Abstract
Epithelial–mesenchymal transition (EMT) is thought to be a crucial event during the early metastasis of tumor cells. Transforming growth factor (TGF)-β1 is involved in the process of EMT in a variety of human malignancies. Matrix metalloproteinase (MMP)-9 plays an important role in tumor invasion and metastasis, and its expression is regulated by various growth factors, including TGF-β1, in different cell types. To date, the role of MMP-9 in TGF-β1-induced EMT in esophageal squamous cell carcinoma (ESCC) remains unclear. In this study, we aimed to elucidate the mechanism underlying MMP-9-mediated TGF-β1 induction of EMT in ESCC. We analyzed the expression of MMP-9, E-cadherin, and vimentin, in ESCC cells (EC-1), before and after the treatment with exogenous TGF-β1 or a broad spectrum MMP inhibitor, GM6001. Additionally, we analyzed the activity of MMP-9 in these cells and performed MMP-9 knockdown experiments. The results obtained in this study demonstrated that the treatment of EC-1 cells with TGF-β1 can induce EMT, together with the upregulation of vimentin and downregulation of E-cadherin expression in a time-dependent manner. The treatment with GM6001 was shown to attenuate TGF-β1-induced EMT. Furthermore, the exposure of EC-1 cells to TGF-β1 increased the expression and activity of MMP-9, while MMP-9 knockdown blocked TGF-β1-induced EMT and inhibited cell invasiveness and migration. Additionally, treatment with the recombinant human MMP-9 was shown to induce EMT and enhance ESCC cell invasion and metastasis. The obtained data suggest that the regulation of MMP-9 by TGF-β1 may represent a novel mechanism underlying TGF-β1-induced EMT in ESCC.
Introduction
Esophageal squamous cell carcinoma (ESCC) is one of the predominant malignant tumors in the world. Its incidence is very high in China, especially in Henan Province. Although a large number of different antitumor therapies are used for the treatment of ESCC patients, the prognosis is usually poor.Citation1,Citation2
Among the most prominent features of malignant tumors are the invasion and metastasis of cancer cells. Numerous studies showed that the epithelial–mesenchymal transition (EMT) is an important process in the embryonic development, which also plays a key role in the invasion and metastasis of a variety of malignant tumors originating from the epithelial cells.Citation3,Citation4 EMT is characterized by the dissociation of cell-to-cell contacts and the loss of cell adhesion and cell polarity, resulting in cells that gain mesenchymal properties.Citation5 During the process of EMT, epithelial markers, including E-cadherin and β-catenin, were shown to be downregulated, while the markers related to the mesenchymal phenotype, including vimentin, N-cadherin, and fibronectin, are upregulated,Citation5 which results in the induction of migratory and invasive properties of cells.Citation6 Therefore, it is very important to elucidate the mechanism underlying the EMT during the invasion and metastasis of ESCCs.
Transforming growth factor (TGF)-β1 is one of the pivotal factors regulating EMT, which is responsible for the initiation and maintenance of the EMT.Citation6–Citation8 Recently, a number of studies demonstrated that TGF-β1 is involved in the EMT in a wide variety of human malignancies, including oral, liver, pancreatic, and esophageal cancers.Citation9–Citation13 Furthermore, it was recently demonstrated that TGF-β1 can lead to an increase in the expression of matrix metalloproteinases (MMPs), such as MMP-2 and MMP-9, in breast and oral cancers.Citation14–Citation17 However, the mechanism of TGF-β1-induced EMT has not been completely understood. MMPs represent a family of zinc-containing proteolytic enzymes that break down the protein components of the extracellular matrix (ECM) and endothelial cell basement membrane.Citation18 MMPs and their specific inhibitors (TIMPs) play an important role in the progression of different types of solid and hematological malignancies by regulating cell growth, cancer cell activation, and immune functions.Citation19,Citation20 In addition to the ability of MMPs to remodel ECM, some of these molecules, such as MMP-3, MMP-9, MMP-14, and MMP-28, are known to induce EMT directly.Citation21–Citation25 However, to date, the role of MMP-9 in TGF-β1-induced EMT in ESCC remains unclear.
In this study, we showed that MMP-9 is involved in TGF-β1-induced EMT. MMP-9 is a downstream mediator of TGF-β1, and we demonstrated that the inhibition of its expression prevents TGF-β1-induced EMT and reduces cell invasion and metastasis. Furthermore, the upregulation of MMP-9 expression is sufficient to induce EMT and enhance cell invasion and metastasis in EC-1 cells. These data indicate that TGF-β1-induced EMT is mediated by MMP-9, which represents a previously unknown mechanism underlying TGF-β1-induced EMT in ESCC.
Materials and methods
Cell lines and cell culture
EC-1 cells (kindly provided by Professor Shihua Cao, University of Hong Kong) were cultured in RPMI 1640 medium (Solarbio, Beijing, People’s Republic of China) with 10% fetal bovine serum (FBS; Gemini Bio, West Sacramento, CA, USA), 100 U/mL penicillin, and 100 µg/mL streptomycin and incubated at 37°C in a humid atmosphere with 5% CO2. The cells were originally purchased from ATCC (Manassas, VA, USA) and that the research had been reviewed and approved by the Zhengzhou University Life Science Ethics Review Committee.
Cell treatment
EC-1 cells were seeded at a low density in six-well plates and maintained for 24 h in the complete medium. Afterward, they were starved overnight and washed in phosphate-buffered saline (PBS), and the medium was replaced with serum-free RPMI 1640 medium, serum-free medium containing 10 ng/mL recombinant human TGF-β1 (PeproTech, Rocky Hill, NJ, USA), serum-free medium containing 25 µmol/L broad spectrum MMP inhibitor GM6001 (Millipore, Darmstadt, Germany), and serum-free medium containing both TGF-β1 and GM6001. Additionally, to explore the role of MMP-9 in EMT, EC-1 cells were seeded at low density (2×105) in six-well plates for 24 h in the complete medium and starved overnight. Recombinant human MMP-9 protein (R&D Systems, Minneapolis, MN, USA) was activated using p-aminophenylmercuric acetate (APMA; Sigma-Aldrich, St Louis, MO, USA) at a final concentration of 1 mM at 37°C overnight, and the cells treated with this protein were incubated at 37°C for 48 h. Gelatin zymography was used to determine the presence of the active form of MMP-9.
Stable transfection
Human MMP-9 short hairpin RNA (shRNA) (GenePharma, Shanghai, People’s Republic of China) was used, and the target sequence was 5′-GCAGATTCCAAACCTTTGATT-3′. Briefly, 1 day before transfection, 2×105 cells/mL were seeded into six-well plates. When the confluence of 60%–80% was reached, EC-1 cells were transfected with 3 µg of MMP-9 shRNA or control shRNA, using Lipofectamine 2000 (Thermo Fisher Scientific, Waltham, MA, USA) and serum-free medium, according to the manufacturer’s instructions. After 6 h of incubation, the medium was replaced with fresh RPMI 1640 with 10% FBS, or medium containing TGF-β1, and the cells were cultured for additional 48 h.
Real-time polymerase chain reaction (PCR)
Total RNA was isolated from EC-1 cells incubated with different molecules using RNApure total RNA Kit (Aidlab, Beijing, People’s Republic of China), according to the manufacturer’s instructions. RNA was reverse transcribed into complementary DNA (cDNA) using HiScript II Q RT SuperMix Kit (Vazyme Biotech, Jiangsu Sheng, People’s Republic of China). Primer sequences used in this study are as follows: MMP-9 (forward [F]: 5′-CCAA CTACGACACCGACGAC-3′, reverse [R]: 5′-TGGAAG ATGAATGGAAACTGG-3′), E-cadherin (F: 5′-TGATTC TGCTGCTCTTGCTG-3′, R: 5′-CAAAGTCCTGG TCCTCTTCTCC-3′), vimentin (F: 5′-ATGTGGATGAGT CCAAGCCTGAC-3′, R: 5′-GAGTGGGTATCAACCAGAG GGAGT-3′), and β-actin (F: 5′-TGACGTGGACATC CGCAACAAAG-3′, R: 5′-CTGGAAGGTGGACAG CGAGG-3′). All reactions were performed using 7500 Fast Real-Time PCR System (Applied Biosystems, Foster City, CA, USA), with qPCR SYBR Green Master Mix (Vazyme Biotech), and normalized to β-actin messenger RNA (mRNA) levels.
Western blot analysis
Total proteins were isolated from the treated EC-1 cells using RIPA buffer (Solarbio) and stored at -80°C, according to the manufacturer’s instructions. Protein concentration was determined by measuring the absorbance (optical density, OD562), using BCA Protein Assay Kit (Biotech Well, Shanghai, People’s Republic of China). Briefly, 40 µg of total protein isolated from each sample was separated on 10%–15% sodium dodecyl sulfate (SDS)-polyacrylamide gels (PAGE) and then transferred to polyvinylidene difluoride membrane (Millipore) at 100 mV for 1.5–2 h. The blots were blocked and incubated with primary antibodies overnight at 4°C, using anti-MMP-9 (1:200; Cell Signaling Technology, Danvers, MA, USA), anti-E-cadherin (1:200; Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-vimentin (1:400), and anti-β-actin (1:1,000; ZsBio, Beijing, People’s Republic of China) antibodies. After washing thoroughly (3×10 min) with Tris-buffered saline–Tween 20 (TBST), the samples were incubated with goat anti-mouse secondary immunoglobulin G (IgG) (1:2,000; Bioss, Shanghai, People’s Republic of China) or goat anti-rabbit secondary IgG (1:2,000; Bioss) for 2 h at room temperature. The bands were detected using enhanced chemiluminescence method (BeyoECL Plus). All experiments were repeated three times.
Immunofluorescence analysis
ESCC EC-1 cells were seeded in six-well plates containing glass coverslips, and they were incubated overnight. After incubating EC-1 cells with different treatments for additional 48 h, cells were washed three times with PBS. Following this, they were fixed in 4% paraformaldehyde for 30 min and then permeabilized in 0.4% Triton X-100 for 10 min, washed three times with PBS, and blocked with 5% bovine serum albumin (BSA) for 30 min at 37°C. Next, the samples were incubated with primary anti-E-cadherin (1:100) and anti-vimentin (1:100) antibodies, at 4°C overnight, followed by the incubation with a CY-3-conjugated goat anti-rabbit secondary antibody (Biotech Well) for 2 h at 37°C and DAPI (Biotech Well) for 5 min at room temperature, in order to detect E-cadherin, vimentin, and cell nuclei. The cells were visualized using fluorescence microscopy (Leica Microsystems, Wetzlar, Germany).
Gelatin zymography
EC-1 cells were treated with TGF-β1, GM6001, or the combination of these two molecules for 48 h. Afterward, MMP-9 activity was determined by gelatin zymography, using Gelatin Zymo Electrophoresis Kit (Genmed Scientifics, Arlington, MA, USA), according to the manufacturer’s instructions.
Cell invasion assay
Cell invasion assay was performed using transwell inserts (Costar Transwell, Corning, People’s Republic of China). Matrigel-coated polycarbonate membranes (8 µm pore size) separated the upper and lower compartments of a transwell chamber. EC-1 cells, not transfected or transfected with MMP-9 shRNA, were incubated with previously described treatments, including serum-free medium, and afterward seeded into the upper compartments, while RPMI 1640 with 10% FBS was added to the lower compartments, as a chemoattractant to induce invasion. Afterward, the plates were incubated at 37°C for 48 h, the cells were fixed with methanol, stained for 20 min with 0.4% Trypan blue (Solarbio), and the number of cells that invaded through the membranes was counted using an optical microscope. Each experiment was performed in duplicate and repeated three times.
Scratch assay
Cell migratory ability was examined using the scratch assay. EC-1 cells, untransfected or transfected with MMP-9 shRNA, were incubated with different treatments and seeded in six-well plates. When 80%–100% of confluence was reached, cell monolayer was scratched with a 200 µL tip at the center of the wells. Following this, the cells were incubated in the presence or absence of TGF-β1 for 48 h, washed with PBS, and RPMI 1640 containing 0.2% FBS was added. After 48 h, the images were captured at 200× magnification under a inverted light microscope. At least three replicates for each treatment were used.
Statistical analysis
All statistical analyses were performed with analysis of variance (ANOVA) and two-tailed Student’s t-test, using SPSS 17.0 (IBM Corp., Armonk, NY, USA) software package. The results are presented as mean ± standard deviation (SD) of three replicate assays. P-values <0.05 were considered statistically significant.
Results
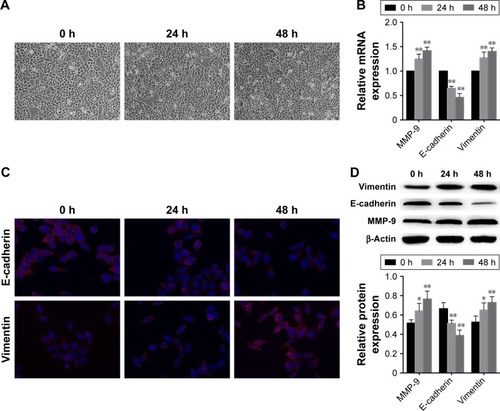
TGF-β1 induces change in the EMT-associated gene expression levels and alters ESCC cell morphology
In order to determine whether TGF-β1 induces EMT, we treated EC-1 cells with TGF-β1 and incubated them for 0 h, 24 h, and 48 h. Compared with the cells incubated for 0 h (control), EC-1 cells treated for 24 h or 48 h displayed morphological changes typical for EMT, as demonstrated by phenotypic transformation from the endothelial cobblestone shape to fibroblastic spindle-shaped morphology (). Real-time PCR analysis showed that E-cadherin levels were downregulated and vimentin expression was upregulated in EC-1 cells treated with TGF-β1, in comparison with those in the cells treated for 0 h (control) (). Immunofluorescence and Western blot analysis indicated that TGF-β1 treatment significantly decreased E-cadherin expression and increased vimentin expression as well (). The changes in E-cadherin and vimentin expression, at both mRNA and protein levels, in response to TGF-β1, were shown to be considerably time-dependent.
Figure 1 TGF-β1 induces EMT in EC-1 cells.
Abbreviations: TGF, transforming growth factor; EMT, epithelial–mesenchymal transition; mRNA, messenger RNA; MMP, matrix metalloproteinase; SD, standard deviation.
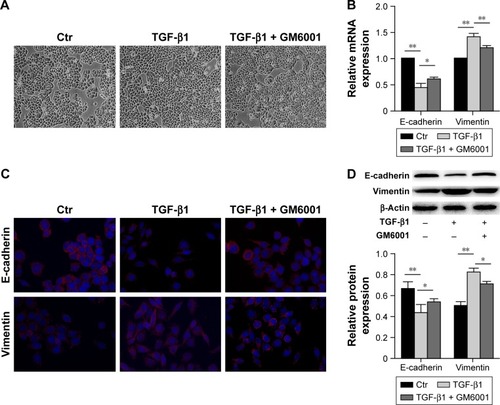
TGF-β1-induced EMT is abrogated by MMP inhibitor GM6001
To elucidate whether MMPs mediate TGF-β1-induced EMT, a broad spectrum MMP inhibitor GM6001 was used. Following the treatment of cells with this inhibitor for 48 h, we observed that TGF-β1-induced EMT was abrogated. EC-1 cells generally maintained endothelial cobblestone shape, but some cells showed fibroblastic spindle-shaped morphology (). Real-time PCR analysis showed that GM6001 inhibited the TGF-β1-induced decrease in E-cadherin expression and increase in vimentin expression compared with those in the TGF-β1-treated cells (). Consistent with this, immunofluorescence staining and Western blot analysis showed that the downregulation of E-cadherin and the induction of vimentin expression were inhibited by GM6001 ().
Figure 2 MMP inhibitor GM6001 inhibits TGF-β1-induced EMT.
Abbreviations: MMP, matrix metalloproteinase; TGF, transforming growth factor; EMT, epithelial–mesenchymal transition; mRNA, messenger RNA; SD, standard deviation.
MMP-9 mediates TGF-β1-induced EMT in ESCC
Gelatin zymography showed that MMP-9 activity was significantly increased following the treatment with TGF-β1. However, after the cells were treated with GM6001, MMP-9 activity was shown to be reduced (). Furthermore, real-time PCR and Western blot analysis demonstrated that a significant increase in MMP-9 mRNA and protein levels is induced in EC-1 cells treated with TGF-β1 in comparison with the untreated cells ().
Figure 3 MMP-9 mediates TGF-β1-induced EMT in ESCC.
Abbreviations: MMP, matrix metalloproteinase; TGF, transforming growth factor; EMT, epithelial–mesenchymal transition; ESCC, esophageal squamous cell carcinoma; shRNA, short hairpin RNA; SD, standard deviation; mRNA, messenger RNA.
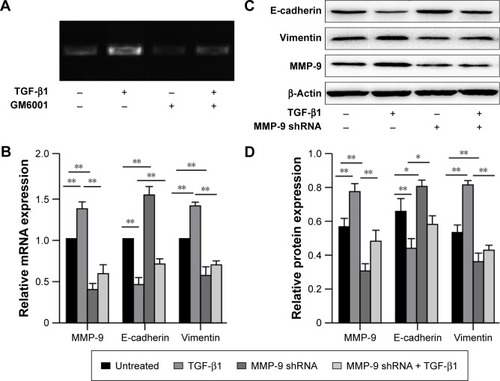
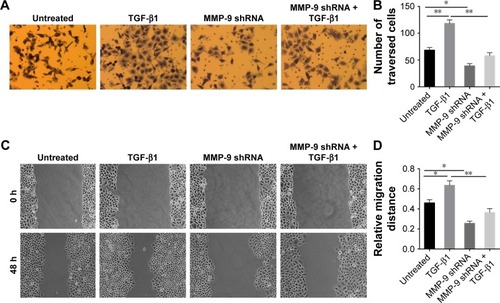
MMP-9 expression knockdown attenuates TGF-β1-induced EMT and inhibits cell invasiveness and migration
MMP-9 shRNA was used to inhibit the expression of endogenous MMP-9 in EC-1 cells. The inhibition of MMP-9 expression was confirmed in EC-1 cells at both mRNA and protein levels (). Furthermore, real-time PCR and Western blot analysis showed that MMP-9 knockdown significantly increases the expression of E-cadherin and decreases vimentin expression compared with those in the untreated cells. MMP-9 knockdown was shown to inhibit the effects of TGF-β1 on E-cadherin and vimentin expression ().
Next, we wanted to determine the role of MMP-9 in TGF-β1-induced tumor cell invasion and metastasis, using invasion assay and scratch assay, respectively. These experiments demonstrated that MMP-9 knockdown dramatically decreased invasive and metastatic potential of cells and blocked TGF-β1-induced cell invasion and metastasis ().
Figure 4 MMP-9 knockdown inhibits invasive and metastatic potential of EC-1 cells and blocks TGF-β1-induced cell invasion and metastasis.
Abbreviations: MMP, matrix metalloproteinase; TGF, transforming growth factor; SD, standard deviation; shRNA, short hairpin RNA.
MMP-9 induces EMT and enhances cell invasion and metastasis in ESCC
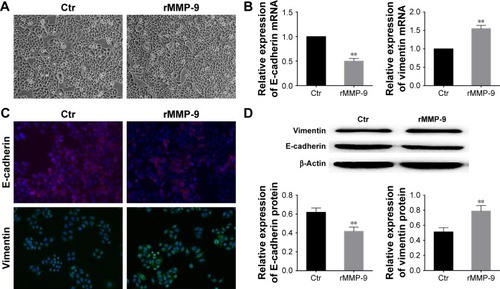
We investigated whether MMP-9 expression is sufficient to induce EMT. After treating the cells with recombinant human MMP-9 for 48 h, we observed morphological changes in cells, which changed from cuboid clustered epithelial cells to fibroblastic spindle-shaped cells (). Real-time PCR analysis showed a significant downregulation of E-cadherin and upregulation of vimentin expression (). Immunofluorescence and Western blot analysis showed similar results, and E-cadherin levels were shown to be decreased, while the levels of mesenchymal marker vimentin were increased in comparison with those in the control cells ().
Figure 5 Upregulation of MMP-9 expression induces EMT.
Abbreviations: MMP, matrix metalloproteinase; EMT, epithelial–mesenchymal transition; rMMP-9, recombinant MMP-9; SD, standard deviation; Ctr, control; mRNA, messenger RNA.
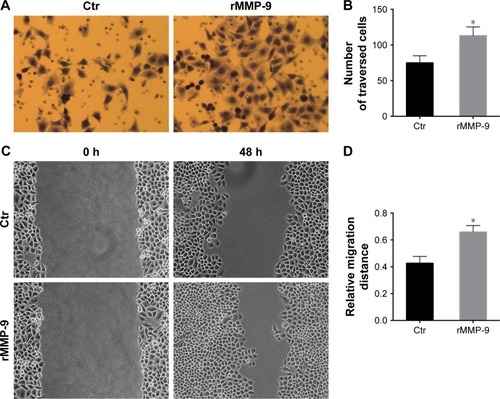
Cell invasion assays showed that the invasiveness of EC-1 cells treated with recombinant MMP-9 significantly increases, compared with that of the control cells (). Scratch assay results demonstrated that EC-1 cells treated with recombinant MMP-9 repopulated the wound area faster than the control cells ().
Figure 6 Upregulation of MMP-9 expression enhances EC-1 invasiveness and metastatic potential.
Abbreviations: MMP, matrix metalloproteinase; rMMP-9, recombinant MMP-9; SD, standard deviation; Ctr, control.
Discussion
Recent investigations demonstrated that EMT contributes to cancer progression, invasion, and metastasis.Citation6,Citation26 A growing number of studies showed that several growth factors, including TGF-β,Citation27,Citation28 epidermal growth factor (EGF),Citation29,Citation30 vascular endothelial growth factor (VEGF),Citation31 and hepatocyte growth factor (HGF),Citation32 induce the process of EMT, which is characterized by the loss of epithelial markers and the induction of the expression of mesenchymal markers. TGF-β1 signaling is induced following the binding of this protein to TGF-β receptor types I and II, which promotes the phosphorylation of SMAD protein family members (SMAD2 and SMAD3), and it binds a cytoplasmic protein SMAD4 and translocates to the nucleus, further activating multiple cellular responses, such as MMP expression.Citation33 Here, we demonstrated that TGF-β1 induces changes in the morphology of ESCC cells and their transition from epithelial to mesenchymal phenotype. This change was shown to be accompanied by the downregulation of E-cadherin and upregulation of vimentin expression, which is consistent with the data obtained in the previous studies.Citation12,Citation13
A previous study showed that TGF-β1 induces the expression of SNAIL in OKF4, OKF6, and UMSCC-1 cells, but not in SCC-25 cells, while SNAIL induces the expression of MMP-9 in oral squamous cell carcinoma (OSCC) cell.Citation15 Additionally, in OSCC cells, TGF-β1 effects on SLUG expression were shown to be mediated by ERK1/2-dependent pathways, and not PI3-kinase signaling, and SLUG was shown to promote OSCC cells invasion by increasing the expression of MMP-9.Citation16 Furthermore, TGF-β1 can promote breast cancer and liver cancer metastases by upregulating MMP-9 expression.Citation34,Citation35 In our study, we showed that TGF-β1 can induce the expression of MMP-9. Furthermore, gelatin zymography results demonstrated that MMP-9 activity is significantly increased in EC-1 cells after the treatment with TGF-β1. Following the treatment of cells with GM6001, MMP-9 activity was shown to be decreased, but MMP-2 expression was not detected (data not shown). The role of MMP-9 in TGF-β1-induced EMT was further examined by MMP-9 shRNA knockdown, and the obtained results showed that this significantly increases E-cadherin and decreases vimentin levels, inhibiting cell invasiveness and migration as well. Taken together, these data suggest that MMP-9 is the downstream target of TGF-β1 and that TGF-β1-induced downregulation of E-cadherin expression is largely mediated by MMP-9. One previous report indicated that MMP-9 is involved in TGF-β1-induced EMT in renal tubular cells, which agrees with our results.Citation36 However, the mechanism underlying the effects of TGF-β1 on MMP-9 expression remains undetermined. Interestingly, an increasing number of studies demonstrated that MMPs, such as MMP-2, MMP-9, and MMP-14, can stimulate TGF-β1 activity by cleaving latent TGF-β-binding protein-1.Citation37 MMP-8 was reported to cooperate with TGF-β1, stimulating EMT and enhancing the invasion and metastasis of hepatocellular carcinoma.Citation38
MMPs are divided into six categories: collagenases, gelatinases, stromelysins, matrilysins, membrane-type MMPs, and other MMPs.Citation19 The expression and activity of MMPs were shown to be increased in almost every type of human cancer; and this increase was shown to correlate with the advanced tumor stage, increased invasion and metastasis, and shortened survival.Citation20 MMP-9, also known as gelatinase B, plays a critical role in the processes that represent the hallmarks of cancer, including migration, invasion, and angiogenesis.Citation39–Citation41 During the EMT, MMPs are crucial for the regulation of cell behavior. The overexpression of stromelysin-1 (MMP-3) can induce EMT in mammary epithelial cells and increase the expression of RAC1, which can result in an increase in cellular reactive oxygen species levels and the subsequent upregulation of the EMT transcription factor SNAIL.Citation22 Epilysin (MMP-28) was shown to induce EMT and cell invasion through the proteolytic processing of latent TGF-β complexes into the active form in lung carcinoma cells.Citation25 Several other MMPs, including MMP-3, MMP-7, MMP-9, ADAM10, and MT1-MMP, were shown to be involved in E-cadherin cleavage.Citation42–Citation46 MMP-2, MMP-9, MMP-13, and MT1-MMP have also been implicated in the regulation of osteoblast, chondrocyte, and epithelial cell functions through TGF-β activation as well.Citation47–Citation50
In this study, we reported that the treatment of cells with the recombinant MMP-9 is sufficient for the reduction of E-cadherin levels and it induced migratory and invasive phenotype of these cells. This indicates that the upregulation of MMP-9 expression can promote the invasion and metastasis of ESCC. It was reported that MMP-8 regulates TGF-β1 expression through the activation of PI3K/Akt/Rac1 signaling.Citation38 but it remains unknown whether MMPs can also induce TGF-β1 expression.
Conclusion
The results obtained in this study showed a novel mechanism of EMT induction in ESCC, showing that TGF-β1 and MMP-9 play significant roles in the EMT and that MMP-9 is a mediator of TGF-β1-induced EMT in ESCC.
Acknowledgments
We thank Professor Shihua Cao (the University of Hong Kong) for providing the EC-1 cell line. This study was funded by the National Natural Science Foundation of China (grant number 81071970).
Disclosure
The authors report no conflicts of interest in this work.
References
- ParkinDMBrayFFerlayJPisaniPGlobal cancer statistics, 2002CA Cancer J Clin20055527410815761078
- LiYSunDLDuanYNAssociation of functional polymorphisms in MMPs genes with gastric cardia adenocarcinoma and esophageal squamous cell carcinoma in high incidence region of North ChinaMol Biol Rep201037119720519562509
- BoyerBVallesAMEdmeNInduction and regulation of epithelial-mesenchymal transitionsBiochem Pharmacol20006081091109911007946
- GuarionMEpithelial-mesenchymal transition and tumour invasionInt J Biochem Cell Biol200739122153216017825600
- LeeJMDedharSKalluriRThompsonEWThe epithelial-mesenchymal transition: new insights in signaling, development, and diseaseJ Cell Biol2006172797398116567498
- ThieryJPAcloqueHHuangRYNietoMAEpithelial-mesenchymal transitions in development and diseaseCell2009139587189019945376
- XuJLamouilleSDerynckRTGF-beta-induced epithelial to mesenchymal transitionCell Res200919215617219153598
- IkushimaHMiyazonoKTGF signalling: a complex web in cancer progressionNat Rev Cancer201010641542420495575
- QuanJElhousinyMJohnsonNWGaoJTransforming growth factor-beta1 treatment of oral cancer induces epithelial-mesenchymal transition and promotes bone invasion via enhanced activity of osteoclastsClin Exp Metastasis201330565967023378237
- ReichlPHaiderCGrubingerMMikulitsWTGF-beta in epithelial to mesenchymal transition and metastasis of liver carcinomaCurr Pharm Des201218274135414722630087
- EllenriederVHendlerSFBoeckWTransforming growth factor beta1 treatment leads to an epithelial-mesenchymal transdifferentiation of pancreatic cancer cells requiring extracellular signal-regulated kinase 2 activationCancer Res200161104222422811358848
- ZhangHYLiSSTransforming growth factor-β1 induced epithelial-mesenchymal transition in human esophageal squamous cell carcinoma via PTEN/PI3K signaling pathwayOncol Rep20143252134214225175594
- PangLLiQWeiCTGF-β1/Smad signaling pathway regulates epithelial-to-mesenchymal transition in esophageal squamous cell carcinoma: in vitro and clinical analyses of cell lines and nomadic Kazakh patients from northwest Xinjiang, ChinaPLoS One2014912e11230025464508
- KatsunoYLamouilleSDerynckRTGF-β signaling and epithelial–mesenchymal transition in cancer progressionCurr Opin Oncol2013251768423197193
- SunLDiamondMEOttavianoAJJosephMJAnanthanarayanVMunshiHGTransforming growth factor-{beta}1 promotes matrix metalloproteinase-9-mediated oral cancer invasion through Snail expressionMol Cancer Res200861102018234959
- JosephMJDangi-GarimellaSShieldsMASlug is a down-stream mediator of transforming growth factor-beta1-induced matrix metalloproteinase-9 expression and invasion of oral cancer cellsJ Cell Biochem2009108372673619681038
- SinpitaksakulSNPimkhaokhamASanchavanakitNPavasantPTGF-β1 induced MMP-9 expression in HNSCC cell lines via Smad/MLCK pathwayBiochem Biophy Res Commun20083714713718
- StamenkovicIExtracellular matrix remodelling: the role of matrix metalloproteinasesJ Pathol2003200444846412845612
- ChaudharyAKPandyaSGhoshKNadkarniAMatrix metalloproteinase and its drug targets therapy in solid and hematological malignancies: an overviewMutat Res2013753172323370482
- KapoorCVaidyaSWadhwanVHiteshKaurGPathakASeesaw of matrix metalloproteinases (MMPs)J Cancer Res Ther2016121283527072206
- RadiskyDCLevyDDLittlepageLERac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instabilityNature2005436704712312716001073
- OrlichenkoLSRadiskyDCMatrix metalloproteinases stimulate epithelial-mesenchymal transition during tumor developmentClin Exp Metastasis200825659360018286378
- YangCCZhuLFXuXHNingTYYeJHLiuLKMembrane Type 1 Matrix Metalloproteinase induces an epithelial to mesenchymal transition and cancer stem cell-like properties in SCC9 cellsBMC Cancer20131317123548172
- PangLLiQLiSMembrane type 1-matrix metalloproteinase induces epithelial-to-mesenchymal transition in esophageal squamous cell carcinoma: observations from clinical and in vitro analysesSci Rep2015622179
- IllmanSALehtiKKeski-OjaJLohiJEpilysin (MMP-28) induces TGF-beta mediated epithelial to mesenchymal transition in lung carcinoma cellsJ Cell Sci2006119pt 183856386516940349
- ChangCJChaoCHXiaWp53 regulates epithelial-mesenchymal transition and stem cell properties through modulating miRNAsNat Cell Biol201113331732321336307
- XiangJFuXRanWWangZGrhl2 reduces invasion and migration through inhibition of TGFβ-induced EMT in gastric cancerOncogenesis201761e28428067907
- XuanXZengQLiYAkt-mediated transforming growth factor-β1-induced epithelial-mesenchymal transition in cultured human esophageal squamous cancer cellsCancer Gene Ther201421623824524874843
- CuiLSongJWuLRole of Annexin A2 in the EGF-induced epithelial-mesenchymal transition in human CaSki cellsOncol Lett201713137738328123570
- KimJKongJChangHKimHKimAEGF induces epithelialmesenchymal transition through phospho-Smad2/3-Snail signaling pathway in breast cancer cellsOncotarget2016751850218503227829223
- LuoMHouLLiJVEGF/NRP-1axis promotes progression of breast cancer via enhancement of epithelial-mesenchymal transition and activation of NF-κB and β-cateninCancer Lett2016373111126805761
- JiaoDWangJLuWCurcumin inhibited HGF-induced EMT and angiogenesis through regulating c-Met dependent PI3K/Akt/mTOR signaling pathways in lung cancerMol Ther Oncolytics201631601827525306
- WendtMKSchiemannWPTherapeutic targeting of the focal adhesion complex prevents oncogenic TGF-beta signaling and metastasisBreast Cancer Res2009115R6819740433
- GomesLRTerraLFWailemannRAMLabriolaLSogayarMCTGF-β1 modulates the homeostasis between MMPs and MMP inhibitors through p38 MAPK and ERK1/2 in highly invasive breast cancer cellsBMC Cancer2012122622260435
- LiJYangBZhouQAutophagy promotes hepatocellular carcinoma cell invasion through activation of epithelial–mesenchymal transitionCarcinogenesis20133461343135123430956
- TanTKZhengGHsuTTMacrophage matrix metalloproteinase-9 mediates epithelial-mesenchymal transition in vitro in murine renal tubular cellsAm J Pathol201017631256127020075196
- TianMNeilJRSchiemannWPTransforming growth factor-beta and the hallmarks of cancerCell Signal201123695196220940046
- QinGLuoMChenJReciprocal activation between MMP-8 and TGF-β1 stimulates EMT and malignant progression of hepatocellular carcinomaCancer Lett20163741859526872724
- HanahanDWeinbergRAHallmarks of cancer: the next generationCell201114464667421376230
- KessenbrockKPlaksVWerbZMatrix metalloproteinases: regulators of the tumor microenvironmentCell20101411526720371345
- GialeliCTheocharisADKaramanosNKRoles of matrix metalloproteinases in cancer progression and their pharmacological targetingFEBS J20112781162721087457
- LochterAGalosySMuschlerJFreedmanNWerbZBissellMJMatrix metalloproteinase stromelysin-1 triggers a cascade of molecular alterations that leads to stable epithelial-to-mesenchymal conversion and a premalignant phenotype in mammary epithelial cellsJ Cell Biol19971397186118729412478
- NoeVFingletonBJacobsKRelease of an invasion promoter E-cadherin fragment by matrilysin and stromelysin-1J Cell Sci2001114pt 111111811112695
- SymowiczJAdleyBPGleasonKJEngagement of collagen-binding integrins promotes matrix metalloproteinase-9-dependent E-cadherin ectodomain shedding in ovarian carcinoma cellsCancer Res20076752030203917332331
- MaretzkyTReissKLudwigAADAM10 mediates E-cadherin shedding and regulates epithelial cell-cell adhesion, migration, and beta-catenin translocationProc Natl Acad Sci U S A2005102269182918715958533
- CovingtonMDBurghardtRCParrishARIschemia-induced cleavage of cadherins in NRK cells requires MT1-MMP (MMP-14)Am J Physiol Renal Physiol20062901F43F5116077081
- YuQStamenkovicICell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesisGenes Dev200014216317610652271
- KarsdalMALarsenLEngsigMTMatrix metalloproteinase-dependent activation of latent transforming growth factor-beta controls the conversion of osteoblasts into osteocytes by blocking osteoblast apoptosisJ Biol Chem200227746440614406712226090
- MuDCambierSFjellbirkelandLThe integrin alpha(v)beta8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-beta1J Cell Biol2002157349350711970960
- DangeloMSarmentDPBillingsPCPacificiMActivation of transforming growth factor beta in chondrocytes undergoing endochondral ossificationJ Bone Miner Res200116122339234711760850
