Abstract
Systemic lupus erythematosus (SLE) is characterized by the second highest prevalence of pulmonary arterial hypertension (PAH), after systemic sclerosis, among the connective tissue diseases. SLE-associated PAH is hemodynamically defined by increased mean pulmonary artery pressure at rest (≥25 mmHg) with normal pulmonary capillary wedge pressure (≤15 mmHg) and increased pulmonary vascular resistance. Estimated prevalence ranges from 0.5% to 17.5% depending on the diagnostic method used and the threshold of right ventricular systolic pressure in studies using transthoracic echocardiogram. Its pathogenesis is multifactorial with vasoconstriction, due to imbalance of vasoactive mediators, leading to hypoxia and impaired vascular remodeling, collagen deposition, and thrombosis of the pulmonary circulation. Multiple predictive factors have been recognized, such as Raynaud’s phenomenon, pleuritis, pericarditis, anti-ribonuclear protein, and antiphospholipid antibodies. Secure diagnosis is based on right heart catheterization, although transthoracic echocardiogram has been shown to be reliable for patient screening and follow-up. Data on treatment mostly come from uncontrolled observational studies and consist of immunosuppressive drugs, mainly corticosteroids and cyclophosphamide, as well as PAH-targeted approaches with endothelin receptor antagonists (bosentan), phosphodiesterase type 5 inhibitors (sildenafil), and vasodilators (epoprostenol). Prognosis is significantly affected, with 1- and 5-year survival estimated at 88% and 68%, respectively.
Introduction
Pulmonary arterial hypertension (PAH) is classified into five distinct categories according to etiology.Citation1 Group 1 PAH may be idiopathic, heritable, or drug or toxin induced, or it could be associated with human immunodeficiency virus, schistosomiasis, portal hypertension, and congenital heart disease as well as with connective tissue diseases (CTDs). PAH related to left heart diseases is classified in group 2, lung diseases in group 3, chronic thromboembolic pulmonary hypertension in group 4, while other, less well-known causes are included in group 5. CTD-associated PAH is hemodynamically defined by increased mean pulmonary artery pressure at rest (mPAP ≥25 mmHg), with normal pulmonary capillary wedge pressure (PCWP ≤15 mmHg) and increased pulmonary vascular resistance (PVR).Citation2 Systemic lupus erythematosus (SLE) is characterized by the second highest prevalence of PAH, after systemic sclerosis, according to the REVEAL registry (Registry to Evaluate Early and Long-term Pulmonary Arterial Hypertension disease management), a 55-center longitudinal US-based registry with nearly 3,500 patients.Citation3 The aim of this review is to present the current knowledge on the epidemiology, pathophysiology, diagnosis and treatment of SLE-associated PAH (SLE-PAH).
Epidemiology
The annual incidence of systemic-autoimmune-disease-associated PAH has been estimated to be between one and three cases per million population, with a prevalence ranging from 5 to 15 cases per million.Citation4,Citation5 Cohort studies have reported that PAH prevalence in SLE varies widely between 0.5% and 43%,Citation6–Citation8 although more recent reports estimate this as being between 0.5% and 17.5%.Citation9 Other investigators estimated the prevalence of PAH to be 14% based on the first transthoracic echocardiogram (TTE) of lupus patients.Citation10 The differences are mainly attributed to the method used for diagnosis (TTE vs right heart catheterization, RHC), the threshold for PAH (right ventricular systolic pressure, RVSP >30 mmHg or >40 mmHg), and the studied population.
The majority of patients with SLE-PAH are women (95% in the REVEAL registry with RHC confirmed disease) with a mean age of 45 years (45.5±11.9) at PAH diagnosis.Citation11 In rare cases, PAH is the first manifestation leading to SLE diagnosis.Citation12 Concerning severity, this is usually moderate with pulmonary artery systolic pressure (PASP) ranging between 40 and 60 mmHg and PVR between 5 and 15 Wood units.Citation11,Citation13
Predictive factors
Certain lupus manifestations and immunologic abnormalities have been identified as predictors of PAH in observational cohort studies. In this context, Raynaud’s phenomenon, active renal disease, and vasculitic manifestations (digital gangrene, cutaneous vasculitis, and livedo reticularis) were shown to independently predict PAH development.Citation14,Citation15 In another small prospective study with 34 patients, Raynaud’s phenomenon was strongly related to increased PASP as assessed by TTE.Citation16 Pleural effusions were also demonstrated to occur with higher frequency in CTD-associated PAH; patients with pleural effusions had higher mean right atrial pressure (mRAP) and lower cardiac output.Citation17 However, most patients in that study had overt right heart failure, which may have contributed to pleural effusion. In a recent analysis of a large Chinese cohort (n=1,934, 74 patients with PAH), pleuritis was an independent predictor of PAH in lupus and showed an odds ratio (OR) =3.06 (95% confidence interval [CI] =1.6–5.85).Citation18 Additional clinical manifestations with an independent predictive ability for PAH were interstitial lung disease (OR =17, 95% CI =3.6–80) and pericardial effusion (OR =21.3, 95% CI =4.1–110.6).Citation13 In the same study, it was shown that, paradoxically, lupus patients with PAH had less severe disease, as assessed by the SLE Disease Activity Index (SLEDAI); actually, a SLEDAI ≤9 was strongly predictive of PAH with an OR =26.4 (95% CI =6.6–105.5).Citation13 Moreover, the lack of acute rash and low erythrocyte sedimentation rate (≤20 mm/h) were also independent predictors. The clinical significance of these findings is difficult to interpret since it is believed that systemic inflammation drives PAH in these patients.Citation18
Immunological variables associated with PAH in lupus patients include positive anti-U1-RNP (ribonuclear protein) and antiphospholipid antibodies.Citation13 Anti-U1-RNP antibodies, in particular, were strong predictors for PAH in several studies with a hazard ratio ranging from 2.6 to 12.4 (95% CI =3.6–42.9).Citation12,Citation13,Citation18 Antiphospholipid antibodies, mainly anticardiolipin antibodies and lupus anticoagulant, were also associated with increased risk for PAH.Citation6,Citation13,Citation19 Recently, anti-Sjogren’s Syndrome related antigen A antibodies were demonstrated as strong predictors with an OR =4.8 (95% CI =1.7–14).Citation13 Details on the predictive factors for PAH in lupus patients are given in .
Table 1 Independent predictors of PAH in SLE
Pathogenesis
The pathogenetic mechanisms in SLE-PAH have not been elucidated yet. However, accumulating evidence suggests that multiple factors, such as genetic predisposition, environmental stimuli, and immune system dysfunction play a significant role.Citation15 The net result is an imbalance between vasoconstrictive and vasodilating soluble mediators, which leads to vasoconstriction with increased PVR.Citation20 Endothelin-1 (ET-1) and thromboxane A2 (TXA-2) are the main mediators that lead to vasoconstriction; their serum levels were significantly elevated in patients with SLE-PAH and correlated with PAH severity.Citation21,Citation22 In addition, about 42% of patients with SLE-PAH were found to have antibodies against the endothelin receptor type A; their titers correlated strongly with PASP.Citation23 These antibodies promote endothelial dysfunction and probably contribute to the inhibition of prostacyclin production by endothelial cells, which is one of the main vasodilators.Citation15,Citation20 In line with the vasoconstriction theory, it was recently demonstrated that lupus patients with PAH have increased arterial stiffness (assessed by brachial–ankle pulse wave velocity), and this was an independent predictor of PAH.Citation24 Vasoconstriction represents an early event in the PAH pathogenic cascade, and vasoresponders (patients with a >10 mmHg decrease in PASP upon inhalation of vasoactive compounds during RHC) have a good response to calcium channel blockers, regardless of PAH etiology.Citation25,Citation26
Pulmonary vasoconstriction will lead to decreased oxygen saturation and hyperexpression of the hypoxia-inducible factor (HIF) and erythropoietin (EPO), which may promote smooth muscle cell proliferation and extensive vascular remodeling.Citation20,Citation27 Several distinct pathways are implicated in vascular remodeling such as impaired apoptosis with upregulation of antiapoptotic proteins and abnormal proliferation of endothelial and adventitial cells.Citation15,Citation20 In parallel, the inflammatory response will drive the accumulation of monocytes, neutrophils, mast cells, and dendritic cells into the elastic lamina, which in turn induce the production of certain chemokines, cytokines, and growth factors that perpetuate abnormal remodeling.Citation28 In later stages of PAH, microthrombi in the pulmonary vasculature will lead to further elevation of PASP.Citation20
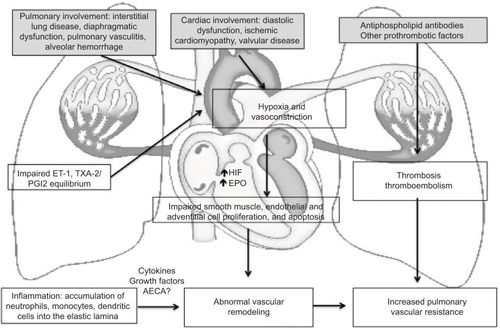
In the aforementioned pathogenetic model of PAH, many characteristics of SLE, such as vasculitis, thrombosis, and interstitial pulmonary fibrosis are implicated.Citation15 Pulmonary vasculitis is mediated through immune complexes; both immunoglobulins and complement have been demonstrated to deposit on the arterial wall.Citation29 Other pulmonary vascular pathologic findings were similar to those of idiopathic PAH and mainly consisted of plexiform lesions, smooth muscle cell hypertrophy, intimal proliferation, and collagen deposition.Citation15,Citation30 In addition, CTD-associated PAH was characterized by more frequent involvement of pulmonary veins and perivascular inflammatory infiltration.Citation31 Thrombosis occurs more frequently in lupus patients, particularly in the presence of antiphospholipid antibodies. Although antiphospholipid syndrome per se may lead to chronic thromboembolic pulmonary hypertension, antiphospholipid antibodies have been shown to mediate endothelial dysfunction and induce the secretion of adhesion molecules.Citation15 This may also occur in the presence of anti-endothelial cell antibodies – their binding to endothelial cells results in increased production of IL-6 and IL-8.Citation32 The role of other immunological abnormalities in SLE-PAH, such as the decreased numbers of T regulatory cells and the more frequent presence of the anti-U1-RNP antibodies, has not been clarified yet.Citation15,Citation33 A schematic representation of the pathogenesis of PAH in the context of SLE is presented in .
Figure 1 A schematic representation of the pathogenesis of PAH in SLE.
Abbreviations: PAH, pulmonary arterial hypertension; SLE, systemic lupus erythematosus; ET-1, endothelin-1; TXA-2, thromboxane A2; HIF, hypoxia-inducible factor; EPO, erythropoietin; PVR, pulmonary vascular resistance; AECA, anti-endothelial cell antibodies.
Clinical presentation and diagnosis
Dyspnea (initially exertional), dry cough, chest pain, exercise intolerance, and fatigue are the cardinal clinical manifestations of PAH. Physical findings may include elevated jugular venous pressure, accentuated S2 cardiac sound, and murmurs indicative of tricuspid and/or pulmonic valve insufficiency. In late stages, right heart failure with liver enlargement due to congestion, ascites, and/or lower limb edema may be seen.
The gold standard for PAH diagnosis is RHC, which precisely assesses PASP, diastolic PAP, PCWP, PVR, and cardiac output.Citation1 Secure diagnosis requires a mean PAP ≥25 mmHg at rest.Citation34 Documentation of all these parameters also allows for rough etiologic characterization of PAH; a PCWP ≤15 mmHg is required to exclude cases of heart failure. However, it was shown that many patients with heart failure and preserved ejection fraction have low PCWP (16–18 mmHg).Citation35 PVR ≥3 Wood units should also be included in the characterization of PAH.Citation34 During the procedure, vasoreactivity can be assessed with the use of nitric oxide, epoprostenol, or adenosine. Most SLE patients are not vasoreactive, and therapy with calcium channel blockers has not proven to be beneficial.Citation15 RHC is not always feasible due to availability and/or procedure-related complications (mild-to-moderate in 1.1%, mortality 0.055%).Citation36
TTE can provide a reliable estimation of the RVSP and tricuspid valve insufficiency.Citation15 RVSP is calculated by the modified Bernoulli equation as RVSP =4v2 + mRAP, where v equals the tricuspid regurgitant jet velocity (a reflection of the right ventricular-to-atrial systolic pressure gradient) and mRAP is estimated from the diameter and respirophasic variability of the inferior vena cava during normal respiration.Citation37 RVSP does not necessarily correlate with the measurement of PASP as obtained with RHC.Citation38 In that study, TTE and RHC provided comparable measurements of PASP (77.2±35 mmHg vs 76.9±21.7 mmHg, respectively). However, TTE was inaccurate (over- or underestimating PASP by >10 mmHg) in 57% of the cases. Other studies showed that the correlation between TTE and RHC was satisfactory, at least for the initial evaluation of such patients.Citation39 Moreover, it was recently demonstrated that two consecutive TTEs with an RVSP ≥40 mmHg were the most accurate predictors for PAH with a sensitivity of 100%, specificity 97%, positive predictive value of 70%, and negative predictive value of 100%.Citation40 In general, TTE is recommended for the initial screening of patients with suspected PAH as well as for the evaluation of response to treatment.Citation34 Of note, TTE may lead to PAH diagnosis in asymptomatic patients, although the threshold used in that study was rather low (RVSP =30 mmHg).Citation41
Additional investigations are warranted for the precise etiologic diagnosis of PAH. High-resolution computed tomography of the thorax will help exclude any concomitant interstitial lung disease, while ventilation/perfusion scan for acute or chronic thromboemboli will rule out chronic thromboembolic pulmonary hypertension.Citation15,Citation34 Rare causes of PAH such as sleep apnea syndrome (assessed by polysomnography), human immunodeficiency virus, schistosomiasis, and portopulmonary hypertension should also be excluded. Pulmonary function tests reveal isolated decreased diffusing capacity for carbon monoxide (DLCO).
Therapeutic approach
Treatment of SLE-PAH should be prompt and aim at PASP normalization in order to maximize survival. Thorough diagnostic evaluation is of utmost importance since patients with no other risk factors (eg, left heart failure, chronic thromboembolic PAH) should be treated accordingly (diuretics, anticoagulants, etc).Citation42 Existing randomized controlled trials have solely assessed the effect of PAH-targeted therapies with endothelin receptor antagonists (bosentan), phosphodiesterase type 5 inhibitors (PDE5 inhibitors, sildenafil), and vasodilators (treprostinil, a synthetic analog of prostacyclin, PGI2).Citation43–Citation45 In these studies, patients with different CTDs, mainly mixed connective tissue disease (MCTD) and systemic scleroderma, were also included, and a subanalysis of the lupus patients was not performed.
Additional data come from observational cohort studies using immunosuppressive therapies that have shown a considerable benefit in such patients. Tanaka et alCitation46 reported a significant reduction of RVSP in seven out of eight patients who received corticosteroids ± cyclophosphamide (CYC) – two patients relapsed and were again treated successfully. Intravenous CYC pulses (dose ranging from 500 mg/month to 1,000 mg/m2/month) were also administered in five cohort studies in conjunction with prednisone (0.5–1 mg/kg/day with slow tapering)Citation47,Citation48 and vasodilatorsCitation49,Citation50 or PDE5 inhibitors.Citation51 Gonzalez-Lopez et alCitation47 showed that CYC in combination with low doses of prednisone (<15 mg/day) was effective in 16 patients with moderate SLE-PAH as assessed by TTE. In another study with CYC, five out of 12 lupus patients responded and showed improved survival.Citation48 More recent studies demonstrated that the addition of vasodilators and supportive treatment with diuretics and anticoagulants may benefit patients with more severe PAH at diagnosis; in those studies, patients with MCTD were also included.Citation49 Finally, Kommireddy et alCitation51 reported a mean reduction of 16 mmHg in PASP (assessed by TTE) with three intravenous CYC pulses plus oral prednisone and PDE5 inhibitors in 11/24 patients with SLE-PAH. Moreover, rituximab was shown to provide benefit in a refractory case of SLE-PAH,Citation52 while another patient was successfully managed with mycophenolate mofetil and cyclosporine.Citation12 Details on the studies that used immunosuppressive treatment for SLE-PAH are given in .
Table 2 Studies with immunosuppressive medications for SLE-PAH
Other studies have used only PAH-targeted approaches in SLE-PAH. Robbins et alCitation53 administered intravenous epoprostenol in six patients and observed a significant improvement in PAH hemodynamics and functional class according to the New York Heart Association. Other investigators reported similar results along with better survival in epoprostenol-treated patients.Citation54 Oudiz et alCitation45 showed a favorable hemodynamic response and trend toward improved quality of life in 25 lupus patients with subcutaneous treprostinil, a prostacyclin analog. Endothelin receptor antagonists, particularly bosentan, have been shown to improve functional New York Heart Association class and exercise tolerance (assessed with the 6-minute walk distance) in a randomized, placebo-controlled trial, which included 16 lupus patients, as well as other small observational studies.Citation43,Citation55,Citation56 Moreover, sildenafil in low doses improved PAH hemodynamics and functional capacity after 12 weeks of treatment; in that study, 84 patients with CTD-associated PAH (19 with SLE) were subanalyzed.Citation44 Finally, a randomized, placebo-controlled trial with riociguat, a soluble guanylate cyclase stimulator with vasoactive, antifibrotic, and anti-inflammatory effects, showed favorable results in PAH associated with CTDs.Citation57 In that study, 18 patients with SLE were included; however, specific details for these patients were not provided. Details on the studies that used PAH-targeted therapies for SLE-PAH are given in .
Table 3 Studies with PAH-targeted therapy medications for SLE-PAH
Prognosis
PAH significantly affects survival and quality of life in CTDs.Citation58 Direct comparisons from the REVEAL registry showed that patients with CTD-associated PAH had poorer 1-year survival as compared to patients with idiopathic PAH (86% vs 93%).Citation11 Among the CTDs group, however, SLE patients had the best rates for 1-year survival (94% vs 82% for scleroderma and 88% for MCTD), although all patients had comparable hemodynamic characteristics at PAH diagnosis. Data from the Korean CTD-PAH registry, with 174 patients enroled, demonstrated that the 1- and 3-year survival were 90.7% and 87.3%, respectively.Citation59 In that study, low DLCO, diabetes, and pleural effusion were poor prognostic factors, while anti-U1-RNP antibodies seemed to be protective. A recent meta-analysis of six studies encompassing 323 lupus patients with PAH demonstrated that the pooled 1-, 3-, and 5-year survival were 88%, 81%, and 68%, respectively.Citation60 Higher mPAP, PVR, and brain natriuretic peptide and lower 6-minute walk distance were related to poor survival. In an older systematic review of 23 observational studies, higher mPAP at diagnosis, Raynaud’s phenomenon, thrombocytopenia, pregnancy, pulmonary vasculitis, and anticardiolipin antibodies were associated with decreased survival.Citation61
Conclusion
PAH affects 0.5%–17.5% of patients with SLE and has significant effects on prognosis. Its pathogenesis is multifactorial and mediated through vasoconstriction, hypoxia, and impaired vascular remodeling. Immunosuppressive treatment is efficacious in selected cases, while PAH-targeted therapies may provide additional benefit.
Disclosure
The authors report no conflicts of interest in this work.
References
- SimonneauGGatzoulisMAAdatiaIUpdated clinical classification of pulmonary hypertensionJ Am Coll Cardiol201362Suppl 25D34D4124355639
- McLaughlinVVArcherSLBadeschBDACCF/AHA 2009 Expert consensus document on pulmonary hypertension. A report of the American College of Cardiology Foundation Task Force on Expert Consensus documents and the American Heart Association developed in collaboration with the American College of Chest Physicians, American Thoracic Society Inc and the Pulmonary Hypertension AssociationJ Am Coll Cardiol2009531573161919389575
- McGoonMDMillerDPREVEAL: a contemporary US pulmonary arterial hypertension registryEur Respir Rev20122181822379169
- PeacockAJMurphyNEMcMurreyJJCaballeroLStewartSAn epidemiological study of pulmonary arterial hypertensionEur Respir J20073010410917360728
- HumbertMSitbonOChaouatAPulmonary arterial hypertension in France: results from a national registryAm J Resp Crit Care Med20061731023103016456139
- PrabuAPatelKYeeCSPrevalence and risk factors for pulmonary arterial hypertension in patients with lupusRheumatology (Oxford)2009481506151119671698
- SimonsonJSSchillerNBPetriMHellmannDBPulmonary hypertension in systemic lupus erythematosusJ Rheumatol1989169189252769664
- WinslowTMOssinovMAFazioGPSimonsonJSRedbergRFSchillerNBFive-year follow-up study of the prevalence and progression of pulmonary hypertension in systemic lupus erythematosusAm Heart J19951295105157872181
- ArnaudLAgardCHarocheJCacoubPPietteJCAmouraZPulmonary arterial hypertension in systemic lupus erythematosusRev Med Interne20113268969721376432
- JohnsonSRGladmanDDUrowitzMBIbanezDDGrantonJTPulmonary hypertension in systemic lupusLupus20041350650915352421
- ChungLLiuJParsonsLCharacterization of connective tissue disease-associated pulmonary arterial hypertension from REVEAL: identifying systemic sclerosis as a unique phenotypeChest20101381383139420507945
- PreteMFatoneMCVaccaARacanelliVPerosaFSevere pulmonary hypertension as the initial manifestation of systemic lupus erythematosus: a case report and review of the literatureClin Exp Rheumatol20143226727424351505
- HuangCLiMLiuYBaseline characteristics and risk factors of pulmonary arterial hypertension in systemic lupus erythematosus patientsMedicine (Baltimore)201695e276126962774
- LianFChenDWangYClinical features and independent predictors of pulmonary arterial hypertension in systemic lupus erythematosusRheumatol Int2012321727173121437686
- DhalaAPulmonary arterial hypertension in systemic lupus erythematosus: current status and future directionsClin Dev Immunol2012201285494122489252
- KasparianAFlorosAGialafosERaynaud’s phenomenon is correlated with elevated systolic pulmonary arterial pressure in patients with systemic lupus erythematosusLupus20071650550817670849
- LuoYERobbinsIMKaratasMBrixeyAGRiceTWLightRWFrequency of pleural effusions in patients with pulmonary arterial hypertension associated with connective tissue diseasesChest2011140424721212140
- LiMWangQZhaoJChinese SLE Treatment and Research Group (CSTAR) registry: II. Prevalence and risk factors of pulmonary arterial hypertension in Chinese patients with systemic lupus erythematosusLupus2014231085109124651670
- XiaYKTuSHHuYHPulmonary hypertension in systemic lupus erythematosus: a systematic review and analysis of 642 cases in Chinese populationRheumatol Int2013331211121722983159
- HuberLCByeHBrockMSwiss Society of Pulmonary HypertensionThe pathogenesis of pulmonary hypertension-an updateSwiss Med Wkly2015145w1420226479975
- LaiYCPotokaKCChampionHCMoraALGladwinMTPulmonary arterial hypertension: the clinical syndromeCirculation Res201411511513024951762
- ShenJYChenSLWuYXPulmonary hypertension in systemic lupus erythematosusRheumatology Int199918147151
- GuoLLiMChenYAnti-endothelin receptor type A autoantibodies in systemic lupus erythematosus-associated pulmonary arterial hypertensionArthritis Rheumatol2015672394240226018988
- LeeJHIm ChoKArterial stiffness, antiphospholipid antibodies and pulmonary arterial hypertension in systemic lupus erythematosusJ Cardiol20146445045524755203
- SitbonOLong-term response to calcium channel blockers in idiopathic pulmonary arterial hypertensionCirculation20051113105311115939821
- MontaniDSavaleLNataliDLong-term response to calcium channel blockers in non-idiopathic pulmonary arterial hypertensionEur Heart J2010311898190720543192
- KaramanianVAHarhayMGrantGRErythropoietin up-regulation in pulmonary arterial hypertensionPulm Circ2014426927925006446
- PullamsettiSSSavaiRJanssenWInflammation, immunological reaction and role of infection in pulmonary hypertensionClin Microbiol Infect20111771420545963
- SasakiNKamatakiASawaiTA histopathological study of pulmonary hypertension in connective tissue diseasesAllergol Int20116041141721918364
- RoncoroniAJAlvarezCMolinasFPlexogenic arteriopathy associated with pulmonary vasculitis in systemic lupus erythematosusRespiration19925952561579720
- DorfmullerPHumbertMPerrosFFibrous remodelling of the pulmonary venous system in pulmonary arterial hypertension associated with connective tissue diseasesHum Pathol20073889390217376507
- ArendsSJDamoiseauxJGDuijvestijnAMFunctional implications of IgG anti-endothelial cell antibodies in pulmonary arterial hypertensionAutoimmunity20134646347024083390
- NicollsMRTaraseviciene-StewartLRaiPRBadeschDBVoelkelNFAutoimmunity and pulmonary hypertension: a perspectiveEur Respir J2005261110111816319344
- HoeperMMBogaardHJCondliffeRDefinitions and diagnosis of pulmonary hypertensionJ Am Coll Cardiol201362Suppl 25D42D5024355641
- FrostAEFarberHWBarstRJMillerDPElliottCGMcGoonMDDemographics and outcomes of patients diagnosed with pulmonary hypertension with pulmonary capillary wedge pressures 16 to 18 mmHg: insights from the REVEAL registryChest201314318519522661451
- HoeperMMLeeSHVoswinckelRComplications of right heart catheterization procedures in patients with pulmonary hypertension in experienced centresJ Am Coll Cardiol2006482546255217174196
- KircherBJHimelmanRBSchillerNBNoninvasive estimation of right atrial pressure from the inspiratory collapse of the inferior vena cavaAm J Cardiol1990664934962386120
- FarberHWForemanAJMillerDPMcgoonMDREVEAL Registry: correlation of right heart catheterization and echocardiography in patients with pulmonary arterial hypertensionCongest Heart Fail201117566421449993
- RichJDShahSJSwamyRSKampARichSInaccuracy of Doppler echocardiographic estimates of pulmonary artery pressures in patients with pulmonary hypertension: implications for clinical practiceChest201113998899320864617
- Ruiz-IrastorzaGGarmendiaMVillarIEgurbideMVAguirreCPulmonary hypertension in systemic lupus erythematosus: prevalence, predictors and diagnostic strategyAutoimmun Rev20131241041522841984
- KamelSROmarGMDarwishAFAsklanyHTEllabbanASAsymptomatic pulmonary hypertension in systemic lupus erythematosusClin Med Insights Arthritis Musculoskelet Disord20114778622084605
- AkdoganAKilicLDoganIPulmonary hypertension in systemic lupus erythematosus: pulmonary thromboembolism is the leading causeJ Clin Rheumatol20131942142524263143
- RubinLJBadeschDBBarstRJBosentan therapy for pulmonary arterial hypertensionN Engl J Med200234689690311907289
- BadeschDBHillNSBurgessGSildenafil for pulmonary arterial hypertension associated with connective tissue diseasesJ Rheumatol2007342417242217985403
- OudizRJSchilzRJBarstRJTreprostinil, a prostacyclin analogue, in pulmonary arterial hypertension associated with connective tissues diseaseChest200412642042715302727
- TanakaEHarigaiMTanakaMKawaguchiYHaraMKamataniMPulmonary hypertension in systemic lupus erythematosus: evaluation of clinical characteristics and response to immunosuppressive treatmentJ Rheumatol20022928228711838845
- Gonzalez-LopezLCardona-MunozEGCelisATherapy with intermittent pulse cyclophosphamide for pulmonary hypertension associated with systemic lupus erythematosusLupus20041310511214995003
- SanchezOSitbonOJaisXSimonneauGHumbertMImmunosuppressive therapy in connective tissue diseases-associated pulmonary arterial hypertensionChest200613018218916840400
- JaisXLaunayDYaiciAImmunosuppressive therapy in lupus-and mixed connective tissue disease-associated pulmonary arterial hypertension: a retrospective analysis of twenty-three casesArthritis Rheum20085852153118240255
- Miyamichi-YamamotoSFukumotoYSugimuraKIntensive immunosuppressive therapy improves pulmonary hemodynamics and long-term prognosis in patients with pulmonary arterial hypertension associated with connective tissue diseaseCirc J2011752668267421873802
- KommireddySBhyravavajhalaSKurimetiKPulmonary arterial hypertension in systemic lupus erythematosus may benefit by addition of immunosuppression to vasodilator therapy: an observational studyRheumatology (Oxford)2015541673167925929760
- HenniganSChannickRNSilvermanGJRituximab treatment of pulmonary arterial hypertension associated with systemic lupus erythematosus: a case reportLupus20081775475618625655
- RobbinsIMGaineSPSchilzRTapsonVERubinLJLoydJEEpoprostenol for treatment of pulmonary hypertension in patients with systemic lupus erythematosusChest2000117141810631192
- ShiraiYYasuokaHTakeuchiTSatohTKuwanaMIntravenous epoprostenol treatment of patients with connective tissue disease and pulmonary arterial hypertension at a single centreMod Rheumatol2013231211122023359006
- DentonCPHumbertMRubinLBlackCMBosentan treatment for pulmonary arterial hypertension related to connective tissue disease: a subgroup analysis of the pivotal clinical trials and their open-label extensionsAnn Rheum Dis2006651336134016793845
- MokMYTsangPLLamYMLoYWongWSLauCSBosentan use in systemic lupus erythematosus patients with pulmonary arterial hypertensionLupus20071627928517439935
- HumbertMCoghlanJGGhofraniHARiociguat for the treatment of pulmonary arterial hypertension associated with connective tissue diseases: results from PATENT-1 and PATENT-2Ann Rheum Dis201610.1136/annrheumdis-2015-209087
- WangHGuoXLaiJPredictors of health-related quality of life in patients with systemic lupus erythematosus associated pulmonary arterial hypertensionClin Exp Rheumatol20163429129526941000
- KangKYJeonCHChoiSJSurvival and prognostic factors in patients with connective tissue disease-associated pulmonary hypertension by echocardiography: results from a Korean nationwide registryInt J Rheum Dis201510.1111/1756-185X.12645
- QianJWangYHuangCSurvival and prognostic factors of systemic lupus erythematosus-associated pulmonary arterial hypertension: a PRISMA-compliant systematic review and meta-analysisAutoimmun Rev20161525025726640159
- ChowSLChandranVFazelzadRJohnsonSRPrognostic factors for survival in systemic lupus erythematosus associated pulmonary hypertensionLupus20122135336422127457
