Abstract
The discovery of epidermal growth-factor receptor (EGFR)-activating mutations and the introduction of oral EGFR tyrosine kinase inhibitors (EGFR-TKIs) have expanded the treatment options for patients with non-small cell lung cancer. The first two reversible EGFR-TKIs, erlotinib and gefitinib, are approved for use in the first-line setting in patients with known EGFR-activating mutations and in the second- and third-line settings for all NSCLC patients. These first-generation EGFR-TKIs improve progression-free survival when compared to chemotherapy in patients with EGFR-activating mutations in the first-line setting. However, nearly all patients develop resistance to EGFR-directed agents. There is a need for further therapy options for patients with disease progression after treatment with reversible EGFR-TKIs. Afatinib is an irreversible ErbB family blocker that inhibits EGFR, HER2, and HER4. In vitro and in vivo, afatinib have shown increased inhibition of the common EGFR-activating mutations as well as the T790M resistance mutation when compared to erlotinib and gefitinib. Clinically, afatinib has been evaluated in the LUX-Lung series of trials, with improvement in progression-free survival reported in patients with EGFR-activating mutations in both first- and second-/third-line settings when compared to chemotherapy. Further investigation is needed to determine the precise role that afatinib will play in the treatment of patients with non-small cell lung cancer and EGFR-activating mutations.
Introduction
Lung cancer is the leading cause of cancer death globally, with a low 5-year survival rate of 15%.Citation1 Non-small cell lung carcinoma (NSCLC) is the most common type, comprising 85% of lung cancers.Citation1 Risk factors for lung cancer are well described, and include first- and secondhand cigarette smoking,Citation2,Citation3 radon gas,Citation4 asbestos,Citation5 and other airborne chemicals and particulates.Citation1 However, among lung cancer patients who have not been exposed to traditional risk factors, a substantial proportion are found to have oncogene-driven malignancies, including patients whose tumors are driven by epidermal growth-factor receptor (EGFR).
The vast majority of NSCLC patients are diagnosed at advanced stages, at which point locoregional therapy is not an option.Citation1 Until recently, cytotoxic chemotherapy administered intravenously was the only treatment option for these patients, with unsatisfactory median overall survival rates in the 12-month range.Citation6 With the discovery of EGFR mutations, and subsequent introduction of oral EGFR tyrosine kinase inhibitors (EGFR-TKIs), the therapeutic options have expanded for NSCLC patients.
Epidermal growth factor receptor-activating mutations
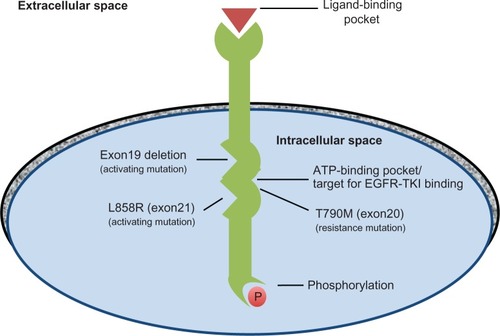
The EGFR family of cell surface-receptor tyrosine kinases controls the intracellular signaling pathways that promote cell growth, proliferation, differentiation, and migration.Citation7 Members of the ErbB family include EGFR (HER1/ErbB1), HER2 (ErbB2), HER3 (ErbB3), and HER4 (ErbB4). These cell-membrane receptors are composed of an extracellular domain containing a ligand-binding pocket and an intracellular catalytic domain.Citation8 Binding of extracellular growth-factor ligands causes dimerization of the receptors, leading to homo- or heterodimers.Citation9 Formation of these dimers activates the receptors’ tyrosine kinase activity, initiating intracellular signaling cascades.
Lung adenocarcinoma with activating EGFR mutations is now a well-described molecular subgroup of lung adenocarcinoma. Multiple aberrations in the signal-transduction pathways controlled by EGFR have been implicated in NSCLC. For example, mutations in genes encoding EGFR pathway proteins result in dysregulation of the proteins’ tyrosine kinase activity and lead to proliferation, survival, and dissemination of malignant cells.Citation10,Citation11 Elevated gene copy number and increased expression of the receptor proteins have also been described.Citation11
Multiple specific EGFR-activating mutations have been identified, including short in-frame single nucleotide mutations, in-frame duplications/insertions, and single-nucleotide substitutions, all surrounding the adenosine triphosphate (ATP)-binding pocket.Citation12 The most common EGFR mutations in patients with lung adenocarcinoma are deletions in exon 19 (the LREA deletion) and a single amino acid substitution in exon 21 – L858R. These mutations are located within the catalytic domain and result in constitutive EGFR activation ().Citation8 Exon 19 deletion and exon 21 L858R mutation account for 10%–15% of Caucasian patients and 50% of Asian patients with NSCLC. Less common mutations include L861Q in exon 21 and G719X in exon 18.Citation13 While EGFR-activating mutations occur at a higher prevalence in certain populations, such as females, never-smokers, and Asians, clinical characteristics alone cannot be used to predict EGFR status, and National Comprehensive Cancer Network (NCCN) guidelines recommend mutational analysis of tumor tissue to verify the presence of EGFR mutations prior to initiating EGFR-directed therapy. Roughly 20,000 patients in the United States are diagnosed with lung adenocarcinoma with activating EGFR mutations yearly.
The era of EGFR-TKIs
There is evidence that tumors with EGFR-activating mutations become completely dependent on EGFR to activate downstream intracellular signaling cascades. When inhibited by EGFR-TKIs, the tumor cells are unable to replicate and undergo apoptosis.Citation14 TKIs compete with ATP at the receptor intracellular catalytic domain, thus preventing ATP binding, autophosphorylation, and downstream intracellular signaling.Citation9,Citation15 Erlotinib and gefitinib, the first-generation EGFR-TKIs, bind reversibly to the kinase domain and effectively inhibit both wild-type and mutated EGFR.Citation13
Initial FDA approval for erlotinib in 2004 was based on the results of the BR21 trial, a phase III international, randomized, double-blind, placebo-controlled trial comparing erlotinib 150 mg daily plus best supportive care (BSC) with BSC alone in second- and third-line settings in 731 unselected patients with stage IIIB or IV NSCLC and Eastern Cooperative Oncology Group performance status of 0–3.Citation16 The response rate (RR) to erlotinib was 9% versus 1% for placebo (P < 0.001). Progression-free survival (PFS) was longer in the erlotinib group, at 2.2 months versus 1.8 months for placebo (P < 0.001). Note that EGFR mutation testing was not part of this trial.
As the biology of EGFR-activating mutations was better clarified, first-generation EGFR-TKIs were tested specifically in patients with EGFR-activating mutations. Tumors with activating EGFR mutations were found to have unique sensitivity to targeted therapy with EGFR-TKIs,Citation17,Citation18 with RRs around 75% in the first-line setting,Citation19,Citation20 a vast improvement over the 9% seen in unselected populations. Some data suggest that patients with EGFR exon 19 deletions are more susceptible to the activity of reversible EGFR-TKIs compared to those with the exon 21 L858R mutation.Citation18,Citation21
Further studies then compared first-generation EGFR-TKIs (erlotinib and gefitinib) to chemotherapy in patients with EGFR-activating mutations in advanced NSCLC. In the first-line setting, a European randomized trial, EURTAC, compared erlotinib 150 mg daily to platinum-containing chemotherapy regimens (cisplatin or carboplatin with docetaxel or gemcitabine) in 174 patients with advanced NSCLC. PFS was 9.7 months in the erlotinib group versus 5.2 months in the chemotherapy group. There was no difference in overall survival (OS). There were fewer adverse events in patients treated with erlotinib.Citation22 Similar results were reported in an analogous trial in Chinese patients – OPTIMAL.Citation23 Based on these studies, the NCCN guidelines were amended in 2011 to recommend erlotinib for first-line use in patients with documented EGFR mutations.
Gefitinib is approved in the European Union for use in advanced-stage EGFR-mutated NSCLC.Citation24 Its approval is based on demonstrated improved PFS when compared to chemotherapy in the first-line setting for Asian patients with EGFR mutations in three phase III randomized controlled trials (IPASS, NEJ002, and WJTPG3405).Citation25–Citation27 While gefitinib is not approved in the United States, the NCCN guidelines comment that “in areas of the world where gefitinib is available, it may be used in place of erlotinib.”Citation1
At the present time, erlotinib and gefitinib are used in the first-line treatment of patients with advanced NSCLC and EGFR-activating mutations. Erlotinib and gefitinib can also be used in second- and third-line settings in unselected patients, regardless of EGFR mutation status.Citation1 While RR and PFS in the EGFR-mutated population favors the use of EGFR-TKIs as compared to chemotherapy in the first-line setting, disease progression typically occurs after a median of 10–14 months on an EGFR-TKI.Citation25,Citation28 Once progression occurs, further treatment options are limited, particularly for patients with moderate to poor performance status who will be unable to tolerate toxicities from cytotoxic chemotherapy. Thus, there is a need for therapy options after progression on first-generation anti-EGFR agents.
Resistance to first-generation EGFR-TKIs
Nearly all EGFR-mutated patients eventually develop resistance to reversible EGFR-TKIs after a median of 14 months.Citation28 In clinical practice, it is not always feasible to obtain tissue sampling with EGFR testing at the time of progression. For these reasons, Jackman et alCitation29 proposed criteria to define acquired resistance that have been used in multiple clinical studies.
The Jackman criteria are as follows: patients who have a tumor known to harbor an EGFR-activating mutation (such as exon 19 deletion or exon 21 L858R mutation, amongst others), or show objective clinical benefit from treatment with EGFR-TKI as defined by objective response or durable stable disease (>6 months), and then have systemic progression of disease while on continuous treatment with EGFR-TKI should be considered to have acquired resistance. These criteria have been noted to have a positive predictive value of 66% for EGFR-sensitizing mutations.Citation29
There are multiple known mechanisms of resistance to first-generation EGFR-TKIs. Most mechanisms are thought to be secondary (acquired). The most common secondary resistance mutation is the T790M missense mutation in exon 20, which accounts for 50%–60% of patients with disease progression while on a first-generation EGFR-TKI.Citation30–Citation32 The T790M mutation is referred to as the gatekeeper mutation, as it occurs within the ATP-binding site in a similar location to known resistance mutations in other tyrosine kinases ().Citation8,Citation14 It is hypothesized to interfere with first-generation EGFR-TKI binding by steric hindrance; the T790M mutation produces a bulky methionine side chain in the receptor kinase domain.Citation33 Besides T790M, other secondary resistance mutations include D761Y in exon 19,Citation34 T854A in exon 21,Citation35 and L747S in exon 19.Citation36
An additional mechanism of resistance is amplification of MET tyrosine kinase, which can occur in up to 22% of patients and can coexist with or be independent of EGFR T790M.Citation37 Less common resistance mechanisms include histologic transformation to a small-cell carcinoma, occurring in up to 14% of patients resistant to EGFR-TKIs,Citation31,Citation32 and morphologic changes consistent with an epithelial– mesenchymal transition, the therapeutic implications of which are unknown.Citation38
There are also reports of primary resistance genotypes, including T790M missense mutation, in a small subset of patients.Citation39 It has been hypothesized that low levels of T790M in the presence of common activating mutations might reduce effectiveness of reversible EGFR-TKIs in first-line treatment.Citation39–Citation41 Indeed, up to 20%–30% of EGFR-mutated patients do not respond to first-generation EGFR-TKIs.Citation18,Citation25
Attempts to overcome resistance
Currently, there is no standard option for advanced NSCLC patients who experience progression after treatment with a reversible EGFR-TKI, and patients who are candidates for further therapy are typically treated with cytotoxic chemotherapy or enrolled in clinical trials investigating novel agents for acquired resistance. Some have advocated continuing an EGFR-TKI, either the same medication or switching to the other first-generation option;Citation42–Citation44 however, there is no consensus surrounding this practice. The rationale for continuing a first-generation TKI is that many tumors remain addicted at least in part to the EGFR signaling pathway despite acquired resistance.Citation42 Riely et alCitation42 demonstrated decreased positron emission tomography avidity and tumor size with reintroduction of an EGFR-TKI even after progression on an EGFR-TKI.
Many drugs have been studied in patients who progressed after treatment with a reversible EGFR-TKI, including XL-647,Citation45 dasatinib,Citation46 and neratinib,Citation47 with little success. Combinations of therapy such as cetuximab plus erlotinibCitation48 and gefitinib plus everolimusCitation42 have also been tried. The most promising drug thus far has been afatinib (BIBW2992; Boehringer-Ingelheim Pharma, Ingelheim, Germany),Citation49,Citation50 an ErbB family blocker with reported in vitro and in vivo activity against EGFR mutant tumors harboring exon 19 deletions, exon 21 L858R mutations and the exon 20 T790M “resistance” mutations.
Pharmacology
Afatinib is a highly selective, irreversible inhibitor of EGFR, ErbB2/HER2, and ErbB4/HER4.Citation49 Like gefitinib and erlotinib, afatinib is an aniline–quinazoline derivative.Citation50 Afatinib covalently binds directly to the ATP-binding site in the kinase domains of both EGFR (Cys 773) and HER2 (Cys 805).Citation49 The irreversible, covalent binding of afatinib leads to longer suppression of receptor kinase activity than with reversible first-generation EGFR-TKIs, as the kinase activity is suppressed until the synthesis of new receptors.Citation9 Afatinib further improves on the activity of first-generation EGFR-TKIs by its activity against multiple receptors. The irreversible binding of afatinib to HER2 inactivates the preferred dimerization partner of EGFR, preventing the dimer formation that promotes the receptors’ tyrosine kinase activity.Citation33,Citation49
Afatinib has shown preclinical activity in both first-line and second-line settings. Both in vivo and in vitro models have shown that afatinib has increased affinity for the EGFR L858R mutation compared to the first-generation EGFR-TKIs.Citation51 In cell-culture models, acquired resistance may develop at a slower rate when irreversible or second-generation EGFR-TKIs such as afatinib are used in the first-line setting.Citation52 Additionally, afatinib has higher potency than reversible EGFR-TKIs in reducing survival of NSCLC cell lines with the T790M resistance mutation ()Citation49 and in cell lines with the less common secondary resistance mutation T854A.Citation35 Finally, afatinib has shown activity in xenograft models with EGFR L858R/T790M double-mutant murine lung tumor.Citation49
Table 1 Inhibition of EGFR cell lines by afatinib compared to erlotinib as shown by EC50 values
The recommended phase II dose of afatinib is 50 mg orally daily based on phase I trials in patients with advanced solid tumor malignancies as well as specifically in patients with advanced NSCLC.Citation50,Citation53,Citation54 At 50 mg, more than 90% of patients experienced a treatment-related adverse event, but dose-limiting adverse events were experienced in an acceptable number of patients.Citation50,Citation53 Because the severity (but not the overall incidence) of adverse events increases with dose increases from 40 mg daily to 50 mg daily, some phase II trials begin at a starting dose of 40 mg daily.Citation54–Citation56
To maximize plasma drug concentrations, afatinib should be taken while fasting.Citation50 Maximal plasma concentrations are attained 3–6 hours after drug administration.Citation50,Citation57 With once-daily dosing, afatinib reaches a steady state after 7 daysCitation50 and has a half-life of 30–40 hours.Citation53 Afatinib undergoes minimal metabolism and has no identified major circulating metabolites. It does not require dose adjustment for renal impairment. Unlike erlotinib, there is no detectable cytochrome P450-mediated metabolism of afatinib.Citation58
Safety and tolerability
EGFR is expressed in the epithelium; it helps maintain mucosal integrity and promote mucosal repair in the gut and maintains the protective barrier of the skin.Citation56,Citation57 Therefore, the most common treatment-related adverse events of EGFR-TKIs in general, and afatinib in particular, are gastrointestinal (GI) and cutaneous side effects, specifically diarrhea and rash.Citation22,Citation55
Almost all patients experience at least one treatment-related adverse event when receiving afatinib therapy, with 90% of patients experiencing either a GI or cutaneous adverse event.Citation50,Citation53,Citation55,Citation57 GI side effects include diarrhea (95%), nausea, vomiting, stomatitis, and decreased appetite. Cutaneous adverse events include rash, acne, dry skin, folliculitis, and palmar-plantar disorders. The rash is usually located on the face and trunk; when severe, it can cause ulceration and desquamation.Citation57 In combined data of treatment-related adverse events from recent phase II/III trials, 88% of patients had diarrhea and 81% experienced a rash (n = 489).Citation55,Citation61 Of these patients, the majority (>80%) had grade 1 or 2, and none had grade 4, adverse events.Citation55,Citation61 Both GI and cutaneous adverse events are usually manageable with supportive care, dose reduction, or interruption of treatment.Citation50,Citation57,Citation59 Afatinib was associated with possible treatment-related interstitial lung disease (4/129 patients) in only one study,Citation55 similar to the infrequent reports of interstitial pneumonia, pneumonitis, acute respiratory distress syndrome, pulmonary fibrosis, and alveolitis associated with erlotinib.Citation60 In phase II/III trials, afatinib is associated with a dose-reduction rate of 38%–67%Citation55,Citation56,Citation61 and a drug-discontinuation rate of 8%–20%.Citation55,Citation62
Efficacy studies
Clinically, afatinib has shown promise in the LUX-Lung series of trials. The complete series of afatinib trials in advanced NSCLC is summarized in .
Table 2 Clinical trials investigating afatinib for advanced stage non-small cell lung carcinoma
LUX-Lung 1 was a phase IIb/III study of 585 patients with stage IIIb or IV NSCLC (adenocarcinoma) who progressed on chemotherapy including at least one platinum-based regimen and at least 12 weeks of erlotinib or gefitinib.Citation61 Patients were randomized to afatinib 50 mg/day plus BSC or placebo plus BSC. Patients were treated until disease progression or undue toxicity. While the primary end point of OS was not statistically significant, OS of 10.78 months in the afatinib group versus 11.96 months in the placebo group (hazard ratio [HR] 1.077, P = 0.74), there was a statistically significant difference in the secondary end point of PFS in favor of afatinib. Median PFS in the group receiving afatinib was 3.3 months versus 1.1 months for patients who received placebo (HR 0.38, P < 0.0001). Partial RR was 7% in the afatinib group versus 0.5% in the placebo group (P < 0.01). Disease-control rate was 58% in the afatinib group versus 19% in the placebo group (P < 0.0001).
There are several potential reasons why LUX-Lung 1 did not show a difference in OS for afatinib. First, the study design was based on the assumption that the control-group OS would be a median of 4.7 months, as observed in the second-line and third-line phase III trial of erlotinib,Citation16 but instead OS survival in the placebo group surprisingly exceeded 10 months. This could be attributable to the additional therapies given after progression on the trial. Notably, more patients in the placebo group (79% versus 68%) received additional chemotherapy upon progression. In an exploratory analysis of the 191 patients who did not receive subsequent systemic treatment upon progression on this trial, there was a survival advantage for patients who received afatinib over placebo (5.8 vs 4.6 months, HR 0.65).
It is important to note that EGFR mutation status was not required for study entry, and so the number of patients with EGFR mutations is unknown. Less than half of the patients in the study had complete or partial response to previous reversible EGFR-TKI therapy, less than would be expected if they all had EGFR-activating mutations. Indeed, a more robust improvement in PFS was seen in the 96 patients who were known to harbor EGFR-activating mutations. Similarly, when analyzing patients who met Jackman criteria for acquired resistance,Citation29 the PFS difference was 4.5 months for those treated with afatinib versus 1.0 month for those who received placebo, suggesting that afatinib may have its greatest impact in subgroups of patients with EGFR mutations.Citation61
LUX-Lung 2 was a phase II open-label, single-arm trial in 129 patients with stage IIIb/IV adenocarcinoma of the lung with confirmed EGFR-activating mutations.Citation55 Sixty-one patients received afatinib as first-line treatment, and 68 patients received afatinib as second-line treatment after progressing following cytotoxic therapy. No patients had been exposed to prior EGFR-TKIs. The patients continued on afatinib 50 mg/day (later decreased to 40 mg/day for improved tolerability) until progression or undue toxicity.
At median follow-up of 22 months, planned analysis was performed. The primary end point, overall RR, was 61%. Notably, 66% of those with exon 19 deletion or exon 21 L858R mutations had a response to treatment, while only 39% of those with less common EGFR mutations did. The vast majority (87%) of responses occurred within 8 weeks. There was no difference in RR based on prior chemotherapy. The median response duration was 12.9 months by independent assessment and 14 months by investigator assessment. Median PFS was slightly shorter in the subset of patients with less common mutations, 10.1 months (95% CI 8.12–13.80 months), and slightly longer in those for whom afatinib was first-line treatment. These results are similar to those seen in analogous trials of first-generation EGFR-TKIs. The median OS was 23.3 months (95% CI 18.53–38.01 months) in those patients receiving afatinib in the second-line setting and was not reached for patients who received afatinib as first-line treatment.
The results of the LUX-Lung 3 trial were presented at the annual American Society of Clinical Oncology meeting in June 2012. In this phase III trial, 345 patients with EGFR mutation-positive advanced NSCLC were randomized to receive afatinib or cisplatin/pemetrexed as first-line therapy. After a median follow-up of 8 months, PFS in the afatinib group was 11.1 months compared to 6.9 months in the chemotherapy arm (HR 0.58, P = 0.0004). Among the 308 patients with the common mutations exon 19 deletion or exon 21 L858R mutation, the difference in PFS was even more striking: 13.6 months with afatinib compared to 6.9 months in the chemotherapy group (HR 0.47, P < 0.0001). OS data will be available in 2 years.Citation62
Upcoming and ongoing trials of afatinib include additional single-agent afatinib trials in the LUX-Lung series () as well as a phase II trial of afatinib in the third-line treatment of EGFR wild-type advanced NSCLCCitation63 and a phase Ib/II combination trial with afatinib and cetuximab after progression on a first-generation EGFR-TKI. Thus far, results have been promising in this latter trial, with disease control reported in the first 26 patients, including 36% with partial responses and four out of 13 responses in T790M-mutated patients.Citation64
Patient-focused perspectives
Despite the frequency of side effects, patients report improved quality of life with afatinib treatment.Citation59,Citation61 When compared with placebo, patients with advanced, non-small cell lung cancer treated with afatinib (vs placebo) reported statistically significant improvement in cough (46% vs 25%), dyspnea (51% vs 36%), and pain (50% vs 32%).Citation61 Preliminary results from LUX-Lung 3 comparing afatinib versus cisplatin and pemetrexed as first-line treatment in patients with advanced lung adenocarcinoma demonstrate a statistically significant delay in onset of cough (HR 0.60) and dyspnea (HR 0.68) with afatinib treatment.Citation62 LUX-Lung 3 also demonstrated improvement in health-related quality of life with afatinib compared to chemotherapy. A higher proportion of patients treated with afatinib (vs placebo) had a 10-point or more improvement in cough (67% vs 60%), dyspnea (64% vs 50%), and pain (59% vs 48%) when analyzed using the European Organisation for Research and Treatment of Cancer (EORTC) standardized quality-of-life questionnaire for lung cancer (QLQ-LC13).Citation65 Quality of life and its determinants were evaluated using the EORTC QLQ-C30 questionnaire, and patients treated with afatinib experienced improvements in their overall well-being and physical, cognitive, and role functioning compared with chemotherapy (P < 0.05).Citation65
Conclusion
The first-generation reversible EGFR-TKIs erlotinib and gefitinib have yielded impressive clinical benefits for patients with EGFR-mutated NSCLC. Unfortunately, these benefits are transient due to the mutability of tumor-cell genomes and the resultant resistance that develops to these agents. Thus, additional treatments that can overcome or prevent resistance are needed. Just as erlotinib and gefitinib are most effective in patients with EGFR-activating mutations, irreversible EGFR-TKIs such as afatinib likely have their own particular niche. At the present time, afatinib’s role is not yet defined. It may be best utilized as a second- or third-line TKI in patients with the most common resistance mutations. Or it may simply prove to be a third EGFR-TKI option for patients with EGFR-activating mutations.
At a minimum, afatinib appears comparable to current first-generation EGFR-TKI options. In the first-line treatment of patients with common activating mutations, phase III studies show PFS of 9.7–13.1 months with erlotinib,Citation22,Citation23 9.2–9.4 months with gefitinib,Citation25,Citation27 and 11.1 months with afatinib.Citation62 Admittedly, cross-trial comparisons do not take into account different patient characteristics such as EGFR mutation status, thus a head-to-head comparison would be prudent. In the meantime, continued efforts to determine the molecular subtype of patients who will most benefit from afatinib are ongoing.
Disclosure
The authors report no conflicts of interest in this work.
References
- National Comprehensive Cancer NetworkNCCN Clinical Practice Guidelines in Oncology: Non-Small Cell Lung Cancer2012 Available from: http://www.nccn.org/professionals/physician_gls/f_guidelines.aspAccessed October 14, 2012
- DollRGrayRHafnerBPetoRMortality in relation to smoking: 22 years’ observations on female British doctorsBMJ198028062199679717417764
- JanerichDTThompsonWDVarelaLRLung cancer and exposure to tobacco smoke in the householdN Engl J Med1990323106326362385268
- TurnerMCKrewskiDChenYPopeCA3rdGapsturSThunMJRadon and lung cancer in the American Cancer Society cohortCancer Epidemiol Biomarkers Prev201120343844821212062
- HeintzNHJanssen-HeiningerYMMossmanBTAsbestos, lung cancers, and mesotheliomas: from molecular approaches to targeting tumor survival pathwaysAm J Respir Cell Mol Biol201042213313920068227
- SandlerAGrayRPerryMCPaclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancerN Engl J Med2006355242542255017167137
- ReidAVidalLShawHde BonoJDual inhibition of ErbB1 (EGFR/HER1) and ErbB2 (HER2/neu)Eur J Cancer200743348148917208435
- HerbstRSHeymachJVLippmanSMLung cancerN Engl J Med2008359131367138018815398
- SpicerJFRudmanSMEGFR inhibitors in non-small cell lung cancer (NSCLC): the emerging role of the dual irreversible EGFR/HER2 inhibitor BIBW 2992Target Oncol20105424525520574858
- GazdarAFActivating and resistance mutations of EGFR in non-small-cell lung cancer: role in clinical response to EGFR tyrosine kinase inhibitorsOncogene200928Suppl 1S24S3119680293
- RayMSalgiaRVokesEEThe role of EGFR inhibition in the treatment of non-small cell lung cancerOncologist200914111116113019892771
- MurraySDahabrehIJLinardouHManoloukosMBafaloukosDKosmidisPSomatic mutations of the tyrosine kinase domain of epidermal growth factor receptor and tyrosine kinase inhibitor response to TKIs in non-small cell lung cancer: an analytical databaseJ Thorac Oncol20083883283918670300
- RielyGJPolitiKAMillerVAPaoWUpdate on epidermal growth factor receptor mutations in non-small cell lung cancerClin Cancer Res200612247232724117189394
- EngelmanJAJannePAMechanisms of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancerClin Cancer Res200814102895289918483355
- SharmaSVBellDWSettlemanJHaberDAEpidermal growth factor receptor mutations in lung cancerNat Rev Cancer20077316918117318210
- ShepherdFARodrigues PereiraJCiuleanuTErlotinib in previously treated non-small-cell lung cancerN Engl J Med2005353212313216014882
- JackmanDMMillerVACioffrediLAImpact of epidermal growth factor receptor and KRAS mutations on clinical outcomes in previously untreated non-small cell lung cancer patients: results of an online tumor registry of clinical trialsClin Cancer Res200915165267527319671843
- RosellRMoranTQueraltCScreening for epidermal growth factor receptor mutations in lung cancerN Engl J Med20093611095896719692684
- InoueASuzukiTFukuharaTProspective phase II study of gefitinib for chemotherapy-naive patients with advanced non-small-cell lung cancer with epidermal growth factor receptor gene mutationsJ Clin Oncol200624213340334616785471
- JannePAWangXSocinskiMARandomized phase II trial of erlotinib alone or with carboplatin and paclitaxel in patients who were never or light former smokers with advanced lung adenocarcinoma: CALGB 30406 trialJ Clin Oncol201230172063206922547605
- JackmanDMYeapBYSequistLVExon 19 deletion mutations of epidermal growth factor receptor are associated with prolonged survival in non-small cell lung cancer patients treated with gefitinib or erlotinibClin Cancer Res200612133908391416818686
- RosellRCarcerenyEGervaisRErlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trialLancet Oncol201213323924622285168
- ZhouCWuYLChenGErlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutationpositive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 studyLancet Oncol201112873574221783417
- GridelliCDe MarinisFDi MaioMCortinovisDCappuzzoFMokTGefitinib as first-line treatment for patients with advanced non-small-cell lung cancer with activating epidermal growth factor receptor mutation: review of the evidenceLung Cancer201171324925721216486
- MokTSWuYLThongprasertSGefitinib or carboplatin-paclitaxel in pulmonary adenocarcinomaN Engl J Med20093611094795719692680
- MaemondoMInoueAKobayashiKGefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFRN Engl J Med2010362252380238820573926
- MitsudomiTMoritaSYatabeYGefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trialLancet Oncol201011212112820022809
- Paz-AresLSoulieresDMelezinekIClinical outcomes in non-small-cell lung cancer patients with EGFR mutations: pooled analysisJ Cell Mol Med2010141–2516920015198
- JackmanDPaoWRielyGJClinical definition of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancerJ Clin Oncol201028235736019949011
- PaoWMillerVAPolitiKAAcquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domainPLoS Med200523e7315737014
- ArcilaMEOxnardGRNafaKRebiopsy of lung cancer patients with acquired resistance to EGFR inhibitors and enhanced detection of the T790M mutation using a locked nucleic acid-based assayClin Cancer Res20111751169118021248300
- SequistLVWaltmanBADias-SantagataDGenotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitorsSci Transl Med201137575ra26
- OuSHSecond-generation irreversible epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs): a better mousetrap? A review of the clinical evidenceCrit Rev Oncol Hematol201283340742122257651
- BalakMNGongYRielyGJNovel D761Y and common secondary T790M mutations in epidermal growth factor receptor-mutant lung adenocarcinomas with acquired resistance to kinase inhibitorsClin Cancer Res200612216494650117085664
- BeanJRielyGJBalakMAcquired resistance to epidermal growth factor receptor kinase inhibitors associated with a novel T854A mutation in a patient with EGFR-mutant lung adenocarcinomaClin Cancer Res200814227519752519010870
- CostaDBHalmosBKumarABIM mediates EGFR tyrosine kinase inhibitor-induced apoptosis in lung cancers with oncogenic EGFR mutationsPLoS Med200741016691679 discussion 168017973572
- EngelmanJAZejnullahuKMitsudomiTMET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signalingScience200731658271039104317463250
- SudaKTomizawaKFujiiMEpithelial to mesenchymal transition in an epidermal growth factor receptor-mutant lung cancer cell line with acquired resistance to erlotinibJ Thorac Oncol2011671152116121597390
- MaheswaranSSequistLVNagrathSDetection of mutations in EGFR in circulating lung-cancer cellsN Engl J Med2008359436637718596266
- RosellRMolinaMACostaCPretreatment EGFR T790M mutation and BRCA1 mRNA expression in erlotinib-treated advanced non-small-cell lung cancer patients with EGFR mutationsClin Cancer Res20111751160116821233402
- SuKYChenHYLiKCPretreatment epidermal growth factor receptor (EGFR) T790M mutation predicts shorter EGFR tyrosine kinase inhibitor response duration in patients with non-small-cell lung cancerJ Clin Oncol201230443344022215752
- RielyGJKrisMGZhaoBProspective assessment of discontinuation and reinitiation of erlotinib or gefitinib in patients with acquired resistance to erlotinib or gefitinib followed by the addition of everolimusClin Cancer Res200713175150515517785570
- CostaDBNguyenKSChoBCEffects of erlotinib in EGFR mutated non-small cell lung cancers with resistance to gefitinibClin Cancer Res200814217060706718981003
- ChaftJEOxnardGRSimaCSKrisMGMillerVARielyGJDisease fare after tyrosine kinase inhibitor discontinuation in patients with EGFR-mutant lung cancer and acquired resistance to erlotinib or gefitinib: implications for clinical trial designClin Cancer Res201117196298630321856766
- PietanzaMCLynchTJJrLaraPNJrXL647 – a multitargeted tyrosine kinase inhibitor: results of a phase II study in subjects with non-small cell lung cancer who have progressed after responding to treatment with either gefitinib or erlotinibJ Thorac Oncol20127121922622011666
- JohnsonMLRielyGJRizviNAPhase II trial of dasatinib for patients with acquired resistance to treatment with the epidermal growth factor receptor tyrosine kinase inhibitors erlotinib or gefitinibJ Thorac Oncol2011661128113121623279
- SequistLVBesseBLynchTJNeratinib, an irreversible pan-ErbB receptor tyrosine kinase inhibitor: results of a phase II trial in patients with advanced non-small-cell lung cancerJ Clin Oncol201028183076308320479403
- JanjigianYYAzzoliCGKrugLMPhase I/II trial of cetuximab and erlotinib in patients with lung adenocarcinoma and acquired resistance to erlotinibClin Cancer Res20111782521252721248303
- LiDAmbrogioLShimamuraTBIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer modelsOncogene200827344702471118408761
- YapTAVidalLAdamJPhase I trial of the irreversible EGFR and HER2 kinase inhibitor BIBW 2992 in patients with advanced solid tumorsJ Clin Oncol201028253965397220679611
- MetroGCrinoLThe LUX-Lung clinical trial program of afatinib for non-small-cell lung cancerExpert Rev Anticancer Ther201111567368221554040
- KwakELSordellaRBellDWIrreversible inhibitors of the EGF receptor may circumvent acquired resistance to gefitinibProc Natl Acad Sci USA2005102217665767015897464
- MurakamiHTamuraTTakahashiTPhase I study of continuous afatinib (BIBW 2992) in patients with advanced non-small cell lung cancer after prior chemotherapy/erlotinib/gefitinib (LUX-Lung 4)Cancer Chemother Pharmacol201269489189922071596
- AgusDBTerlizziEStopferPAmelsbergAGordonMSA phase I dose escalation study of BIBW 2992, an irreversible dual EGFR/HER2 receptor tyrosine kinase inhibitor, in a continuous schedule in patients with advanced solid tumoursJ Clin Oncol2006241897S97S
- YangJCShihJYSuWCAfatinib for patients with lung adenocarcinoma and epidermal growth factor receptor mutations (LUX-Lung 2): a phase 2 trialLancet Oncol201213553954822452895
- SchulerMAwadaAHarterPA phase II trial to assess efficacy and safety of afatinib in extensively pretreated patients with HER2-negative metastatic breast cancerBreast Cancer Res Treat201213431149115922763464
- EskensFAMomCHPlantingASA phase I dose escalation study of BIBW 2992, an irreversible dual inhibitor of epidermal growth factor receptor 1 (EGFR) and 2 (HER2) tyrosine kinase in a 2-week on, 2-week off schedule in patients with advanced solid tumoursBr J Cancer2008981808518026190
- CohenMHJohnsonJRChenYFSridharaRPazdurRFDA drug approval summary: erlotinib (Tarceva) tabletsOncologist200510746146616079312
- LinNUWinerEPWheatleyDA phase II study of afatinib (BIBW 2992), an irreversible ErbB family blocker, in patients with HER2-positive metastatic breast cancer progressing after trastuzumabBreast Cancer Res Treat201213331057106522418700
- BarberNAGantiAKPulmonary toxicities from targeted therapies: a reviewTarget Oncol20116423524322076388
- MillerVAHirshVCadranelJAfatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): a phase 2b/3 randomised trialLancet Oncol201213552853822452896
- YangCHSchulerMHYamamotoNLUX-Lung 3: a randomized, open-label, phase III study of afatinib versus pemetrexed and cisplatin as first-line treatment for patients with advanced adenocarcinoma of the lung harboring EGFR-activating mutationsJ Clin Oncol201230SupplLBA7500
- Boehringer Ingelheim PharmaceuticalsA phase II trail of afatinib (BIBW 2992) in third-line treatment for patients with stage IIIB/IV adenocarcinoma of the lung harbouring wild-type epidermal growth factor receptor (EGFR)ClinicalTrialsgov [website on the Internet]Bethesda, MDUS National Library of Medicine2012 [updated April 13, 2012]. Available from: http://clinicaltrials.gov/ct2/show/NCT01003899 NLM identifier: NCT01003899. Accessed October 14, 2012
- JanjigianYY GHHornLSmitEFActivity and tolerability of afatinib (BIBW 2992) and cetuximab in NSCLC patients with acquired resistance to erlotinib and gefitinibJ Clin Oncol201129Suppl7525
- SequistLVSchulernYamamotoMLUX-Lung 3: Symptom and health-related quality of life results from a randomized phase III study in 1st-line advanced NSCLC patients harbouring EGFR mutations37th ESMO CongressSeptember 28–October 2, 2012Vienna, Austria