Abstract
Studies demonstrate that lipid mediator 20-Hydroxyeicosatetraenoic acid (20-HETE) synthesis and signaling are associated with the growth of cancer cells in vitro and in vivo. Stable 20-HETE agonists promote the proliferation of cancer cells, whereas selective inhibitors of the 20-HETE-producing enzymes of the Cytochrome (CYP450)4A and CYP4F families can block the proliferation of glioblastoma, prostate, renal cell carcinoma, and breast cancer cell lines. A recent observation that the expression of CYP4A/4F genes was markedly elevated in thyroid, breast, colon, and ovarian cancer further highlights the significance of 20-HETE-producing enzymes in the progression of different types of human cancer. These findings provide the rationale for targeting 20-HETE-producing enzymes in human cancers and set the basis for the development of novel therapeutic strategies for anticancer treatment.
Introduction
The eicosanoid, 20-hydroxyeicosatetraenoic acid (20-HETE), has emerged as a novel signaling molecule contributing to the progression of cancer.Citation1–Citation3 Eicosanoids are 20-carbon bioactive lipid mediators generated by enzymatic oxidation of arachidonic acid (AA). These include prostaglandins (products of cyclooxygenases), leukotrienes (products of lipoxygenases), and hydroxyeicosatetraenoic (HETEs) and epoxyeicosatrienoic acids (EETs) (products of cytochrome P450 enzymes).Citation4 Even though eicosanoid-mediated modulation of ion transport, renal and pulmonary functions, as well as vascular tone and reactivity have been universally acknowledged,Citation5,Citation6 not until recently has it become evident that these lipid mediators are also involved in carcinogenesis.Citation7,Citation8 Prostaglandins have subsequently been the most widely and intensely studied group of eicosanoids in cancer biology.Citation8 Among prostaglandins, prostaglandin E2 (PGE2) has received the most attention as a potential contributor to cancer progression.Citation9–Citation11 Indeed, PGE2 has a potent proproliferative effect, is involved in conferring a multidrug resistance phenotype,Citation12,Citation13 and it increases tumor growth in ApcMin/+ and azoxymethane mouse models of colorectal cancer.Citation14 PGE2 also reversed nonsteroidal anti-inflammatory drug-induced adenoma regression in these mice. Furthermore, inhibition of endogenous PGE2 resulted in the suppression of intestinal tumorogenesis.Citation15 These findings are consistent with established PGE2-mediated signaling, which includes, among others, transactivation of endothelial growth factor (EGF) receptor,Citation16–Citation18 and peroxisome proliferator-activated receptor δ.Citation19 Activation of these signaling cascades resulted in stimulation of cell migration through increased PI3K-Akt signaling in colon cancer cells and increased intestinal epithelial tumor cell survival. Concordantly, PGE2 has also been shown to induce expression of such antiapoptotic proteins as Bcl-2,Citation20 and increase transcriptional activity of a key antiapoptotic regulator, nuclear factor-kappa B (NFκB).21 It has also been reported that PGE2 possesses an angiogenic effect.Citation22,Citation23 PGE2 reversed the antiangiogenic activity of nonsteroidal anti-inflammatory drugs, whereas homozygous deletion of PGE2 receptor EP2 completely abrogated the induction of vascular endothelial growth factor (VEGF) in APCΔ716 mouse polyps.Citation24 This is consistent with earlier studies showing that PGE2 upregulates VEGF in cultured human fibroblasts,Citation25 and increases VEGF and basic fibroblast growth factor expression through the stimulation of extracellular-signal-regulated kinase (ERK)2/c-Jun N-terminal kinase 1 signaling pathways in endothelial cells.Citation26 Similarly, while not as well studied as PGE2, PGF2α has been demonstrated to enhance carcinogen-induced transformation of fibroblasts in vitro,Citation7 while thromboxane A2 was reported to promote angiogenesis.Citation27
Compared with prostaglandins, much less is known about the role of lipoxygenases (LOXs) in cancer. Data are accumulating that support the role of 15-LOX-1 as a tumor suppressor, especially in colon cancer.Citation28 On the other hand overexpression of 12-LOX was strongly associated with poor differentiation and invasiveness of prostate cancer.Citation29 Further, it has been shown that leukotriene B4 (LTB4) levels are increased in human colon and prostate cancers,Citation30,Citation31 and the expression of LTB4 receptors is upregulated in human pancreatic cancer.Citation32 Additionally, it has been shown that inhibition of LTB4 synthesis leads to reduced esophageal adenocarcinoma in a rat model and that blocking the receptor of LTB4 suppressed the LTB4-stimulated expression of ERK in colon cancer cells.Citation33 Other LOX byproducts, such as 12(S) HETE have been reported to mediate the activation of NFκB,34 induce angiogenesis through stimulating VEGF expression in prostate cancer cells,Citation35,Citation36 and increase adhesion of B16 murine melanoma cells to endothelial cells via upregulation of ανβ3 integrin expression.Citation37
The role of HETEs and EETs in cancer has been neglected until recently.Citation38 There are mounting data that suggest that products of ω-hydroxylases of the cytochrome P450 (CYP) family of proteins, notably 20-HETE, can play an important role in cell growth and cancer development.Citation38 In this review, we will summarize the findings that provide the rationale for considering 20-HETE producing enzymes as novel targets for anticancer therapy, describe the potential of available pharmacological agents for interfering with 20-HETE synthesis and signaling, and discuss the potential of their clinical application for cancer treatment.
Cellular synthesis of 20-HETE and other eicosanoids
AA is metabolized to eicosanoids through three major pathways: the cyclooxygenase (COX), the LOX, and the CYP-450 monooxygenase pathways, which insert oxygen at different positions in AA to generate the wide variety of lipid mediators. AA metabolized by the COX pathway forms prostaglandins (PGs) and thromboxanes. AA metabolized by LOX pathway produces 15(S), 12(S), 12(R), 8(S), 5(S) HETEs, leukotrienes, and lipoxins. Finally, AA metabolized by the CYP-450 pathway generates 16-, 17-, 18-, 19-, and 20-HETEs and 5-, 6-, 8-, 9-, 11-, 12-, 14-, and 15-EETs.Citation4
The COX enzymes (COX1/COX2) catalyze the conversion of AA to an unstable cyclic endoperoxide (prostaglandin H2, PGH2), which is further catalytically converted to the various prostanoids including PGD2, PGE2, PGF2α, prostacyclin, and thromboxane A2 via reduction, rearrangement, or isomerization by the terminal synthase enzymes.Citation4 It should be noted that 5-, 8-, 12-, and 15-LOX enzymes metabolize AA to several regioisomeric allylic hydroperoxides, which are further reduced into the corresponding leukotrienes and HETEs.Citation4 The cytochrome P-450 ω-hydroxylases (CYP4A and CYP4F) metabolize AA to produce HETEs, including 19- and 20-HETE (), and cytochrome P-450 epoxygenases (CYP2C and CYP2J) convert AA to the various EETs. To generate 19/20-HETE the ω-hydroxylases of the 4A and 4F gene families of CYP450 introduce an hydroxyl (OH) group at or near the terminal spCitation3 carbon group of AA.Citation4,Citation39,Citation40 The pathways involved in the metabolism of AA are depicted in .
Figure 1 Synthesis of 20-HETE.
Abbreviations: 20-HETE, 20-hydroxyeicosatetraenoic acid; CYP, cytochrome P450; 19-HETE, 19-hydroxyeicosatetraenoic acid.
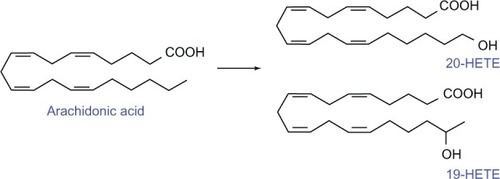
Figure 2 An overview of eicosanoid synthesis pathways.
Abbreviations: CYP, cytochrome P450; LOX, lipoxygenase; EET, epoxyeicosatrienoic acid; PGI2, prostacyclin synthase; PGE2, prostaglandin E2; PGF2, prostaglandin F2; PGD2, prostaglandin D2; TXA2, thromboxane A2; LTA4, leukotriene A4; LTB4, leukotriene B4; PGH2, prostaglandin H2; COX1/2, cyclooxygenase 1/2; HETE, hydroxyeicosatetraenoic acid.
Methods of detecting 20-HETE in cells and tissues
Due to the chemical and biological complexity of eicosanoids, the measurement of their concentrations in biological samples must be carefully validated and controlled in the laboratory. There are several analytical techniques available when it is necessary to determine eicosanoid concentrations in biological samples. In this chapter, we discuss each of these techniques and evaluate the advantages and drawbacks of each methodology. There are several analytical techniques available when it is necessary to determine eicosanoid concentrations in biological samples. In this chapter, we discuss each of these techniques and evaluate the advantages and drawbacks of each methodology.
Radioimmunoassay (RIA) is a commonly used method for the quantitative analysis of eicosanoid levels in biological samples. The assay is based on the competition between the antigen (the eicosanoid being measured) and a constant amount of radioactive homologous antigen for a limited amount of specific eicosanoid antibody. Thus, the necessary reagents include a monospecific antibody, an unlabelled eicosanoid to be used as a standard, and radioactively labeled eicosanoid. The antibody-bound radioactivity is inversely proportional to the concentration of the eicosanoid in the sample. RIA is rapid, sensitive, and relatively inexpensive;Citation4,Citation41 however, there are risks associated with using radioactive materials, which accompany all RIA assays.
Similarly, enzyme immunoassay (EIA) is also based on the competition between an eicosanoid and a constant amount of chemiluminescent (eg, acetylcholinesterase or alkaline phosphatase) conjugated eicosanoid for a limited amount of antibodies raised against a particular eicosanoid. The product of the enzymatic reaction is read spectrophotometrically at a certain wavelength after the addition of a corresponding substrate and is inversely proportional to the concentration of the eicosanoid in the samples. EIA kits for 20-HETE measurement (Eagle Biosciences, Nashua, NH, USA; R&D Inc, Detroit, MI, USA; and ALPCO Diagnostics, Salem, NH, USA), as well as kits for other eicosanoids, are commercially available. While EIA is similar to RIA with regard to sensitivity and specificity, the cost of EIA is much higher. Further, both assays are limited by the fact that antibodies to all the known AA metabolites are not readily available. Another drawback of these assays is nonspecific binding, which results from the cross-reactivity of the antibodies with other eicosanoids of similar structure leading the investigator to overestimate the concentrations of analytes. Further, both of these techniques allow for the analysis of only one analyte at a time, which is time consuming.Citation4,Citation41
Gas chromatography-mass spectrometry (GC-MS) is another technique utilized in the analysis of eicosanoids and is based on the partition of the analyte between the stationary and gaseous phases. This requires molecules that are being analyzed to be volatile and thermally stable at high GC temperatures. To achieve this, polar groups on eicosanoids, which cause tailing during GC-MS, need to be derivatized to more stable trimethylsilyl groups, which is time consuming and complicated.Citation4,Citation41
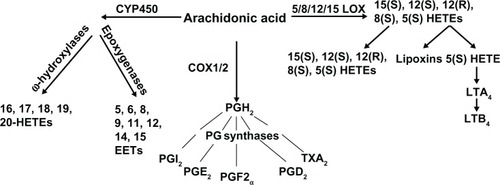
Liquid chromatography-mass spectrometry-mass spectrometry (LC-MS-MS) is a more practical analysis of eicosanoids. In the analysis of eicosanoids by LC-MS-MS, eicosanoids have to be initially extracted by a solid phase, reverse phase, normal phase (silica), and ion exchange columns. Eicosanoids can also be further extracted using liquid extraction, and depending on the eicosanoid being analyzed, one or both of the extraction methods may need to be utilized. The complex mixture of eicosanoids will then need to be separated by liquid chromatography before being introduced into the source of the mass spectrometer, where ionization takes place allowing for the identification of different eicosanoids. Additionally, the use of stable isotopelabeled internal standards in LC-MS-MS prior to extraction is essential for quantitative analysis of the eicosanoids present in cells, urine, plasma, or tissue homogenates.Citation4,Citation41 An example of the detection of eicosanoids by LC-MS-MS is shown in . We have used this technique quite routinely for the measurement of 20-HETE in tissue and cellular samples. While GC-MS and LC-MS-MS are both sensitive and allow for the analysis of multiple analytes simultaneously, the cost is prohibitive, which limits the usage of this method for 20-HETE detection.
Figure 3 LC-MS-MS detection of HETE standards.
Abbreviations: LC-MS-MS, liquid chromatography-mass spectrometry-mass spectrometry; HETE, hydroxyeicosatetraenoic acid; PG, prostaglandin; DiHETE, diastereomeric 5,6-dihydroxyeicosatetraenoic acid; EET, epoxyeicosatrienoic acid.
In summary, LC-MS-MS and GC-MS are the most reliable and accurate methods for identifying and quantifying 20-HETE and other eicosanoid levels. However, the high cost of these techniques limits the number of replicates per sample, sometimes leading to inconclusive results. Immunoassays, which are less expensive, recently became an alternative detection method for 20-HETE and other eicosanoids for which antibodies are available. They are more practical, as samples can be run in replicates and a larger amount of samples can be assayed; however, immunoassays can also be problematic due to nonspecific binding. Overall, as each of the detection methods listed above has its drawbacks, it is desirable to combine these assays to accurately measure and confirm levels of 20-HETE and other eicosanoids in biological samples.
20-HETE-producing enzymes
There are four human CYP450 isoforms that catalyze the hydroxylation of AA at the ω- or 20-carbon to produce 20-HETE; they are CYP4A11, CYP4A22, CYP4F2, and CYP4F3.Citation39,Citation40,Citation42 These CYP450 enzymes are encoded by distinct genes and are highly homologous. Analysis of the CYP4A11 gene revealed that, like CYP4A22, it contains 12 exons and eleven introns, and its overall length and exon/ intron structure were similar to those of CYP4A22. However, the physical alignment of CYP4A11 and CYP4A22 genes exposed small insertions and deletions in the introns, with the most conserved being intron 8, while introns 3, 6, and 9 showed the highest degree of divergence, resulting in a 96% overall sequence identity between CYP4A11 and CYP4A22 genes. Furthermore, the translated sequences for CYP4A11 and CYP4A22 show that these proteins are of the same length and differ only by 25 residues.Citation43 Similarly, CYP4F2 and CYP4F3 genes share 80% amino acid identity, with highly similar organization of genes.Citation44 Of the four 20-HETE-producing enzymes, CYP4F2 is considered the major enzyme involved in the production of 20-HETE in humans accounting for about 70% of 20-HETE production, with an apparent KM of 23.5 μM and Vmax of 7.4 min–1 while CYP4A11 has a tenfold higher KM, of 228.2 μM and Vmax of 49.1 min–1. Besides kinetic studies, the role of CYP4F2 as the predominant AA ω-hydroxylase in the production of 20-HETE, has been further confirmed by immunoinhibition studies, which showed that antibodies to CYP4F2 elicited marked inhibition (93%) of 20-HETE formation, whereas antibodies to CYP4A11 only elicited 13% inhibition.Citation42 It was recently reported that overexpression of a novel CYP4 family member, CYP4Z1, was associated with increased levels of 20-HETE in breast cancer cells.Citation45
The abundance of 20-HETE-producing enzymes in mammals makes it hard to use the knockout approach to study their relevance for cancer progression.Citation39 In mice, the two enzymes primarily responsible for 20-HETE production are CYP4A10 and CYP4A12, with CYP4A12 being the predominant 20-HETE synthase in the mouse kidney.Citation46 In rats, there are four isoforms of 20-HETE-producing enzymes: CYP4A1, CYP4A2, CYP4A3, and CYP4A8. Among them, CYP4A1 has the highest catalytic activity toward AA ω–hydroxylation. It is followed by CYP4A2 and CYP4A3, which display similar activity. CYP4A8 also contributed to 20-HETE formation in rat kidney.Citation47
Disruption of the mouse CYP4A10 gene caused hypertension, which was dietary salt sensitive.Citation48 CYP4A12 knockout mice were not described. Recently, rat knockouts of CYP4A2 and CYP4A3 were generated using a zinc fnger nuclease approach as a part of the PhysGen Knockout program (“Mechanistic Characterization of Genes for Hypertension and Renal Disease”) at the Medical College of Wisconsin (Milwaukee, WI, USA). This program aimed to knock out a large number of genes, nominated by genomewide association studies as relevant for the development of hypertension and renal disease, and the generated rat strains are available for distribution. So far, neither mouse nor rat knockouts of 20-HETE-producing enzymes have been utilized in cancer-related studies.
20-HETE actions and signaling
It should be noted that 20-HETE, being a potent vasoconstrictor, plays a complex role in the regulation of blood pressure and vascular tone. The vasoconstriction activity of 20-HETE in rat aortic rings was among the first 20-HETE actions described.Citation49 Specifically, 20-HETE mediates autoregulation of cerebral blood fow.Citation50 Renal actions of 20-HETE are linked to the regulation of Na+ transport and the pressure-natriuresis response.Citation51,Citation52 Importantly, 20-HETE inhibits Na+ transport in renal proximal tubules,Citation53 but not in cortical collecting ducts, where inhibition is likely to be mediated by EETs.Citation54,Citation55 Inhibitors of 20-HETE formation promote salt-sensitive hypertension and renal injury in the rat model.Citation56 For an excellent summary of the renal and vascular actions of 20-HETE and the role of 20-HETE in the control of hypertension, see the review by Williams et al.Citation52 The role of 20-HETE in physiological and pathological vascularization was also recently reviewed.Citation57
In addition to vasoactive effects, 20-HETE stimulates mitogenic and angiogenic responses both in vitro and in vivo.Citation39 Both mitogenic and angiogenic activities are important for the ability of 20-HETE to support the proliferation of cancer cells and tumor growth; 20-HETE has been shown to induce cell proliferation and DNA synthesis in vascular smooth muscle and proximal tubule cell cultures, as well as in a number of cancer cell lines. The proangiogenic activity of 20-HETE with regard to endothelial cell population is through the promotion of proliferation, migration, and cell survival. These 20-HETE actions were accompanied by increased VEGF expression and release.Citation1,Citation58 VEGF is the major driver of tumor angiogenesis.Citation59 These studies support the idea that 20-HETE signaling contributed to cell growth, invasion, and angiogenesis in the regulation of tumor development.
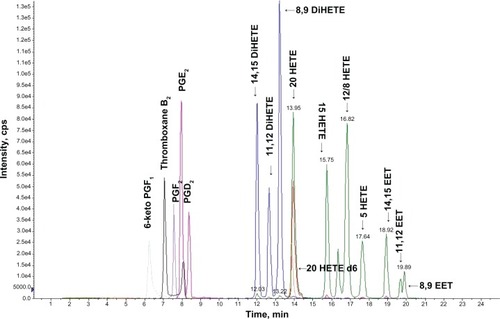
The biggest challenge with attempts to define 20-HETE signaling is that to date, the specific cellular receptor of 20-HETE has not been identified. In striking contrast to PGs (whose G-protein coupled receptors are well studied and characterized), no reliable information about the nature of HETE receptors in general and the 20-HETE receptor in particular, is available. Correspondingly, specific 20-HETE antagonists preventing the binding of 20-HETE to its receptor are missing from the arsenal of tools for the treatment of 20-HETE-mediated pathologies, a circumstance which continues to delay the development of 20-HETE-focused therapeutic strategies. The situation was improved with the introduction of specific inhibitors of 20-HETE synthesis and antagonists of 20-HETE signaling, which will be discussed below. It also must be taken into consideration that enzymes that synthesize 20-HETE are microsomal enzymes, and 20-HETE, when released intracellularly, could act as an intracellular second messenger and interact directly with intracellular targets. Therefore, the possibility that 20-HETE and other HETEs act without binding to canonical transmembrane receptors cannot be ruled out, even though it would make them different from other eicosanoids.
In the absence of an identified 20-HETE receptor, studies of 20-HETE signaling are limited to description of cellular signaling pathways being activated following addition of 20-HETE to cultured cells and administration of 20-HETE to animals. Multiple signaling cascades were reported to be activated by 20-HETE in a variety of cell systems.Citation57,Citation60,Citation61 In addition, 20-HETE induced activation of Raf/MEK/ERK, signaling a cascade secondary to the activation of c-Src and EGF receptor in renal epithelial cells.Citation62 Moreover, 20-HETE triggers NF κB and ERK activation in human endothelial cells.Citation60 Furthermore, 20-HETE can also activate the small-GTPase Ras, which plays a critical role in the regulation of cell growth;Citation63 20-HETE was reported to signal through the cyclic adenosine monophosphate/ protein kinase A (PKA)-phosphorylase kinase-glycogen phosphorylase pathway to induce hyperglycemia.Citation64 NAHPH-oxidase-derived superoxide mediated 20-HETE-induced activation of L-type Ca2+ channels via a PKC-dependent mechanism in cardiomyocytes.Citation61 A diagram showing the various 20-HETE-mediated signaling pathways and their (probable) interactions is included ().
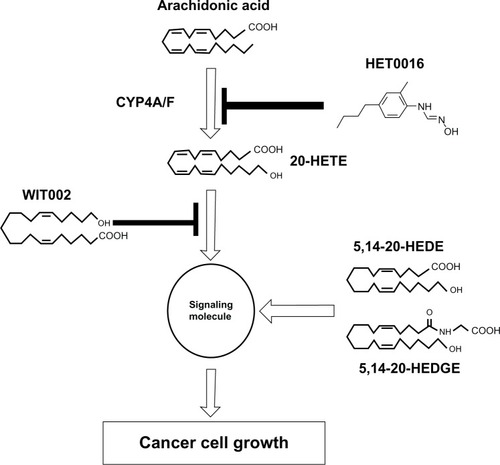
Figure 4 Proposed mechanism of action of 20-HETE.
Abbreviations: 20-HETE, 20-hydroxyeicosatetraenoic acid; GPCR, G protein-coupled receptor; EGFR, endothelial growth factor; PLA2, phospholipase A2; NADPH, nicotinamide adenine dinucleotide phosphate; SOS, salt overly sensitive; AA, arachidonic acid; CYP, cytochrome P450; PKC, protein kinase C; PKA, protein kinase A; PhK, phosphorylase kinase; GP, glycogen phosphorylase pathway; cAMP, cyclic adenosine monophos phate; MEK, MAP kinase kinase; ERK, extracellular signal-regulated kinases; IκBα, I-kappaB-alpha; NFκB, nuclear factor-kappaB; VEGF, vascular endothelial growth factor; HET0016, N-Hydroxy-N′-(4-butyl-2-methylphenyl) formamidine; WIT002, 20-hydroxyeicosa-6(Z),15(Z)-dienoic acid.
Expression of 20-HETE-producing enzymes in human cancers
While 20-HETE induces mitogenic and angiogenic responses in cancer cells and inhibitors of the 20-HETE pathway have been shown to reduce the growth of brain and kidney tumors at least in animal models,Citation65,Citation66 the expression pattern of 20-HETE-producing enzymes in human cancers has not been addressed. We have demonstrated that the 20-HETE-producing enzymes CYP4A/F are upregulated in human cancer tissue samples.Citation63 In this study, TissueScan cancer survey panels obtained from OriGene (OriGene Technologies, Inc, Rockville, MD, USA) containing prenormalized cDNA from multiple cancer tissues were used to run real-time polymerase chain reaction (Sybr Green®, Life Technologies, Carlsbad, CA) to analyze the expression of CYP4A and CYP4F genes at the messenger ribonucleic acid (mRNA) level. The tissue samples used for each type of TissueScan panel were obtained from independent patients, covered various disease stages, and matched control normal tissues, and these samples are included for comparison between normal and disease samples. Analysis of 20-HETE-producing CYP enzymes in various human cancer tissues demonstrated that CYP4A/F enzymes were upregulated at the mRNA level in human thyroid, breast, colon, and ovary cancers when compared to corresponding normal tissue.Citation63 Analysis of human lung cancer tissue samples also revealed increased levels of CYP4F/A enzymes; even though a dramatic increase of expression of 4F2 and 4F3 were detected only in one of nine cancer tissue samples, two other cancer samples (indicated as E8 and E10) demonstrated increased expression of 4F2, which was not detectable in matched control normal tissues (). Most CYP450 enzymes are expressed in the liver and are involved in the metabolism of drugs and foreign chemicals. Both normal and cancerous liver cells are characterized by the increased expression of 20-HETE-producing enzymes. The prostate is also rich with AA-metabolizing enzymes. Any increase in the expression of 20-HETE-producing enzymes in the liver and prostate will be more difficult to detect because it will be masked by substantial expression in normal cells; this is not the case with other tissues. Primers used in real-time polymerase chain reaction experiments to detect the expression of CYP4A/F genes at the mRNA level are listed in . Using Western blot analysis of an OncoPair INSTA-Blot™ PVDF membrane (IMGENEX Corporation, San Diego, CA, USA) containing 14 lanes of alternating ovarian tumor and matched normal adjacent tissue lysates from seven patient donors (IMGENEX Corporation) and immunohistochemistry analysis, we further demonstrated that the expression of CYP4F2 (the major 20-HETE-producing enzyme) was increased at the protein level in ovarian cancer when compared to normal adjacent tissue.Citation63
Figure 5 Expression of CYP4A and CYP4F genes at the mRNA level is elevated in human lung cancer tissue samples.
Abbreviations: CYP, cytochrome P450; mRNA, messenger ribonucleic acid; PCR, polymerase chain reaction; RNA, ribonucleic acid.
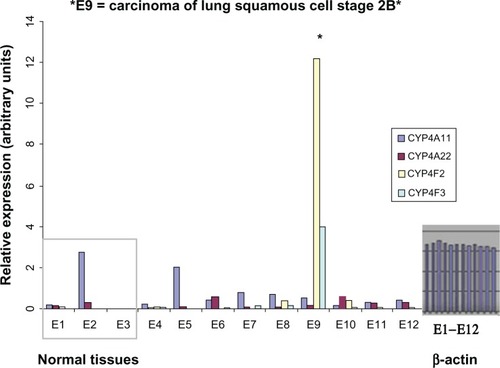
Table 1 Primers used to detect human genes encoding CYP4A/F by real-time PCR
Agonists, antagonists, and inhibitors of the synthesis of 20-HETE
A hydroxyphenylformamidine derivative, HET0016 [N-hydroxy-N′-(4-butyl-2-methylphenyl)-formamidine], was shown to be a potent and selective inhibitor of 20-HETE synthesis in rat and human renal microsomes.Citation67 To retain high inhibitory activity, N-hydroxyformamidine had to be unsubstituted, with the substituent at the paraposition of N-hydroxyformamidine decreasing the inhibitory activity most drastically.Citation68
In the absence of antagonists preventing the binding of 20-HETE to its probable transmembrane receptor, 20-hydroxyeicosa-6(Z), 15(Z)-dienoic acid (WIT002) has emerged as an antagonist of 20-HETE signaling ().Citation69 WIT002 is a noncyclooxygenase metabolizable stable analog of 20-HETE, which antagonizes the vasoconstrictor effects of 20-HETE in vitro and in vivo.Citation69,Citation70 WIT002 also inhibits the stimulatory effect of 20-HETE upon the platelet-derived growth factor-mediated migration of vascular smooth muscle cells.Citation71 N-[20-hydroxyeicosa-6(Z),15(Z)-dienoyl]glycine (6,15-20-HEDGE) is an analog of WIT002 that may be more efficacious because of the addition of a glycine group, which may help the compound enter cells or prolong its action by protecting it from β-oxidation. In addition, synthetic stable analogs of 20-HETE N-[20-hydroxyeicosa-5(Z),14(Z)-dienoyl]glycine (5,14-HEDGE) and 20-hydroxyeicosa-5(Z), 14(Z)-dienoic acid (5,14-20-HEDE) (also termed WIT003) were shown to prevent the impairment of cardiorenal functions suggesting a therapeutic potential for 20-HETE analogs.Citation72,Citation73
Figure 6 Inhibitors, agonists, and antagonists of 20-HETE.
Abbreviations: 20-HETE, 20-hydroxyeicosatetraenoic acid; 5,14-20-HEDGE, N-[20-hydroxyeicosa-5(Z), 14(Z)-dienoyl]glycine; 5,14–20, -HEDE, -20-hydroxyeicosa-5(Z), 14(Z)-dienoic acid. CYP450, Cytochrome P450; WIT002-20-hydroxyeicosa-6(Z),15(Z)-dienoic acid; HET0016, N-Hydroxy-N′-(4-butyl-2-methylphenyl) formamidine.
The question of the specificity of inhibitors of 20-HETE synthesis and signaling is a particularly important one. The ability of HET0016 to specifically inhibit the formation of 20-HETE in vivo has been confirmed using LC-MS-MS profiling of all the COX, LOX, and CYP450 eicosanoids in a variety of tissues following in vitro and in vivo administration.Citation74 The data supporting the specificity of the agonist WIT003 and the antagonist 6,15-20-HEDGE is less direct.Citation69
20-HETE in cancer cell proliferation and tumor growth
Despite multiple reports of the anticancer activity of inhibitors of 20-HETE synthesis and signaling, there is a shortage of data that demonstrate increased levels of 20-HETE in human cancers. One example is provided by prostate cancer patients, who were shown to have urinary levels of free 20-HETE that were significantly higher than those of normal men, even though a correlation between the concentration of 20-HETE and grade of tumor was not found.Citation75
The human glioblastoma cell line, U251, is a malignant glioma cell model, which recapitulates the most prominent histological and immunohistochemical features of human glioblastoma multiforme, a highly aggressive brain tumor with a very poor prognosis.Citation76 HET0016 decreased both basal and EGF-induced U251 cell proliferation, an effect that was nullified by the addition of the 20-HETE agonist, WIT003.Citation77 HET0016 also reduced angiogenic response to U251 cells measured by a cornea pocket angiogenesis assay.Citation78 The 9L gliosarcoma cell line has been extensively used to study chemotherapy and radiation effects upon growth of glioma cells, even though the 9L glioma model is characterized by significant differences from human glioblastoma multiforme.Citation76 HET0016 efficiently inhibited 9L cell proliferation, induced apoptosis in these cells, and prevented the proliferative effect of platelet-derived growth factor.Citation65 Furthermore, HET0016 also prevented the growth of 9L cells in vivo, when 9L cells were implanted into the forebrain of Fisher rats. In the absence of HET0016, control animals implanted with 9L cells display rapidly growing tumor, whereas rats treated with HET0016 exhibited much smaller tumors with a lesser degree of vascularization.Citation65 Nevertheless, careful consideration should be implemented when interpreting HET0016 anticancer actions in U251 and 9L cells, because both U251 cells and 9L cells failed to synthesize 20-HETE in the presence of AA.Citation77 One possible explanation is that 20-HETE synthesized in these cells is rapidly metabolized either by β-oxidation or by COX, and therefore escapes detection by LC-MS-MS.Citation39 The other possibility is that mechanisms of HET0016 action are independent of the inhibition of 20-HETE synthesis in U251 and 9L cells. However, the enforced expression of CYP4A1 in U251 cells caused an increased cell growth both in vitro and in vivo, strongly supporting the significant role of 20-HETE in the progression of glioblastomas.Citation79
Non-small-cell lung cancer is among the leading causes of cancer death in the world; 20-HETE generated by CYP4A11 promoted lung cancer angiogenesis and metastasis by upregulation of VEGF and Matrix metallopeptidase 9 (MMP-9) via PI3 kinase and ERK1/2 signaling in human non-small cell lung cancer cells.Citation80 In addition, 20-HETE signaling was also shown to play a role in tumor angiogenesis and growth in human breast cancer.Citation45 Analysis of mRNA levels encoding 20-HETE-producing CYP enzymes revealed increased levels of CYP 4F2 in some human lung cancer tissue samples ().
With an estimated 64,770 new cases of cancers arising from the kidney and renal pelvis in the USA in 2012, renal cell carcinoma is among the leading causes of cancer death. Renal cancer is notoriously resistant to conventional therapies (chemotherapy, hormonal therapy, and radiotherapy), and metastatic forms of clear-cell renal carcinoma are usually associated with very poor patient survival rates.Citation81 Thus, the lack of an effective therapy demands the urgent investigation and development of any novel treatment strategy. Notably, HET0016 and WIT002 inhibit the proliferation of two different human renal cell carcinoma cell lines, 786-O and 769-P, in vitro, whereas the proliferation of primary normal human proximal tubule epithelial cells was not affected by these drugs.Citation66 Both the TNF-related apoptosis- inducing ligand (TRIAL) -sensitive renal cell carcinoma line 769-P and the TRAIL-resistant renal cell carcinoma line 786-O were equally sensitive to the antiproliferative effects of HET0016. These selective antiproliferative effects of HET0016 and WIT002 on renal cancer cells are of particular interest, since renal cell carcinomas are highly refractory to conventional chemotherapy and even radiation therapy. WIT002 was able to decrease the growth of tumors arising from human kidney cancer epithelial cells in vivo.Citation66 These data were the first to indicate the ability of this class of agents to suppress the growth of tumors of human origin.
Overall, 20-HETE plays an important role in the proliferation of prostate cancer cells, both androgen-responsive LNcAP and androgen-nonresponsive PC3 (personal communication from R Roman, Jackson, MS, USA).Citation82 CYP4F2/3 might be the source of androgen-driven 20-HETE synthesis via androgen receptor-dependent pathways.
In summary, the addition of inhibitors of 20-HETE synthesis and signaling significantly inhibited the growth of cancer cells both in vitro and sometimes in vivo. Such results have been obtained using human prostate cancer cells, MDA-MB 231 breast cancer cells (personal communication from R Roman, Jackson, MS, USA), U235, 9L and RG3 glioblastoma cell lines, as well as 780P and 769P renal epithelial cancer cell lines. Together with data demonstrating an increased level of mRNA encoding enzymes responsible for 20-HETE generation, these results provide a compelling reason to study the role of 20-HETE in promoting the proliferation of several forms of human cancer.
Clinical potential of targeting 20-HETE-producing enzymes
Despite dramatic advancements in the treatment of some types of cancer, effective clinical therapies for common human cancers such as lung, ovarian, and renal cancers remain limited. An involvement of AA pathways in the proliferation of different forms of cancer is well documented and there are multiple studies addressing the role of cyclooxygenases and LOX in particular types of cancer.Citation83–Citation87 These results have raised enthusiasm for the use of COX-2 inhibitors for anticancer therapy, but the risk of cardiovascular thrombotic events associated with the usage of selective COX-2 inhibitors is a significant concern.Citation88,Citation89 Recent studies that have demonstrated the antiproliferative effect of inhibitors of 20-HETE synthesis and the overexpression of CYP4A/F enzymes in human cancers () suggest that targeting enzymes responsible for the generation of 20-HETE could be an alternative therapeutic approach for cancer treatment. So far, the role of the products of cytochrome P450 enzymes in control of cancer is insufficiently studied,Citation87 and there are no cancer-related clinical trials focusing on 20-HETE synthesis and signaling. The tumor histological type could influence the pharmaceutical agents’ capacity to decrease 20-HETE production or signaling.Citation80 It is necessary to confirm the mechanism of HET0016 action and identify molecules that are the direct targets of 20-HETE in cancer cells. Furthermore, even though an overexpression of CYP4A/F enzymes has been shown in different types of human cancer (), studies using a larger number of human tumor samples will be needed to ascertain that the upregulation of CYP4A/F expression occurs in different types of human cancers. These deficiencies notwithstanding, the potential of targeting 20-HETE-producing enzymes to develop into a novel therapeutic strategy for anticancer treatment is high.
Table 2 Expression of CYP4A and CYP4F in human cancer samples and the effects of inhibitors of 20-HETE synthesis and signaling upon cancer cells of different origin
Summary
The CYP4A and CYP4F families of cytochrome P450 enzymes catalyze the omega-hydroxylation of AA to form the lipid mediator, 20-HETE.Citation90,Citation91 Furthermore, 20-HETE stimulates mitogenic and angiogenic responses in vitro and in vivo;Citation92 it also contributes to cancer cell proliferation and tumor growth.Citation65 The stable 20-HETE agonists promote the proliferation of cancer cells, whereas selective inhibitors of the CYP4A and CYP4F families prevent the proliferation of glioblastoma, prostate, renal adenocarcinoma, and breast cancer cell lines. mRNAs encoding CYP4A/4F enzymes are markedly elevated in thyroid, breast, colon, and ovarian cancer. These findings provide the rationale for targeting 20-HETE-producing enzymes in human cancers and could be the basis for the development of novel therapeutic strategies for anticancer treatment.
Acknowledgments
Supported by following grants: Johnson & Johnson grant; Wisconsin Breast Cancer Showhouse Research Grant; NIH grant R21DK088018; Fraternal Order of Eagles Research Grant; and a grant from the Advancing a Healthier Wisconsin Program and The Medical College of Wisconsin Cancer Center.
Disclosure
The authors report no conflicts of interest in this work.
References
- GuoAMArbabASFalckJRActivation of vascular endothelial growth factor through reactive oxygen species mediates 20-hydroxyeicosatetraenoic acid-induced endothelial cell proliferationJ Pharmacol Exp Ther20073211182717210799
- StecDEGannonKPBeairdJSDrummondHA20-hydroxyeicosatetraenoic acid (20-HETE) stimulates migration of vascular smooth muscle cellsCell Physiol Biochem2007191–412112817310106
- LinFRiosAFalckJRBelosludtsevYSchwartzmanML20-hydroxyeicosatetraenoic acid is formed in response to EGF and is a mitogen in rat proximal tubuleAm J Physiol19952696 Pt 2F806F8168594874
- O’DonnellVBMaskreyBTaylorG WEicosanoids: generation and detection in mammalian cellsMethods Mol Biol200946252319160658
- ImigJDEicosanoid regulation of the renal vasculatureAm J Physiol Renal Physiol20002796F965F98111097615
- ChatziantoniouCArendshorstWJRenal vascular reactivity to vasodilator prostaglandins in genetically hypertensive ratsAm J Physiol19922621 Pt 2F124F1301733288
- WangDDuBoisRNProstaglandins and cancerGut200655111512216118353
- WangDDuBoisRNEicosanoids and cancerNat Rev Cancer201010318119320168319
- RigasBGoldmanISLevineLAltered eicosanoid levels in human colon cancerJ Lab Clin Med199312255185238228569
- WangDDuboisRNCyclooxygenase-2: a potential target in breast cancerSemin Oncol2004311 Suppl 3647315052544
- McLemoreTLHubbardWCLitterstCLProfiles of prostaglandin biosynthesis in normal lung and tumor tissue from lung cancer patientsCancer Res19884811314031473130187
- SorokinACyclooxygenase-2: potential role in regulation of drug efflux and multidrug resistance phenotypeCurr Pharm Des200410664765714965327
- PatelVADunnMJSorokinARegulation of MDR-1 (P-glycoprotein) by cyclooxygenase-2J Biol Chem200227741389153892012138126
- MyungSJRerkoRMYanM15-hydroxyprostaglandin dehydrogenase is an in vivo suppressor of colon tumorigenesisProc Natl Acad Sci USA200610332120981210216880406
- NakanishiMMontroseDCClarkPGenetic deletion of mPGES-1 suppresses intestinal tumorigenesisCancer Res20086893251325918451151
- BuchananFGWangDBargiacchiFDuBoisRNProstaglandin E2 regulates cell migration via the intracellular activation of the epidermal growth factor receptorJ Biol Chem200327837354513545712824187
- PaiRSoreghanBSzaboILPavelkaMBaatarDTarnawskiASProstaglandin E2 transactivates EGF receptor: a novel mechanism for promoting colon cancer growth and gastrointestinal hypertrophyNat Med20028328929311875501
- ShengHShaoJWashingtonMKDuBoisRNProstaglandin E2 increases growth and motility of colorectal carcinoma cellsJ Biol Chem200127621180751808111278548
- WangDWangHShiQProstaglandin E(2) promotes colorectal adenoma growth via transactivation of the nuclear peroxisome proliferator-activated receptor deltaCancer Cell20046328529515380519
- ShengHShaoJMorrowJDBeauchampRDDuBoisRNModulation of apoptosis and Bcl-2 expression by prostaglandin E2 in human colon cancer cellsCancer Res19985823623669443418
- PoligoneBBaldwinASPositive and negative regulation of NF-kappaB by COX-2: roles of different prostaglandinsJ Biol Chem200127642386583866411509575
- FosslienEReview: molecular pathology of cyclooxygenase-2 in cancer-induced angiogenesisAnn Clin Lab Sci200131432534811688844
- GatelySThe contributions of cyclooxygenase-2 to tumor angiogenesisCancer Metastasis Rev2000191–2192711191059
- TakakuKSonoshitaMSasakiNSuppression of intestinal polyposis in Apc(delta 716) knockout mice by an additional mutation in the cytosolic phospholipase A(2) geneJ Biol Chem200027544340133401610969066
- TrompezinskiSPernetISchmittDViacJUV radiation and prostaglandin E2 up-regulate vascular endothelial growth factor (VEGF) in cultured human fibroblastsInflamm Res200150842242711556523
- PaiRSzaboILSoreghanBAAtaySKawanakaHTarnawskiASPGE(2) stimulates VEGF expression in endothelial cells via ERK2/ JNK1 signaling pathwaysBiochem Biophys Res Commun2001286592392811527387
- PradonoPTazawaRMaemondoMGene transfer of thromboxane A(2) synthase and prostaglandin I(2) synthase antithetically altered tumor angiogenesis and tumor growthCancer Res2002621636611782360
- Il LeeSZuoXShureiqiI15-Lipoxygenase-1 as a tumor suppressor gene in colon cancer: is the verdict in?Cancer Metastasis Rev2011303–448149122037943
- GaoXGrignonDJChbihiTElevated 12-lipoxygenase mRNA expression correlates with advanced stage and poor differentiation of human prostate cancerUrology19954622272377624992
- LarréSTranNFanCPGE2 and LTB4 tissue levels in benign and cancerous prostatesProstaglandins Other Lipid Mediat2008871–4141918577464
- DreylingKWHoppeUPeskarBAMorgenrothKKozuschekWPeskarBMLeukotriene synthesis by human gastrointestinal tissuesBiochim Biophys Acta198687821841933019409
- HennigRDingXZTongWG5-lipoxygenase and leukotriene B(4) receptor are expressed in human pancreatic cancers but not in pancreatic ducts in normal tissueAm J Pathol2002161242142812163367
- IharaAWadaKYonedaMFujisawaNTakahashiHNakajimaABlockade of leukotriene B4 signaling pathway induces apoptosis and suppresses cell proliferation in colon cancerJ Pharmacol Sci20071031243217220595
- KandouzMNieDPidgeonGPKrishnamoorthySMaddipatiKRHonnKVPlatelet-type 12-lipoxygenase activates NF-kappaB in prostate cancer cellsProstaglandins Other Lipid Mediat2003713–418920414518561
- NieDKrishnamoorthySJinRMechanisms regulating tumor angiogenesis by 12-lipoxygenase in prostate cancer cellsJ Biol Chem200628127186011860916638750
- McCabeNPSelmanSHJankunJVascular endothelial growth factor production in human prostate cancer cells is stimulated by overexpression of platelet 12-lipoxygenaseProstate200666777978716482570
- TangDGGrossiIMChenYQDiglioCAHonnKV12(S)-HETE promotes tumor-cell adhesion by increasing surface expression of alpha V beta 3 integrins on endothelial cellsInt J Cancer19935411021118478136
- PanigrahyDKaipainenAGreeneERHuangSCytochrome P450-derived eicosanoids: the neglected pathway in cancerCancer Metastasis Rev201029472373520941528
- RomanRJP-450 metabolites of arachidonic acid in the control of cardiovascular functionPhysiol Rev200282113118511773611
- CapdevilaJHFalckJRHarrisRCCytochrome P450 and arachidonic acid bioactivation. Molecular and functional properties of the arachidonate monooxygenaseJ Lipid Res200041216318110681399
- WangDDuBoisRNMeasurement of eicosanoids in cancer tissuesMethods Enzymol2007433275017954227
- PowellPKWolfIJinRLaskerJMMetabolism of arachidonic acid to 20-hydroxy-5,8,11, 14-eicosatetraenoic acid by P450 enzymes in human liver: involvement of CYP4F2 and CYP4A11J Pharmacol Exp Ther19982853132713369618440
- BellamineAWangYWatermanMRCharacterization of the CYP4A11 gene, a second CYP4A gene in humansArch Biochem Biophys2003409122122712464262
- ChristmasPJonesJPPattenCJAlternative splicing determines the function of CYP4F3 by switching substrate specificityJ Biol Chem200127641381663817211461919
- YuWChaiHLiYIncreased expression of CYP4Z1 promotes tumor angiogenesis and growth in human breast cancerToxicol Appl Pharmacol20122641738322841774
- MullerDNSchmidtCBarbosa-SicardEMouse Cyp4a isoforms: enzymatic properties, gender- and strain-specific expression, and role in renal 20-hydroxyeicosatetraenoic acid formationBiochem J2007403110911817112342
- YamaguchiYKiritaSHasegawaHContribution of CYP4A8 to the formation of 20-hydroxyeicosatetraenoic acid from arachidonic acid in rat kidneyDrug Metab Pharmacokinet200217210911615618658
- NakagawaKHollaVRWeiYSalt-sensitive hypertension is associated with dysfunctional Cyp4a10 gene and kidney epithelial sodium channelJ Clin Invest200611661696170216691295
- EscalanteBSessaWCFalckJRYadagiriPSchwartzmanMLVasoactivity of 20-hydroxyeicosatetraenoic acid is dependent on metabolism by cyclooxygenaseJ Pharmacol Exp Ther198924812292322492340
- HarderDRNarayananJGebremedhinDPressure-induced myogenic tone and role of 20-HETE in mediating autoregulation of cerebral blood fowAm J Physiol Heart Circ Physiol20113005H1557H156521257913
- WilliamsJMSarkisALopezBRyanRPFlaschAKRomanRJElevations in renal interstitial hydrostatic pressure and 20-hydroxyeicosatetraenoic acid contribute to pressure natriuresisHypertension200749368769417210834
- WilliamsJMMurphySBurkeMRomanRJ20-hydroxyeicosatetraeonic acid: a new target for the treatment of hypertensionJ Cardiovasc Pharmacol201056433634420930591
- QuigleyRBaumMReddyKMGrienerJCFalckJREffects of 20-HETE and 19(S)-HETE on rabbit proximal straight tubule volume transportAm J Physiol Renal Physiol20002786F949F95310836982
- WangSMengFXuJGuYEffects of lipids on ENaC activity in cultured mouse cortical collecting duct cellsJ Membr Biol20092272778519122972
- PavlovTSIlatovskayaDVLevchenkoVMattsonDLRomanRJStaruschenkoAEffects of cytochrome P-450 metabolites of arachidonic acid on the epithelial sodium channel (ENaC)Am J Physiol Renal Physiol20113013F672F68121697242
- HoaglandKMFlaschAKRomanRJInhibitors of 20-HETE formation promote salt-sensitive hypertension in ratsHypertension200342466967312874093
- ChenLAckermanRGuoAM20-HETE in neovascularizationProstaglandins Other Lipid Mediat2012983–4636822227460
- AmaralSLMaierKGSchippersDNRomanRJGreeneASCYP4A metabolites of arachidonic acid and VEGF are mediators of skeletal muscle angiogenesisAm J Physiol Heart Circ Physiol20032845H1528H153512521947
- RapisardaAMelilloGRole of the VEGF/VEGFR axis in cancer biology and therapyAdv Cancer Res201211423726722588059
- IshizukaTChengJSinghH20-hydroxyeicosatetraenoic acid stimulates nuclear factor-kappaB activation and the production of infammatory cytokines in human endothelial cellsJ Pharmacol Exp Ther2008324110311017947496
- ZengQHanYBaoY20-HETE increases NADPH oxidasederived ROS production and stimulates the L-type Ca2+ channel via a PKC-dependent mechanism in cardiomyocytesAm J Physiol Heart Circ Physiol20102994H1109H111720675568
- AkbulutTRegnerKRRomanRJAvnerEDFalckJRParkF20-HETE activates the Raf/MEK/ERK pathway in renal epithelial cells through an EGFR- and c-Src-dependent mechanismAm J Physiol Renal Physiol20092973F662F67019570883
- AlexanianAMillerBRomanRJSorokinA20-HETE-producing enzymes are up-regulated in human cancersCancer Genomics Proteomics20129416316922798501
- LaiGWuJLiuXZhaoY20-HETE induces hyperglycemia through the cAMP/PKA-PhK-GP pathwayMol Endocrinol201226111907191622918876
- GuoMRomanRJFenstermacherJD9L gliosarcoma cell proliferation and tumor growth in rats are suppressed by N-hydroxy-N′-(4-butyl-2-methylphenol) formamidine (HET0016), a selective inhibitor of CYP4AJ Pharmacol Exp Ther200631719710816352703
- AlexanianARufanovaVAMillerBFlaschARomanRJSorokinADown-regulation of 20-HETE synthesis and signaling inhibits renal adenocarcinoma cell proliferation and tumor growthAnticancer Res200929103819382419846914
- MiyataNTaniguchiKSekiTHET0016, a potent and selective inhibitor of 20-HETE synthesizing enzymeBr J Pharmacol2001133332532911375247
- SatoMIshiiTKobayashi-MatsunagaYDiscovery of a N′-hydroxyphenylformamidine derivative HET0016 as a potent and selective 20-HETE synthase inhibitorBioorg Med Chem Lett200111232993299511714595
- YuMCambj-SapunarLKehlFEffects of a 20-HETE antagonist and agonists on cerebral vascular toneEur J Pharmacol2004486329730614985052
- QinXKwansaHBucciERomanRJKoehlerRCRole of 20-HETE in the pial arteriolar constrictor response to decreased hematocrit after exchange transfusion of cell-free polymeric hemoglobinJ Appl Physiol2006100133634216166237
- StecDEGannonKPBeairdJSDrummondHA20-hydroxyeicosatetraenoic acid (20-HETE) stimulates migration of vascular smooth muscle cellsCell Physiol Biochem2007191–412112817310106
- TunctanBKorkmazBBuharaliogluCKA 20-hydroxyeicosatetraenoic acid agonist, N-[20-hydroxyeicosa-5(Z),14(Z)-dienoyl]glycine, opposes the fall in blood pressure and vascular reactivity in endotoxin-treated ratsShock200830332933518323740
- RegnerKRZukAVan WhySKProtective effect of 20-HETE analogues in experimental renal ischemia reperfusion injuryKidney Int200975551151719052533
- ParkFSweeneyWEJiaGRomanRJAvnerED20-HETE mediates proliferation of renal epithelial cells in polycystic kidney diseaseJ Am Soc Nephrol2008208101929193918596124
- NithipatikomKIsbellMASeeWACampbellWBElevated 12- and 20-hydroxyeicosatetraenoic acid in urine of patients with prostatic diseasesCancer Lett2006233221922515882928
- JacobsVLValdesPAHickeyWFDe LeoJACurrent review of in vivo GBM rodent models: emphasis on the CNS-1 tumour modelASN Neuro201133e0006321740400
- GuoMRomanRJFalckJREdwardsPAScicliAGHuman U251 glioma cell proliferation is suppressed by HET0016 [N-hydroxy-N′-(4-butyl-2-methylphenyl)formamidine], a selective inhibitor of CYP4AJ Pharmacol Exp Ther2005315252653316081682
- ChenPGuoMWygleDInhibitors of cytochrome P450 4A suppress angiogenic responsesAm J Pathol2005166261562415681843
- GuoAMShengJScicliGMExpression of CYP4A1 in U251 human glioma cell induces hyperproliferative phenotype in vitro and rapidly growing tumors in vivoJ Pharmacol Exp Ther20083271101918591218
- YuWChenLYangYQCytochrome P450 ω-hydroxylase promotes angiogenesis and metastasis by upregulation of VEGF and MMP-9 in non-small cell lung cancerCancer Chemother Pharmacol201168361962921120482
- WeissRHLinPYKidney cancer: identification of novel targets for therapyKidney Int200669222423216408110
- WuCCLiuYChenJCYP4F isoform expression and 20-HETE synthesis in prostate cancer cells are regulated by androgen and contribute to growth [abstract]Cancer Res2012728 Suppl 1LB-160
- ArunBGossPThe role of COX-2 inhibition in breast cancer treatment and preventionSemin Oncol2004312 Suppl 7222915179621
- BadawiAFThe role of prostaglandin synthesis in prostate cancerBJU Int200085445146210691825
- GuptaRADuBoisRNTranslational studies on Cox-2 inhibitors in the prevention and treatment of colon cancerAnn N Y Acad Sci200091019620410911914
- MyersCEGhoshJLipoxygenase inhibition in prostate cancerEur Urol1999355–639539810325495
- SorokinAEicosanoids and resistance of cancer cells to chemotherapeutic agentsBonavidaBSensitization of Cancer Cells for Chemo/Immuno/Radio-therapyLos Angeles, CAHumana Press2008133156
- KhanMFraserACox-2 inhibitors and the risk of cardiovascular thrombotic eventsIr Med J2012105411912122708229
- Garcia RodriguezLACea-SorianoLTacconelliSPatrignaniPCoxibs: pharmacology, toxicity and effcacy in cancer clinical trialsRecent Results Cancer Res2013191679322893200
- GibsonGGComparative aspects of the mammalian cytochrome P450 IV gene familyXenobiotica19891910112311482683413
- McGiffJCCarrollMACytochrome P450-dependent arachidonate metabolites, renal function and blood pressure regulationAdv Prostaglandin Thromboxane Leukot Res199121B6756821847570
- MiyataNRomanRJRole of 20-hydroxyeicosatetraenoic acid (20-HETE) in vascular systemJ Smooth Muscle Res200541417519316258232