
- SchwartzARGoldARSchubertNEffect of weight loss on upper airway collapsibility in obstructive sleep apneaAm Rev Respir Dis19911443 Pt 14944981892285
- SchwartzARSmithPLWiseRAGoldARPermuttSInduction of upper airway occlusion in sleeping individuals with subatmospheric nasal pressureJ Appl Physiol19886425355423372411
- SchwabRJPro: sleep apnea is an anatomic disorderAm J Respir Crit Care Med20031683270271 discussion27312888606
- KatzIStradlingJSlutskyASZamelNHoffsteinVDo patients with obstructive sleep apnea have thick necks?Am Rev Respir Dis19901415 Pt 1122812312339843
- DaviesRJStradlingJRThe relationship between neck circumference, radiographic pharyngeal anatomy, and the obstructive sleep apnoea syndromeEur Respir J1990355095142376247
- LybergTKrogstadODjupeslandGCephalometric analysis in patients with obstructive sleep apnoea syndrome. I. Skeletal morphologyJ Laryngol Otol198910332872922703770
- GleadhillICSchwartzARSchubertNWiseRAPermuttSSmithPLUpper airway collapsibility in snorers and in patients with obstructive hypopnea and apneaAm Rev Respir Dis19911436130013032048817
- GoldARMarcusCLDipaloFGoldMSUpper airway collapsibility during sleep in upper airway resistance syndromeChest200212151531154012006440
- MathewOPAbu-OsbaYKThachBTGenioglossus muscle responses to upper airway pressure changes: afferent pathwaysJ Appl Physiol19825224454507061298
- MathewOPAbu-OsbaYKThachBTInfluence of upper airway pressure changes on genioglossus muscle respiratory activityJ Appl Physiol19825224384447037716
- HornerRLInnesJAHoldenHBGuzAAfferent pathway(s) for pharyngeal dilator reflex to negative pressure in man: a study using upper airway anaesthesiaJ Physiol199143631442061834
- van LunterenEMuscles of the pharynx: structural and contractile propertiesEar Nose Throat J19937212729338444123
- FogelRBTrinderJMalhotraAWithin-breath control of genioglossal muscle activation in humans: effect of sleep-wake stateJ Physiol2003550Pt 389991012807995
- JelevASoodSLiuHNolanPHornerRLMicrodialysis perfusion of 5-HT into hypoglossal motor nucleus differentially modulates genioglossus activity across natural sleep-wake states in ratsJ Physiol2001532Pt 246748111306665
- Van de GraaffWBThoracic influence on upper airway patencyJ Appl Physiol1988655212421313209556
- Van de GraaffWBThoracic traction on the trachea: mechanisms and magnitudeJ Appl Physiol1991703132813362033000
- StanchinaMLMalhotraAFogelRBThe influence of lung volume on pharyngeal mechanics, collapsibility, and genioglossus muscle activation during sleepSleep200326785185614655919
- BallardRDIrvinCGMartinRJPakJPandeyRWhiteDPInfluence of sleep on lung volume in asthmatic patients and normal subjectsJ Appl Physiol1990685203420412361905
- YounesMOstrowskiMThompsonWLeslieCShewchukWChemical control stability in patients with obstructive sleep apneaAm J Respir Crit Care Med200116351181119011316657
- HudgelDWGordonEAThanakitcharuSBruceENInstability of ventilatory control in patients with obstructive sleep apneaAm J Respir Crit Care Med19981584114211499769273
- CherniackNSRespiratory dysrhythmias during sleepN Engl J Med198130563253307017417
- WhiteDPPathogenesis of obstructive and central sleep apneaAm J Respir Crit Care Med2005172111363137016100008
- AsyaliMHBerryRBKhooMCAssessment of closed-loop ventilatory stability in obstructive sleep apneaIEEE Trans Biomed Eng200249320621611878312
- LongobardoGSGotheBGoldmanMDCherniackNSSleep apnea considered as a control system instabilityRespir Physiol19825033113336819618
- OnalEBurrowsDLHartRHLopataMInduction of periodic breathing during sleep causes upper airway obstruction in humansJ Appl Physiol1986614143814433781958
- WellmanAJordanASMalhotraAVentilatory control and airway anatomy in obstructive sleep apneaAm J Respir Crit Care Med2004170111225123215317668
- JordanASWellmanAEdwardsJKRespiratory control stability and upper airway collapsibility in men and women with obstructive sleep apneaJ Appl Physiol20059952020202715994243
- WheatleyJRWhiteDPThe influence of sleep on pharyngeal reflexesSleep199316Suppl 8S87S898178040
- StanchinaMLMalhotraAFogelRBGenioglossus muscle responsiveness to chemical and mechanical stimuli during non-rapid eye movement sleepAm J Respir Crit Care Med2002165794594911934719
- MalhotraATrinderJFogelRPostural effects on pharyngeal protective reflex mechanismsSleep20042761105111215532204
- YounesMContributions of upper airway mechanics and control mechanisms to severity of obstructive apneaAm J Respir Crit Care Med2003168664565812773321
- RyanCMBradleyTDPathogenesis of obstructive sleep apneaJ Appl Physiol20059962440245016288102
- KimoffRJSforzaEChampagneVOfaraLGendronDUpper airway sensation in snoring and obstructive sleep apneaAm J Respir Crit Care Med2001164225025511463596
- SvanborgEUpper airway nerve lesions in obstructive sleep apneaAm J Respir Crit Care Med2001164218718911463584
- FribergDGazeliusBHokfeltTNordlanderBAbnormal afferent nerve endings in the soft palatal mucosa of sleep apnoics and habitual snorersRegul Pept199771129369299639
- BoydJHPetrofBJHamidQFraserRKimoffRJUpper airway muscle inflammation and denervation changes in obstructive sleep apneaAm J Respir Crit Care Med2004170554154615151922
- IbrahimLHPatelSRModarresMA measure of ventilatory variability at wake-sleep transition predicts sleep apnea severityChest20081341737818347208
- FriedmanJMHalaasJLLeptin and the regulation of body weight in mammalsNature199839567047637709796811
- O’DonnellCPSchaubCDHainesASLeptin prevents respiratory depression in obesityAm J Respir Crit Care Med19991595 Pt 11477148410228114
- ChristouKMarkoulisNMoulasANPastakaCGourgoulianisKIReactive oxygen metabolites (ROMs) as an index of oxidative stress in obstructive sleep apnea patientsSleep Breath20037310511014569521
- SchulzRMahmoudiSHattarKEnhanced release of superoxide from polymorphonuclear neutrophils in obstructive sleep apnea. Impact of continuous positive airway pressure therapyAm J Respir Crit Care Med20001622 Pt 156657010934088
- LavieLVishnevskyALaviePEvidence for lipid peroxidation in obstructive sleep apneaSleep200427112312814998248
- RyanSTaylorCTMcNicholasWTSelective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndromeCirculation2005112172660266716246965
- MinoguchiKTazakiTYokoeTElevated production of tumor necrosis factor-alpha by monocytes in patients with obstructive sleep apnea syndromeChest200412651473147915539715
- RihaRLBranderPVennelleMTumour necrosis factor-alpha (-308) gene polymorphism in obstructive sleep apnoea-hypopnoea syndromeEur Respir J200526467367816204600
- YokoeTMinoguchiKMatsuoHElevated levels of C-reactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressureCirculation200310781129113412615790
- CiftciTUKokturkOBukanNBilgihanAThe relationship between serum cytokine levels with obesity and obstructive sleep apnea syndromeCytokine2004282879115381186
- AlzoghaibiMABahammamASLipid peroxides, superoxide dismutase and circulating IL-8 and GCP-2 in patients with severe obstructive sleep apnea: a pilot studySleep Breath20059311912615988615
- OhgaENagaseTTomitaTIncreased levels of circulating ICAM-1, VCAM-1, and L-selectin in obstructive sleep apnea syndromeJ Appl Physiol1999871101410409552
- OhgaETomitaTWadaHYamamotoHNagaseTOuchiYEffects of obstructive sleep apnea on circulating ICAM-1, IL-8, and MCP-1J Appl Physiol200394117918412391099
- UrsavasAKaradagMRodopluEYilmaztepeAOralHBGozuROCirculating ICAM-1 and VCAM-1 levels in patients with obstructive sleep apnea syndromeRespiration200774552553217148932
- KohlerMCraigSNicollDLeesonPDaviesRJStradlingJREndothelial function and arterial stiffness in minimally symptomatic obstructive sleep apneaAm J Respir Crit Care Med2008178998498818658111
- DragerLFBortolottoLALorenziMCFigueiredoACKriegerEMLorenzi-FilhoGEarly signs of atherosclerosis in obstructive sleep apneaAm J Respir Crit Care Med2005172561361815901608
- LibbyPInflammation in atherosclerosisNature2002420691786887412490960
- KobayashiKNishimuraYShimadaTEffect of continuous positive airway pressure on soluble CD40 ligand in patients with obstructive sleep apnea syndromeChest2006129363263716537861
- IpMSTseHFLamBTsangKWLamWKEndothelial function in obstructive sleep apnea and response to treatmentAm J Respir Crit Care Med2004169334835314551167
- FletcherECLesskeJQianWMillerCCIIIUngerTRepetitive, episodic hypoxia causes diurnal elevation of blood pressure in ratsHypertension1992196 Pt 15555611592450
- BrooksDHornerRLKozarLFRender-TeixeiraCLPhillipsonEAObstructive sleep apnea as a cause of systemic hypertension. Evidence from a canine modelJ Clin Invest19979911061099011563
- FletcherECMillerJSchaafJWFletcherJGUrinary catecholamines before and after tracheostomy in patients with obstructive sleep apnea and hypertensionSleep198710135443563246
- JennumPWildschiodtzGChristensenNJSchwartzTBlood pressure, catecholamines, and pancreatic polypeptide in obstructive sleep apnea with and without nasal Continuous Positive Airway Pressure (nCPAP) treatmentAm J Hypertens1989211 Pt 18478522686712
- LapinskiMPrzybylowskiTLewandowskiJDiurnal blood pressure rhythm and urinary catecholamine excretion in obstructive sleep apnoea and essential hypertensionJ Hypertens Suppl1993115S292S2938158391
- MarroneORiccobonoLSalvaggioAMirabellaABonannoABonsignoreMRCatecholamines and blood pressure in obstructive sleep apnea syndromeChest199310337227278449058
- DimsdaleJECoyTZieglerMGAncoli-IsraelSClausenJThe effect of sleep apnea on plasma and urinary catecholaminesSleep199561853773817676172
- ZieglerMGMillsPJLoredoJSAncoli-IsraelSDimsdaleJEEffect of continuous positive airway pressure and placebo treatment on sympathetic nervous activity in patients with obstructive sleep apneaChest2001120388789311555525
- BixlerEOVgontzasANLinHMAssociation of hypertension and sleep-disordered breathingArch Intern Med2000160152289229510927725
- NietoFJYoungTBLindBKAssociation of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health StudyJAMA2000283141829183610770144
- PeppardPEYoungTPaltaMSkatrudJProspective study of the association between sleep-disordered breathing and hypertensionN Engl J Med2000342191378138410805822
- O’ConnorGTCaffoBNewmanABProspective study of sleep-disordered breathing and hypertension: the Sleep Heart Health StudyAm J Respir Crit Care Med2009179121159116419264976
- HaasDCFosterGLNietoFJAge-dependent associations between sleep-disordered breathing and hypertension: importance of discriminating between systolic/diastolic hypertension and isolated systolic hypertension in the Sleep Heart Health StudyCirculation2005111561462115699282
- BeckerHFJerrentrupAPlochTEffect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apneaCirculation20031071687312515745
- FaccendaJFMackayTWBoonNADouglasNJRandomized placebo-controlled trial of continuous positive airway pressure on blood pressure in the sleep apnea-hypopnea syndromeAm J Respir Crit Care Med2001163234434811179104
- HeitmannJEhlenzKPenzelTSympathetic activity is reduced by nCPAP in hypertensive obstructive sleep apnoea patientsEur Respir J200423225526214979500
- Martinez-GarciaMAGomez-AldaraviRSoler-CatalunaJJMartinezTGBernacer-AlperaBRoman-SanchezPPositive effect of CPAP treatment on the control of difficult-to-treat hypertensionEur Respir J200729595195717301092
- NormanDLoredoJSNelesenRAEffects of continuous positive airway pressure versus supplemental oxygen on 24-hour ambulatory blood pressureHypertension200647584084516585412
- PepperellJCRamdassingh-DowSCrosthwaiteNAmbulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised parallel trialLancet2002359930220421011812555
- RobinsonGVSmithDMLangfordBADaviesRJStradlingJRContinuous positive airway pressure does not reduce blood pressure in nonsleepy hypertensive OSA patientsEur Respir J20062761229123516455835
- Campos-RodriguezFGrilo-ReinaAPerez-RonchelJEffect of continuous positive airway pressure on ambulatory BP in patients with sleep apnea and hypertension: a placebo-controlled trialChest200612961459146716778262
- ChobanianAVBakrisGLBlackHRThe Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 reportJAMA2003289192560257212748199
- ShamsuzzamanASGershBJSomersVKObstructive sleep apnea: implications for cardiac and vascular diseaseJAMA2003290141906191414532320
- HanlyPSassonZZuberiNLunnKST-segment depression during sleep in obstructive sleep apneaAm J Cardiol19937115134113458498378
- FranklinKANilssonJBSahlinCNaslundUSleep apnoea and nocturnal anginaLancet19953458957108510877715342
- Alonso-FernandezAGarcia-RioFRacioneroMACardiac rhythm disturbances and ST-segment depression episodes in patients with obstructive sleep apnea-hypopnea syndrome and its mechanismsChest20051271152215653957
- SorajjaDGamiASSomersVKBehrenbeckTRGarcia-TouchardALopez-JimenezFIndependent association between obstructive sleep apnea and subclinical coronary artery diseaseChest2008133492793318263678
- TurmelJSeriesFBouletLPRelationship between atherosclerosis and the sleep apnea syndrome: an intravascular ultrasound studyInt J Cardiol2009132220320918221805
- MooeTRabbenTWiklundUFranklinKAErikssonPSleep-disordered breathing in men with coronary artery diseaseChest199610936596638617073
- MooeTFranklinKAHolmstromKRabbenTWiklundUSleep-disordered breathing and coronary artery disease: long-term prognosisAm J Respir Crit Care Med200116410 Pt 11910191311734445
- PekerYCarlsonJHednerJIncreased incidence of coronary artery disease in sleep apnoea: a long-term follow-upEur Respir J200628359660216641120
- YuminoDTsurumiYTakagiASuzukiKKasanukiHImpact of obstructive sleep apnea on clinical and angiographic outcomes following percutaneous coronary intervention in patients with acute coronary syndromeAm J Cardiol2007991263017196456
- MehraRPrincipe-RodriguezKKirchnerHLStrohlKPSleep apnea in acute coronary syndrome: high prevalence but low impact on 6-month outcomeSleep Med20067652152816931151
- MarinJMCarrizoSJVicenteEAgustiAGLong-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational studyLancet200536594641046105315781100
- YoungTFinnLPeppardPESleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohortSleep20083181071107818714778
- MarshallNSWongKKLiuPYCullenSRKnuimanMWGrunsteinRRSleep apnea as an independent risk factor for all-cause mortality: the Busselton Health StudySleep20083181079108518714779
- PunjabiNMCaffoBSGoodwinJLSleep-disordered breathing and mortality: a prospective cohort studyPLoS Med200968e100013219688045
- BassettiCAldrichMSSleep apnea in acute cerebrovascular diseases: final report on 128 patientsSleep199922221722310201066
- DykenMESomersVKYamadaTRenZYZimmermanMBInvestigating the relationship between stroke and obstructive sleep apneaStroke19962734014078610303
- MohseninVValorRSleep apnea in patients with hemispheric strokeArch Phys Med Rehabil199576171767811179
- ParraOArboixABechichSTime course of sleep-related breathing disorders in first-ever stroke or transient ischemic attackAm J Respir Crit Care Med20001612 Pt 137538010673174
- WessendorfTETeschlerHWangYMKonietzkoNThilmannAFSleep-disordered breathing among patients with first-ever strokeJ Neurol20002471414710701896
- ArztMYoungTFinnLSkatrudJBBradleyTDAssociation of sleep-disordered breathing and the occurrence of strokeAm J Respir Crit Care Med2005172111447145116141444
- YaggiHKConcatoJKernanWNLichtmanJHBrassLMMohseninVObstructive sleep apnea as a risk factor for stroke and deathN Engl J Med2005353192034204116282178
- ValhamFMooeTRabbenTStenlundHWiklundUFranklinKAIncreased risk of stroke in patients with coronary artery disease and sleep apnea: a 10-year follow-upCirculation2008118995596018697817
- MunozRDuran-CantollaJMartinez-VilaESevere sleep apnea and risk of ischemic stroke in the elderlyStroke20063792317232116888274
- PartinenMJamiesonAGuilleminaultCLong-term outcome for obstructive sleep apnea syndrome patients. MortalityChest1988946120012043191760
- PartinenMGuilleminaultCDaytime sleepiness and vascular morbidity at seven-year follow-up in obstructive sleep apnea patientsChest199097127322295260
- KanekoYHajekVEZivanovicVRaboudJBradleyTDRelationship of sleep apnea to functional capacity and length of hospitalization following strokeSleep200326329329712749548
- SahlinCSandbergOGustafsonYObstructive sleep apnea is a risk factor for death in patients with stroke: a 10-year follow-upArch Intern Med2008168329730118268171
- RedlineSYenokyanGGottliebDJObstructive sleep apnea hypopnea and incident stroke: The Sleep Heart Health StudyAm J Respir Crit Care Med2010182226927720339144
- CherniackNSLongobardoGSCheyne-Stokes breathing. An instability in physiologic controlN Engl J Med1973288189529574571351
- KhooMCKronauerREStrohlKPSlutskyASFactors inducing periodic breathing in humans: a general modelJ Appl Physiol19825336446597129986
- SinDDFitzgeraldFParkerJDNewtonGFlorasJSBradleyTDRisk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failureAm J Respir Crit Care Med199916041101110610508793
- JavaheriSParkerTJLimingJDSleep apnea in 81 ambulatory male patients with stable heart failure. Types and their prevalences, consequences, and presentationsCirculation19989721215421599626176
- NarkiewiczKvan de BornePJPesekCADykenMEMontanoNSomersVKSelective potentiation of peripheral chemoreflex sensitivity in obstructive sleep apneaCirculation19999991183118910069786
- DimsdaleJELoredoJSProfantJEffect of continuous positive airway pressure on blood pressure: a placebo trialHypertension2000351 Pt 114414710642289
- NarkiewiczKvan de BornePJCooleyRLDykenMESomersVKSympathetic activity in obese subjects with and without obstructive sleep apneaCirculation19989887727769727547
- MehraRBenjaminEJShaharEAssociation of nocturnal arrhythmias with sleep-disordered breathing: the Sleep Heart Health StudyAm J Respir Crit Care Med2006173891091616424443
- MonahanKStorfer-IsserAMehraRTriggering of nocturnal arrhythmias by sleep-disordered breathing eventsJ Am Coll Cardiol200954191797180419874994
- MehraRStoneKLVarosyPDNocturnal arrhythmias across a spectrum of obstructive and central sleep-disordered breathing in older men: outcomes of sleep disorders in older men (MrOS sleep) studyArch Intern Med2009169121147115519546416
- KanagalaRMuraliNSFriedmanPAObstructive sleep apnea and the recurrence of atrial fibrillationCirculation2003107202589259412743002
- GamiASHodgeDOHergesRMObstructive sleep apnea, obesity, and the risk of incident atrial fibrillationJ Am Coll Cardiol200749556557117276180
- HoffmanRPSinkeyCADoppJMPhillipsBGSystemic and local adrenergic regulation of muscle glucose utilization during hypoglycemia in healthy subjectsDiabetes200251373474211872674
- PorteDJrWilliamsRHInhibition of insulin release by norepinephrine in manScience1966152726124812505327883
- BratelTWennlundACarlstromKPituitary reactivity, androgens and catecholamines in obstructive sleep apnoea. Effects of continuous positive airway pressure treatment (CPAP)Respir Med19999311710464840
- GrunsteinRRHandelsmanDJLawrenceSJBlackwellCCatersonIDSullivanCENeuroendocrine dysfunction in sleep apnea: reversal by continuous positive airways pressure therapyJ Clin Endocrinol Metab19896823523582493027
- MinoguchiKYokoeTTazakiTIncreased carotid intima-media thickness and serum inflammatory markers in obstructive sleep apneaAm J Respir Crit Care Med2005172562563016120716
- YuanMKonstantopoulosNLeeJReversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of IkkbetaScience200129355351673167711533494
- MokdadAHBowmanBAFordESVinicorFMarksJSKoplanJPThe continuing epidemics of obesity and diabetes in the United StatesJAMA2001286101195120011559264
- CerielloAMotzEIs oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisitedArterioscler Thromb Vasc Biol200424581682314976002
- PunjabiNMSorkinJDKatzelLIGoldbergAPSchwartzARSmithPLSleep-disordered breathing and insulin resistance in middle-aged and overweight menAm J Respir Crit Care Med2002165567768211874813
- IpMSLamBNgMMLamWKTsangKWLamKSObstructive sleep apnea is independently associated with insulin resistanceAm J Respir Crit Care Med2002165567067611874812
- PunjabiNMShaharERedlineSGottliebDJGivelberRResnickHESleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health StudyAm J Epidemiol2004160652153015353412
- LamJCLamBLamCLObstructive sleep apnea and the metabolic syndrome in community-based Chinese adults in Hong KongRespir Med2006100698098716337115
- OkadaMTakamizawaATsushimaKUrushihataKFujimotoKKuboKRelationship between sleep-disordered breathing and lifestyle-related illnesses in subjects who have undergone health-screeningIntern Med2006451589189616946570
- SulitLStorfer-IsserAKirchnerHLRedlineSDifferences in polysomnography predictors for hypertension and impaired glucose toleranceSleep200629677778316796216
- PeltierACConsensFBSheikhKWangLSongYRussellJWAutonomic dysfunction in obstructive sleep apnea is associated with impaired glucose regulationSleep Med20078214915517236808
- KonoMTatsumiKSaibaraTObstructive sleep apnea syndrome is associated with some components of metabolic syndromeChest200713151387139217494788
- CoughlinSRMawdsleyLMugarzaJACalverleyPMWildingJPObstructive sleep apnoea is independently associated with an increased prevalence of metabolic syndromeEur Heart J200425973574115120883
- StrohlKPNovakRDSingerWInsulin levels, blood pressure and sleep apneaSleep19941776146187846459
- VgontzasANPapanicolaouDABixlerEOSleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemiaJ Clin Endocrinol Metab20008531151115810720054
- MakinoSHandaHSuzukawaKObstructive sleep apnoea syndrome, plasma adiponectin levels, and insulin resistanceClin Endocrinol (Oxf)2006641121916402923
- ElmasryAJansonCLindbergEGislasonTTageldinMABomanGThe role of habitual snoring and obesity in the development of diabetes: a 10-year follow-up study in a male populationJ Intern Med20002481132010947876
- Al-DelaimyWKMansonJEWillettWCStampferMJHuFBSnoring as a risk factor for type II diabetes mellitus: a prospective studyAm J Epidemiol2002155538739311867347
- ReichmuthKJAustinDSkatrudJBYoungTAssociation of sleep apnea and type II diabetes: a population-based studyAm J Respir Crit Care Med2005172121590159516192452
- HarschIASchahinSPRadespiel-TrogerMContinuous positive airway pressure treatment rapidly improves insulin sensitivity in patients with obstructive sleep apnea syndromeAm J Respir Crit Care Med2004169215616214512265
- WestSDNicollDJWallaceTMMatthewsDRStradlingJREffect of CPAP on insulin resistance and HbA1c in men with obstructive sleep apnoea and type 2 diabetesThorax2007621196997417557769
- BrooksBCistulliPABorkmanMObstructive sleep apnea in obese noninsulin-dependent diabetic patients: effect of continuous positive airway pressure treatment on insulin responsivenessJ Clin Endocrinol Metab1994796168116857989475
- CoughlinSRMawdsleyLMugarzaJAWildingJPCalverleyPMCardiovascular and metabolic effects of CPAP in obese males with OSAEur Respir J200729472072717251237
- BabuARHerdegenJFogelfeldLShottSMazzoneTType 2 diabetes, glycemic control, and continuous positive airway pressure in obstructive sleep apneaArch Intern Med2005165444745215738376
- SmurraMPhilipPTaillardJGuilleminaultCBioulacBGinHCPAP treatment does not affect glucose-insulin metabolism in sleep apneic patientsSleep Med20012320721311311683
- SaarelainenSLahtelaJKallonenEEffect of nasal CPAP treatment on insulin sensitivity and plasma leptinJ Sleep Res1997621461479377535
- RedlineSMehraRWangXBabineauDAylorJIsmail-BeigiFA double-blind cross-over trial of CPAP vs sham-CPAP on metabolic control in individuals with impaired glucose tolerance and sleep apneaAm J Respir Crit Care Med2010181A2539
- RedlineSMehraRWXBabineauDAylorJIsmail-BeigiFA double-blind cross-over trial of CPAP vs sham-CPAP on metabolic control in individuals with impaired glucose tolerance and sleep apneaAmerican Thoracic Society Abstracts2010
- SavranskyVNanayakkaraALiJChronic intermittent hypoxia induces atherosclerosisAm J Respir Crit Care Med2007175121290129717332479
- LiJSavranskyVNanayakkaraASmithPLO’DonnellCPPolotskyVYHyperlipidemia and lipid peroxidation are dependent on the severity of chronic intermittent hypoxiaJ Appl Physiol2007102255756317082365
- SavranskyVJunJLiJDyslipidemia and atherosclerosis induced by chronic intermittent hypoxia are attenuated by deficiency of stearoyl coenzyme A desaturaseCirc Res2008103101173118018832746
- SteiropoulosPTsaraVNenaEEffect of continuous positive airway pressure treatment on serum cardiovascular risk factors in patients with obstructive sleep apnea-hypopnea syndromeChest2007132384385117573492
- RobinsonGVPepperellJCSegalHCDaviesRJStradlingJRCirculating cardiovascular risk factors in obstructive sleep apnoea: data from randomised controlled trialsThorax200459977778215333855
- GozalDCapdevilaOSKheirandish-GozalLMetabolic alterations and systemic inflammation in obstructive sleep apnea among nonobese and obese prepubertal childrenAm J Respir Crit Care Med2008177101142114918276939
- BarceloABarbeFLlompartEEffects of obesity on C-reactive protein level and metabolic disturbances in male patients with obstructive sleep apneaAm J Med2004117211812115234648
- EnglemanHMDouglasNJSleep 4: Sleepiness, cognitive function, and quality of life in obstructive sleep apnoea/hypopnoea syndromeThorax200459761862215223874
- QuanSFWrightRBaldwinCMObstructive sleep apnea-hypopnea and neurocognitive functioning in the Sleep Heart Health StudySleep Med20067649850716815753
- EnglemanHMKingshottRNWraithPKMackayTWDearyIJDouglasNJRandomized placebo-controlled crossover trial of continuous positive airway pressure for mild sleep Apnea/Hypopnea syndromeAm J Respir Crit Care Med199915924614679927358
- WeaverTEMaislinGDingesDFRelationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioningSleep200730671171917580592
- PatelSRWhiteDPMalhotraAStanchinaMLAyasNTContinuous positive airway pressure therapy for treating sleepiness in a diverse population with obstructive sleep apnea: results of a meta-analysisArch Intern Med2003163556557112622603
- YoungTBlusteinJFinnLPaltaMSleep-disordered breathing and motor vehicle accidents in a population-based sample of employed adultsSleep19972086086139351127
- HackMDaviesRJMullinsRRandomised prospective parallel trial of therapeutic versus subtherapeutic nasal continuous positive airway pressure on simulated steering performance in patients with obstructive sleep apnoeaThorax200055322423110679542
- EllenRLMarshallSCPalayewMMolnarFJWilsonKGMan-Son-HingMSystematic review of motor vehicle crash risk in persons with sleep apneaJ Clin Sleep Med20062219320017557495
- YangEHHlaKMMcHorneyCAHavighurstTBadrMSWeberSSleep apnea and quality of lifeSleep200023453554110875560
- BaldwinCMGriffithKANietoFJO’ConnorGTWalslebenJARedlineSThe association of sleep-disordered breathing and sleep symptoms with quality of life in the Sleep Heart Health StudySleep20012419610511204058
- BrionesBAdamsNStraussMRelationship between sleepiness and general health statusSleep19961975835888899938
- WhitneyCWEnrightPLNewmanABBonekatWFoleyDQuanSFCorrelates of daytime sleepiness in 4578 elderly persons: the Cardiovascular Health StudySleep199821127369485530
- GrunsteinRRStenlofKHednerJASjostromLImpact of self-reported sleep-breathing disturbances on psychosocial performance in the Swedish Obese Subjects (SOS) StudySleep19951886356438560128
- MillmanRPFogelBSMcNamaraMECarlisleCCDepression as a manifestation of obstructive sleep apnea: reversal with nasal continuous positive airway pressureJ Clin Psychiatry19895093483512768203
- WareJThe SF-36 health surveySpilkerBQuality of Life in Pharmacoeconomic in Clinical Trials2nd edPhiladelphia, PALippincott-Raven1996337345
- FlemonsWWReimerMADevelopment of a disease-specific health-related quality of life questionnaire for sleep apneaAm J Respir Crit Care Med199815824945039700127
- MoyerCASonnadSSGaretzSLHelmanJIChervinRDQuality of life in obstructive sleep apnea: a systematic review of the literatureSleep Med20012647749114592263
- JenkinsonCStradlingJPetersenSComparison of three measures of quality of life outcome in the evaluation of continuous positive airways pressure therapy for sleep apnoeaJ Sleep Res1997631992049358398
- D’AmbrosioCBowmanTMohseninVQuality of life in patients with obstructive sleep apnea: effect of nasal continuous positive airway pressure – a prospective studyChest199911511231299925072
- BennettLSBarbourCLangfordBStradlingJRDaviesRJHealth status in obstructive sleep apnea: relationship with sleep fragmentation and daytime sleepiness, and effects of continuous positive airway pressure treatmentAm J Respir Crit Care Med199915961884189010351935
- PiccirilloJFGatesGAWhiteDLSchectmanKBObstructive sleep apnea treatment outcomes pilot studyOtolaryngol Head Neck Surg199811868338449627245
- JokicRKlimaszewskiACrossleyMSridharGFitzpatrickMFPositional treatment vs continuous positive airway pressure in patients with positional obstructive sleep apnea syndromeChest1999115377178110084491
- BolitschekJSchmeiser-RiederASchobersbergerRRosenbergerAKunzeMAignerKImpact of nasal continuous positive airway pressure treatment on quality of life in patients with obstructive sleep apnoeaEur Respir J19981148908949623693
- Practice parameters for the indications for polysomnography and related procedures. Polysomnography Task Force, American Sleep Disorders Association Standards of Practice CommitteeSleep19972064064229302725
- ChediakADAcevedo-CrespoJCSeidenDJKimHHKielMHNightly variability in the indices of sleep-disordered breathing in men being evaluated for impotence with consecutive night polysomnogramsSleep19961975895928899939
- AarabGLobbezooFHamburgerHLNaeijeMVariability in the apnea-hypopnea index and its consequences for diagnosis and therapy evaluationRespiration2009771323718957843
- BittencourtLRSucheckiDTufikSThe variability of the apnoea-hypopnoea indexJ Sleep Res200110324525111696078
- AhmadiNShapiroGKChungSAShapiroCMClinical diagnosis of sleep apnea based on single night of polysomnography vs two nights of polysomnographySleep Breath200913322122619067010
- RichardWKoxDden HerderCLamanMvan TinterenHde VriesNThe role of sleep position in obstructive sleep apnea syndromeEur Arch Otorhinolaryngol20062631094695016802139
- WiegandLZwillichCWWiegandDWhiteDPChanges in upper airway muscle activation and ventilation during phasic REM sleep in normal menJ Appl Physiol19917124884971938720
- FlemonsWWDouglasNJKunaSTRodensteinDOWheatleyJAccess to diagnosis and treatment of patients with suspected sleep apneaAm J Respir Crit Care Med2004169666867215003950
- CollopNAAndersonWMBoehleckeBClinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep MedicineJ Clin Sleep Med20073773774718198809
- IberCRedlineSKaplan GilpinAMPolysomnography performed in the unattended home versus the attended laboratory setting – Sleep Heart Health Study methodologySleep200427353654015164911
- Tonelli de OliveiraACMartinezDVasconcelosLFDiagnosis of obstructive sleep apnea syndrome and its outcomes with home portable monitoringChest2009135233033619201709
- MulgrewATFoxNAyasNTRyanCFDiagnosis and initial management of obstructive sleep apnea without polysomnography: a randomized validation studyAnn Intern Med2007146315716617283346
- CollopNAAndersonWMBoehleckeBClinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep MedicineJ Clin Sleep Med20073773774718198809
- SullivanCEIssaFGBerthon-JonesMEvesLReversal of obstructive sleep apnoea by continuous positive airway pressure applied through the naresLancet1981182258628656112294
- BallesterEBadiaJRHernandezLEvidence of the effectiveness of continuous positive airway pressure in the treatment of sleep apnea/hypopnea syndromeAm J Respir Crit Care Med199915924955019927363
- BarbeFMayoralasLRDuranJTreatment with continuous positive airway pressure is not effective in patients with sleep apnea but no daytime sleepiness: a randomized, controlled trialAnn Intern Med2001134111015102311388814
- BarnesMHoustonDWorsnopCJA randomized controlled trial of continuous positive airway pressure in mild obstructive sleep apneaAm J Respir Crit Care Med2002165677378011897643
- BarnesMMcEvoyRDBanksSEfficacy of positive airway pressure and oral appliance in mild to moderate obstructive sleep apneaAm J Respir Crit Care Med2004170665666415201136
- ChakravortyICaytonRMSzczepuraAHealth utilities in evaluating intervention in the sleep apnoea/hypopnoea syndromeEur Respir J20022051233123812449179
- EnglemanHMMcDonaldJPGrahamDRandomized crossover trial of two treatments for sleep apnea/hypopnea syndrome: continuous positive airway pressure and mandibular repositioning splintAm J Respir Crit Care Med2002166685585912231497
- HenkeKGGradyJJKunaSTEffect of nasal continuous positive a irway pressure on neuropsychological function in sleep apnea-hypopnea syndrome. A randomized, placebo-controlled trialAm J Respir Crit Care Med2001163491191711282765
- JenkinsonCDaviesRJMullinsRStradlingJRComparison of therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised prospective parallel trialLancet199935391702100210510382693
- MansfeldDRGolloglyNCKayeDMRichardsonMBerginPNaughtonMTControlled trial of continuous positive airway pressure in obstructive sleep apnea and heart failureAm J Respir Crit Care Med2004169336136614597482
- MarshallNSNeillAMCampbellAJSheppardDSRandomised controlled crossover trial of humidified continuous positive airway pressure in mild obstructive sleep apnoeaThorax200560542743215860720
- McArdleNDevereuxGHeidarnejadHEnglemanHMMackayTWDouglasNJLong-term use of CPAP therapy for sleep apnea/hypopnea syndromeAm J Respir Crit Care Med19991594 Pt 11108111410194153
- EnglemanHMMartinSEDearyIJDouglasNJEffect of continuous positive airway pressure treatment on daytime function in sleep apnoea/hypopnoea syndromeLancet199434388975725757906330
- McArdleNDouglasNJEffect of continuous positive airway pressure on sleep architecture in the sleep apnea-hypopnea syndrome: a randomized controlled trialAm J Respir Crit Care Med20011648 Pt 11459146311704596
- MontserratJMFerrerMHernandezLEffectiveness of CPAP treatment in daytime function in sleep apnea syndrome: a randomized controlled study with an optimized placeboAm J Respir Crit Care Med2001164460861311520724
- SinDDMayersIManGCGhaharyAPawlukLCan continuous positive airway pressure therapy improve the general health status of patients with obstructive sleep apnea? a clinical effectiveness studyChest200212251679168512426271
- RedlineSAdamsNStraussMERoebuckTWintersMRosenbergCImprovement of mild sleep-disordered breathing with CPAP compared with conservative therapyAm J Respir Crit Care Med19981573 Pt 18588659517603
- KushidaCALittnerMRHirshkowitzMPractice parameters for the use of continuous and bilevel positive airway pressure devices to treat adult patients with sleep-related breathing disordersSleep200629337538016553024
- AyasNTPatelSRMalhotraAAuto-titrating versus standard continuous positive airway pressure for the treatment of obstructive sleep apnea: results of a meta-analysisSleep200427224925315124718
- KasaiTUsuiYYoshiokaTEffect of flow-triggered adaptive servo-ventilation compared with continuous positive airway pressure in patients with chronic heart failure with coexisting obstructive sleep apnea and Cheyne-Stokes respirationCirc Heart Fail20103114014819933407
- PhilippeCStoica-HermanMDrouotXCompliance with and effectiveness of adaptive servoventilation versus continuous positive airway pressure in the treatment of Cheyne-Stokes respiration in heart failure over a six month periodHeart200692333734215964943
- PepinJLKriegerJRodensteinDEffective compliance during the first 3 months of continuous positive airway pressure. A European prospective study of 121 patientsAm J Respir Crit Care Med199916041124112910508797
- KribbsNBPackAIKlineLRObjective measurement of patterns of nasal CPAP use by patients with obstructive sleep apneaAm Rev Respir Dis199314748878958466125
- EnglemanHMWildMRImproving CPAP use by patients with the sleep apnoea/hypopnoea syndrome (SAHS)Sleep Med Rev200371819912586532
- EnglemanHMAsgari-JirhandehNMcLeodALRamsayCFDearyIJDouglasNJSelf-reported use of CPAP and benefits of CPAP therapy: a patient surveyChest19961096147014768769496
- KakkarRKBerryRBPositive airway pressure treatment for obstructive sleep apneaChest200713231057107217873201
- RyanCFLoveLLPeatDFleethamJALoweAAMandibular advancement oral appliance therapy for obstructive sleep apnoea: effect on awake calibre of the velopharynxThorax1999541197297710525554
- NgATGotsopoulosHQianJCistulliPAEffect of oral appliance therapy on upper airway collapsibility in obstructive sleep apneaAm J Respir Crit Care Med2003168223824112724125
- FergusonKACartwrightRRogersRSchmidt-NowaraWOral appliances for snoring and obstructive sleep apnea: a reviewSleep200629224426216494093
- FergusonKAOnoTLoweAAKeenanSPFleethamJAA randomized crossover study of an oral appliance vs nasal-continuous positive airway pressure in the treatment of mild-moderate obstructive sleep apneaChest19961095126912758625679
- FergusonKAOnoTLoweAAal-MajedSLoveLLFleethamJAA short-term controlled trial of an adjustable oral appliance for the treatment of mild to moderate obstructive sleep apnoeaThorax19975243623689196520
- TanYKL’EstrangePRLuoYMMandibular advancement splints and continuous positive airway pressure in patients with obstructive sleep apnoea: a randomized cross-over trialEur J Orthod200224323924912143088
- FujitaSConwayWZorickFRothTSurgical correction of anatomic azbnormalities in obstructive sleep apnea syndrome: uvulopalatopharyngoplastyOtolaryngol Head Neck Surg19818969239346801592
- SherAESchechtmanKBPiccirilloJFThe efficacy of surgical modifications of the upper airway in adults with obstructive sleep apnea syndromeSleep19961921561778855039
- FergusonKAHeighwayKRubyRRA randomized trial of laserassisted uvulopalatoplasty in the treatment of mild obstructive sleep apneaAm J Respir Crit Care Med20031671151912502473
- LarrosaFHernandezLMorelloABallesterEQuintoLMontserratJMLaser-assisted uvulopalatoplasty for snoring: does it meet the expectations?Eur Respir J2004241667015293606
- WoodsonBTStewardDLWeaverEMJavaheriSA randomized trial of temperature-controlled radiofrequency, continuous positive airway pressure, and placebo for obstructive sleep apnea syndromeOtolaryngol Head Neck Surg2003128684886112825037
- StuckBASauterAHormannKVerseTMaurerJTRadiofrequency surgery of the soft palate in the treatment of snoring. A placebo-controlled trialSleep200528784785016124664
- CeylanKEmirHKizilkayaZFirst-choice treatment in mild to moderate obstructive sleep apnea: single-stage, multilevel, temperature-controlled radiofrequency tissue volume reduction or nasal continuous positive airway pressureArch Otolaryngol Head Neck Surg2009135991591919770425
- BackLJLiukkoTRantanenIRadiofrequency surgery of the soft palate in the treatment of mild obstructive sleep apnea is not effective as a single-stage procedure: a randomized single-blinded placebo-controlled trialLaryngoscope200911981621162719504550
- LiHYWangPCLeeLAChenNHFangTJPrediction of uvulopalatopharyngoplasty outcome: anatomy-based staging system versus severity-based staging systemSleep200629121537154117252884
- National Commission on Sleep Disorders Research. Wake up America: a national sleep alert. BethesdaMD19952
- PunjabiNMThe epidemiology of adult obstructive sleep apneaProc Am Thorac Soc20085213614318250205
- YoungTEvansLFinnLPaltaMEstimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and womenSleep19972097057069406321