Abstract
Nimotuzumab is a humanized monoclonal antibody that binds specifically to human epidermal growth factor receptor, blocking receptor activation. Evidence of its radiosensitizing capacity has been widely evaluated. This article integrates published research findings regarding the role of nimotuzumab in the treatment of high grade glioma in combination with radiotherapy or radiochemotherapy in adult and pediatric populations. First, the mechanisms of action of nimotuzumab and its current applications in clinical trials containing both radiation and chemoradiation therapies are reviewed. Second, a comprehensive explanation of potential mechanisms driving radiosensitization by nimotuzumab in experimental settings is given. Finally, future directions of epidermal growth factor receptor targeting with nimotuzumab in combination with radiation containing regimens, based on its favorable toxicity profile, are proposed. It is hoped that this review may provide further insight into the rational design of new approaches employing nimotuzumab as a useful alternative for the therapeutic management of high grade glioma.
Keywords:
Introduction
Gliomas are the most frequently occurring primary tumor of the central nervous system, classified as grade 1 to 4 on the basis of histopathological features and clinical criteria established by the World Health Organization. This classification includes pilocytic astrocytoma (grade 1), diffuse astrocytoma (grade 2), anaplastic astrocytoma ([AA] grade 3), and glioblastoma multiform ([GBM] grade 4).Citation1 Grade 3 and 4 tumors are considered malignant or high grade gliomas (HGG). HGG are the most aggressive form of primary brain tumor without an effective therapy. Despite its relatively low incidence, which is approximately 5 cases per 100,000 people,Citation2 the highly aggressive nature of this tumor remains a challenge for oncologists. HGG usually proliferate and invade extensively into surrounding areas in the brain yielding short life expectancies despite new aggressive modalities of treatment. Therefore, a need for further therapy options, as well as new approaches that evaluate potential combinations of existing modality treatments, is urgently needed. The aim of this review is to integrate published research findings regarding the role of nimotuzumab, a monoclonal antibody against the epidermal growth factor receptor (EGFR) in combination with radiotherapy and chemoradiation in the treatment of HGG, focusing on its additional value for enhancing the efficacy of radiotherapy through accumulated nonclinical and clinical evidence.
Current standard therapies in HGG
The current standard treatment in HGG consists of a combined approach of surgery and radiation, or combined radiation and chemotherapy, depending on the site of the disease as well as a patient’s health condition.Citation3,Citation4 Surgery is the first treatment choice and a maximal surgical resection is indicated whenever possible. However, because of their highly infiltrative nature, HGG cannot be completely eliminated surgically. Indeed, the value of surgery in prolonging patient survival is still controversial.Citation5–Citation8
Subsequent to an optimal surgical resection or biopsy, ionizing radiation is the dominant form of therapy administered postoperatively, prolonging median survival for a maximum of 6 to 8 months.Citation2 Indeed, ionizing radiation is prescribed in the majority of patients with HGG. However, despite the fact that new methods have increased the therapeutic potential of radiation in oncology, a curative treatment remains dismal. The local failure of radiotherapy has been previously outlined by others with respect to the application of sublethal doses of irradiation that may promote the migration and invasiveness of glioma cells.Citation9 Tumor recurrences at the original site invariably occur after radiation therapy impairing its efficacy.Citation10 Migrating tumor cells may reach the edges of the target volume of postoperative radiotherapy, escape delivery of a cumulatively lethal dose, and form the basis for locoregional relapse during or after a few months of radiotherapy.Citation10 Radiotherapy is also frequently indicated in glioblastoma patients with palliative intention, however, with significant limitations. Such limitations include intrinsic resistance of glioma cells to damage induced by ionizing radiation.Citation11 Furthermore, an important proportion of glioma cells can survive irradiation, inducing their proliferation to accelerate tumor cell repopulation during radiation challenge.Citation12,Citation13
More recently, chemotherapy has gained prominence in the management of malignant gliomas. The 1-year patient survival rate increased from 6% to 10% after adjuvant chemotherapy.Citation14 However, despite the moderate success of several agents such as temozolomide, an oral alkylating agent with encouraging results, current conventional protocols still demonstrate a high incidence of locoregional failure and poor overall survival (OS) rates. The introduction of temozolomide has significantly prolonged patient survival, but its efficacy strongly depends on the presence of the DNA repair enzyme, OCitation6-methyl-guanine-DNA methyltransferase (MGMT). DNA promoter methylation status of MGMT is emblematic of repair enzyme activity in the tumor,Citation15 particularly in GBM.Citation14,Citation16 Patients with unmethylated MGMT promoters, which accounts for approximately half of GBM patients,Citation17 who receive concurrent and adjuvant temozolomide chemotherapy with radiotherapy have 2- and 5-year survival rates of 15% and 8%, respectively.Citation18 The success of this modality is mainly limited due to the appearance of a remarkable resistance of tumors to cytotoxic agents. Attempts to overcome such resistance with higher doses of chemotherapeutics result in unacceptable toxicity and bystander damage to the normal tissues.
Due to the limited efficacy of conventional therapies, further attempts to increase patient survival have recently focused on molecular pathways that underlie malignant processes in HGG. The identification of specific molecular traits that underlie the processes of malignant progression has allowed the development of new therapeutic approaches targeting specific differences between normal and malignant cells. A better understanding of molecular mechanisms employed by tumor cells to evade the inhibitory activity of cytotoxic therapies is essential to the rational design of novel strategies that may help to improve their effectiveness in HGG treatment.Citation12,Citation19
One of the well known molecular features of HGG is the amplification of the EGFR gene, which occurs in approximately 40% to 50% of patients.Citation20,Citation21 EGFR overexpression has been found in approximately half of HGG patients and shows a significant association with EGFR gene amplification.Citation22 Furthermore, EGFR overexpression has been reported to correlate with more aggressive disease, resistance to both radio- and chemotherapy, and a poor prognosis in patients.Citation23,Citation24 In the HGG pediatric population, despite being less frequent, EGFR expression has been also correlated with a more aggressive phenotype and worse patient prognosis.Citation25 EGFR has been considered a promising target in the treatment of HGG and several therapeutic agents, such as tyrosine kinase inhibitors and specific anti-EGFR monoclonal antibodies, are currently under evaluation.Citation26 The EGFR class of molecularly targeted agents is attractive for several reasons. First, EGFR is frequently found to be overexpressed in a substantial proportion of human tumors, warranting broad application. Second, the activation of signal transduction pathways driven by the EGFR family is central to many malignant processes. Third, EGFR overexpression has been largely associated with a poor prognosis and resistance to conventional therapies in many tumor types. In line with this, promising preclinical studies have prompted the development of several clinical trials testing the tolerability and efficacy of various EGFR inhibitors, both as a single agent therapy, and in combination with conventional cytotoxic therapies (radiotherapy and chemotherapy). However, despite the large number of compounds under evaluation, the success of these agents in the management of HGG has been limited and clinical results are still modest.
Nimotuzumab: a monoclonal antibody to EGFR
Nimotuzumab is a humanized IgG1 monoclonal antibody which recognizes the extracellular domain of EGFR.Citation27 It competitively binds to the receptor preventing further ligand binding and subsequent EGFR activation. As a result of such a blockade, an antagonistic biological effect on the tumor cell proliferation is exerted.Citation28,Citation29 Also in response to EGFR blockade by nimotuzumab, tumor cells decrease their capacity to secrete proangiogenic factors, such as vascular endothelial growth factor (VEGF), leading to decreased blood vessel formation and increased apoptotic cell death in human tumor xenografts overexpressing EGFR.Citation30,Citation31 In addition, nimotuzumab has shown an ability to recruit other immunological mechanisms such as antibody mediated cellular cytotoxicity and complement dependent cytotoxic effects.Citation28 Currently, nimotuzumab has been granted approval for use in patients with advanced squamous cell carcinoma of the head and neck,Citation32,Citation33 HGG,Citation34,Citation35 and advanced esophageal carcinoma.Citation36 In all these indications, the efficacy of nimotuzumab is based on the combination of the antibody with radiotherapy or radiochemotherapy.
Our group has previously demonstrated the ability of nimotuzumab to increase the antitumor activity of radiation in the U87MG human glioblastoma xenografted mouse model.Citation37 Based on these studies it was determined that adding nimotuzumab to radiation treatment significantly increased the inhibition of EGFR related signaling pathway activation, increasing the antiproliferative activity of both therapies. Such inhibition was not apparent for tumors treated with radiation alone, suggesting a rationale for combining the antibody with radiotherapy in this tumor model. Based upon such observations of a synergistic effect of nimotuzumab in xenograft models, nimotuzumab has been administered in combination with radiation therapy, enhancing its antitumor activity in a number of clinical trials.
Clinical experience of nimotuzumab in combination with radiotherapy or radiochemotherapy in HGG
Several clinical trials have evaluated nimotuzumab concomitant with radiation containing regimens in HGG patients, demonstrating a clinical benefit of the combination therapy in terms of response rate, control disease rate, and OS. and summarize the main results of those clinical trials.
Table 1 Clinical trials of nimotuzumab in combination with radiation and chemoradiation in adult high grade glioma
Table 2 Clinical trials of nimotuzumab in combination with radiation and chemoradiation in pediatric high grade glioma
Adult population
The first clinical trial regarding the use of nimotuzumab in HGG was a multicenter, one arm study that evaluated the safety and preliminary efficacy of the antibody in combination with radiation in newly diagnosed HGG ().Citation34 This study included 29 patients: 12 patients with AA, 16 patients with GBM, and one patient with anaplastic oligodendroglioma. The objective response rate for patients treated with the combination therapy was 37.9% (11 of 29 patients) and the control disease rate was 79.3% (23 patients). The progression free survival to 1 year was 68.8% for patients with GBM and 91.7% for patients with AA. The median survival time for patients with GBM was 17.5 months, whereas the median survival time for AA patients had not been reached at the time the final report was written. Moreover, the survival rate at 2 years was 24% among patients with GBM and 55% in the AA group. Antibody infusions were well tolerated, and the most commonly reported adverse events were headache, chills, fever, nausea, and transaminase elevation, all of them classified as mild according to Common Toxicity Criteria (CTCAE, version 3.0).Citation38 However, the study was limited in that the trial did not compare the nimotuzumab combination with a temozolomide based chemoradiotherapy treatment, which is currently the standard of care for patients with HGG. Based on the response rate and improvement in survival, nimotuzumab was approved as a radiation sensitizing agent for HGG patients undergoing primary radiation based treatment.
A new randomized multicenter clinical trial was conducted in HGG patients that received radiation plus nimotuzumab or placebo ().Citation39 This study included 70 patients: 41 AA and 29 GBM. The median survival time for patients treated with nimotuzumab and radiation was 17.8 months, while the median survival time in the control arm was 12.6 months. According to histologic stratum analysis, the median survival among AA patients was 44.6 months for nimotuzumab group versus 14.6 months in the control group; whereas among GBM patients, the median survival was 16.1 months in nimotuzumab group versus 8.4 months in the control group. Moreover, the combination of nimotuzumab and radiation was safe and the most frequent related adverse events were nausea, fever, tremors, and anorexia, all of them classified as grade 1 and 2.
The fact that patients with HGG who failed to respond to temozolomide based therapy had a 2-year survival rate of 15% (patients treated with radiation only have a 2-year survival of 2%),Citation18 has led to clinical trials of nimotuzumab in combination with temozolomide and radiation therapy in patients with GBM. A Phase I/II trial evaluated whether nimotuzumab enhanced the effect of radiochemotherapy in HGG ().Citation40 A total of 41 patients were randomized to receive 6 weekly infusions of nimotuzumab (200 mg), or placebo in addition to radiotherapy (46–66 Gy) and a temozolomide based regime. Temozolomide was administered daily (75 mg/mCitation2) during radiation therapy. On completion of radiation therapy, there was a 28-day treatment break, followed by a second phase of up to six 28-day cycles of adjuvant temozolomide treatment at 150 mg/mCitation2 once daily for 5 days followed by 23 days without treatment. At the start of cycle 2, the dose was escalated to 200 mg/mCitation2/day if hematological toxicity was within prescribed limits. Mean and median survival time of the treatment group was 14.3 and 16.5 months, respectively, compared to 10.4 and 10.5 months in the control group. The 1-year survival rates of the treatment and control groups were 81.3% and 69.1%, respectively. Nimotuzumab also showed a synergistic effect in combination with chemoradiation; 14 of 20 evaluable patients achieved major responses (70%), including three patients with complete responses (15%).
A confirmatory Phase III study was conducted in Germany in 149 patients with newly diagnosed GBM, 142 of them evaluable for efficacy ().Citation41 In this study patients were randomized to receive nimotuzumab/temozolomide/radiation versus temozolomide/radiation. After daily dosage during radiotherapy, the temozolomide schedule was changed to a 5-day, 4-weekly schedule until week 36. In the nimotuzumab arm, patients received weekly infusions of the antibody (400 mg) until week 11, which was changed to a biweekly application at week 13 (consolidation phase), and discontinued after 1 year of treatment. The median survival time for the nimotuzumab arm was 22.3 months in comparison to 19.6 months in the radiation/temozolomide arm. The difference in median OS in both arms did not achieve a statistical significance, probably due to the unexpected survival registered in the control arm. A subsequent subgroup analysis showed a longer survival time when patients with EGFR positive tumors, nonmethylated MGMT promoters, and incompletely resected GBM received nimotuzumab in addition to standard treatment ().Citation41 This subgroup of patients had an OS of 23.8 months when treated with nimotuzumab in comparison with 13.8 months in the control group (P = 0.03). Patients with nonmethylated MGMT (unresponsive to temozolomide) that received nimotuzumab had a median survival time of 19.6 months, as compared to 15.0 months for the same patients not receiving the antibody. The results compared favorably with results of the study by Hegi et al in which the median survival time was 12.7 months in the temozolomide/radiation arm.Citation42 These therapeutic outcomes reinforce the importance of prospectively identified efficacy predictors in targeted therapies, a hypothesis that should be evaluated in future clinical trials. As in other trials, nimotuzumab was well tolerated and did not exacerbate the toxicity of standard therapy. The most common adverse events were headache, fatigue, nausea, vomiting, thrombocytopenia, aphasia, and mild to moderate acneiform rash.
Pediatric population
A landmark multinational Phase III trial recently evaluated the safety and efficacy of nimotuzumab in combination with radiotherapy in newly diagnosed diffuse intrinsic pontine glioma (DIPG) ().Citation43 Forty-two patients (of which 41 were evaluable) were treated with a 12-week induction therapy of nimotuzumab given weekly at a dose of 150 mg/mCitation2, concomitantly with standard radiotherapy at weeks 3 to 8 (total dose of 54 Gy). In case of nonprogressive disease, the antibody was subsequently administered biweekly in a consolidation phase until disease progression. The best response was a partial response in four patients (9.8%) and stable disease in 27 patients (65.8%). The median progression free survival and OS were 5.8 and 9.4 months, respectively. Two patients were alive 2 years after the start of treatment, and one patient was stable and well 6 years after the primary treatment.Citation43 Moreover, significantly longer survival times were documented in radiological responder patients (partial response, stable disease) when compared to nonresponder (progressive disease) patients (P < 0.005). Repeated applications of nimotuzumab in combination with radiotherapy were safe and well tolerated. There were, again, only minor side effects like fever and erythema (grade 1 and 2) due to the antibody and no patient discontinued the treatment due to adverse events.
Two other ongoing Phase II clinical trials are testing the safety and efficacy of nimotuzumab in combination with different cytotoxic regimens in DIPG.Citation35,Citation44 A Phase II multinational study is being conducted in Cuba and Brazil to evaluate the safety and efficacy of nimotuzumab in combination with radiotherapy ().Citation44 So far, nine patients have been evaluated. Despite the small number of patients under evaluation, six evaluable patients were reported with stabilized disease after 24 weeks of treatment. The combination therapy was well tolerated and only one mucositis (grade 1–2) has been reported.
Vinorelbine has shown efficacy in the treatment of gliomas, and a new trial is evaluating the feasibility of including it with radiotherapy and nimotuzumab in a pediatric population as part of a Phase II study conducted at the Instituto Nazionale Tumori, in Milan ().Citation35 So far, 22 patients have been treated and the 1- and 2-year PFS is 26% and 20%, while the 1-year OS is 73%, and the 2-year OS is 25%. Prior to final evaluation of the study, preliminary results are encouraging and suggest the feasibility of combining nimotuzumab with radiotherapy and vinorelbine for the treatment of DIPG. Therefore, these results suggest that combination therapy with nimotuzumab may prolong patient survival without adding toxicity to the standard therapy.
Potential mechanisms driving the radiosensitization of nimotuzumab
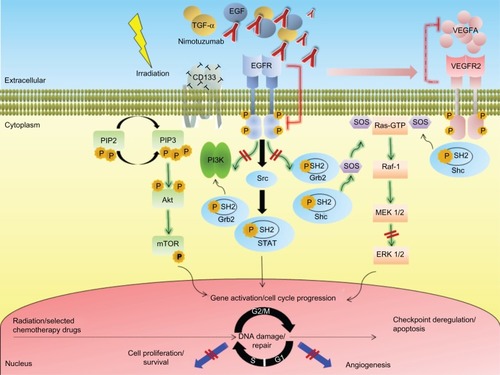
A series of nonclinical studies, including head and neck squamous cell carcinoma (HNSCC), non-small-cell lung carcinoma (NSCLC), and GBM xenografts, have shown the radiosensitizing capability of nimotuzumab and therefore its ability to synergize with ionizing radiation.Citation37,Citation45,Citation46 Indeed, the clearest benefit of nimotuzumab treatment to date comes from studies where the antibody was combined with radiotherapy to treat advanced tumors. A schematic illustration depicting different mechanisms involved in radiosensitization of nimotuzumab is shown in . Interesting findings could explain why nimotuzumab has a positive and different quality to potentiate the effects of radiotherapy.Citation12,Citation47
Figure 1 Simplified schematic illustration depicting different mechanisms implicated in radiosensitization by the anti-epidermal growth factor receptor monoclonal antibody nimotuzumab in high grade glioma.
Abbreviations: Akt, protein kinase B; CD, cluster differentiation; EGF, epidermal growth factor; EGFR, epidermal growth factor receptor; ERK, extracellular signal-regulated kinase; Grb2, growth factor receptor-bound protein 2; GTP, guanosine-5′-triphosphate; MEK, mitogen activated protein kinase kinase; mTOR, mammalian target of rapamycin; PI3K, phosphatidyl inositol 3 kinase; PIP2, phosphatidylinositol 4,5-bisphosphate; PIP3, phosphatidylinositol 3,4,5-trisphosphate; Raf, rapidly accelerated fibrosarcoma; Ras, rat sarcoma; SH2, Src homology-2; shc, Src-homology collagen protein; SOS, son of sevenless homolog 1; Src, sarcoma; STAT, signal transducer and activator of transcription; TGF-α, transforming growth factor alpha; VEGFA, vascular endothelial growth factor type A; VEGFR2, VEGF receptor 2.
Antiproliferative effects of nimotuzumab
The effect of radiation on tumor cell proliferation has been extensively evaluated in the preclinical setting using different tumor models. Previous findings indicate that in cell lines that overexpress EGFR, the receptor becomes rapidly phosphorylated and activated after the administration of clinically relevant doses of radiation.Citation48 A rapid repopulation after radiotherapy, by increased cell proliferation, shortens the time to recurrence of the disease, impairs local tumor control, and decreases the OS of patients. Antiproliferative effects of anti-EGFR monoclonal antibodies have also been shown consistently in preclinical experiments.Citation49 In combination with radiotherapy, the inhibition of cell proliferation induced by anti-EGFR monoclonal antibodies would be expected to lead to tumor regression or slower tumor growth. Among the several mechanisms of activation that have been proposed, at least one of them involves ligand stimulated activation. EGFR radiation induced activation was associated with increased tumor cell proliferation, through a mechanism involving the processing and release of transforming growth factor alpha.Citation50 Thus, it is expected that molecular agents that effectively block EGFR activation might potentiate the radiation induced antiproliferative activity, especially in those tumors overexpressing the receptor. In line with this consideration, nimotuzumab was shown to prevent the radiation induced activation of EGFR, as well as to increase its antiproliferative activity in U87MG GBM xenografts when administered in combination with radiation.Citation37 The antiproliferative activity of the combination therapy in this tumor model was shown to be higher than that exerted by each individual therapy, suggesting that nimotuzumab may improve radiation response, at least in part, by blocking radiation induced proliferation.
Targeting of CD133+ cancer stem cells by nimotuzumab
Cancer stem cells (CSC) are defined as those cancer cells that have the capacity to self-renew and to cause the heterogeneous lineages of cells that comprise the tumor.Citation51 Gliomas were among the first solid cancers in which CSC were identified.Citation52,Citation53 In these tumors, the CD133+ but not CD133− cells were reported to be responsible for tumor outgrowth.Citation11,Citation54,Citation55 Furthermore, the CSC population was shown to be a source of radiation resistance within brain tumors.Citation11,Citation56,Citation57 Thus, additional targeting of CSC by a further treatment would be expected to have additive effects on local tumor control. Several studies have established a potential relationship between CSC and the EGFR system and its ligands in brain tumors. The capacity of nimotuzumab to decrease the population of CD133+ CSC in U87MG xenografts has been reported.Citation37 Such inhibition was associated with the ability of the antibody to increase the cytotoxic activity of radiation in this tumor model. Subsequent studies have shown that GBM CSC that express EGFR have a more aggressive functional and molecular phenotype than noncancer stem cells, and modulation of EGFR expression markedly decreases its tumorigenic potential.Citation58,Citation59 Actually, GBM CSC are more sensitive to Akt signaling inhibition than noncancer stem cells.Citation60 The ability of anti-EGFR monoclonal antibodies to target the CSC population may occur as a consequence of a direct inhibition of the EGFR expressed on these tumor cells, since the latest studies conducted in our group revealed that the CSC markers nestin and CD133 are coexpressed alongside EGFR on the cell surface of the U87MG cells (Diaz-Miqueli, unpublished data, 2009). Alternatively, an indirect targeting of CSC by nimotuzumab might also result from the disruption of the vascular microenvironment of tumors as a result of the antiangiogenic activity of nimotuzumab on tumor xenografts. Since the hypothesis that CSC depends critically upon the presence of an intact vascular niche microenvironment for self-renewal, the disruption of these niche microenvironments by drugs with antiangiogenic potential may ablate the fraction of CSC in brain tumors and arrest tumor growth.Citation61
Perturbations in cell cycle control
Perturbations in cell cycle control induced by monoclonal antibodies may also increase tumor cell response to ionizing radiation.Citation62 Previous separate studies have consistently shown nimotuzumab induces G0/G1 cell cycle arrest in the human epithelial cell line A431.Citation28,Citation30 Ionizing radiation also induces cell cycle arrest, involving checkpoint control at several phases of the cell cycle.Citation63 Thus, the radiosensitizing effect of nimotuzumab may arise from decreasing the fraction of cells in the relatively radioresistant S-phase, with a concomitant increase in the more radiosensitive G1 phase of the cell cycle. This point of view constituted an early premise for rationale combining EGFR inhibitors with ionizing radiation.Citation64 In addition, the combined therapy may prolong the cell cycle, contributing to avoidance of a rapid repopulation after the administration of ionizing radiation.Citation49 Alternatively, a simultaneous cell cycle arrest at both G0/G1 phases induced by nimotuzumab, and G2/M arrest mediated by ionizing radiation might result in cell cycle checkpoint deregulation and subsequent apoptosis.Citation65
Antiangiogenic effects of nimotuzumab
The radiosensitization effects of nimotuzumab, and EGFR inhibitors in general, are more pronounced in vivo, suggesting a potential association with its ability to interfere in tumor– stromal interactions. Complicated interactions between ionizing radiation, EGFR, and the angiogenic processes have been postulated. VEGF and EGFR are key elements in the growth and dissemination of epithelial tumors. Both pathways are closely related, sharing common downstream signaling molecules.Citation66,Citation67 Furthermore, epidermal growth factor (EGF) is one of the growth factors that drive VEGF expression.Citation68 Whereas radiation induced EGFR activation has been postulated to upregulate the secretion of VEGF, a previous study has demonstrated that nimotuzumab decreases VEGF secretion in A431 tumor cells after incubation with the antibody.Citation30 Similar findings have also been consistently demonstrated with other EGFR inhibitors.Citation9,Citation69 Moreover, VEGF receptor expression is increased in A431 mutant cells and the mutant cells acquired resistance to nimotuzumab therapy, despite persistence of antibody treatment.Citation31 Therefore, EGFR inhibition caused by nimotuzumab treatment might sensitize endothelial cells to radiation. However, in contrast to these findings, we found that administration of nimotuzumab concomitant with radiation did not decrease the number of tumor associated vessels in U87MG xenografts when compared to those mice treated with the antibody alone.Citation37 These observations could be explained by the fact that EGFR inhibition exerted by nimotuzumab did not block VEGF, thereby allowing tumor angiogenesis to continue. These observations suggest that the potential mechanistic relevance of the antiangiogenic effect of nimotuzumab should be further evaluated in brain tumors as a radiosensitizer agent.
Signaling pathways affected by nimotuzumab
Aberrant EGF mediated signaling plays an essential role to increase the capacity of tumor cells to proliferate and migrate during the process of tumor growth. The main activated EGFR downstream signaling pathways include the Ras mitogen activated protein kinase (MAPK) cascade, the phosphatidyl inositol 3 kinase (PI3K) cascade, and the signal transducer and activator of transcription (STAT) cascade (). Interestingly, activation of EGFR signaling can also be mediated by radiation in a ligand-independent fashion.Citation70 As a consequence, exposure of tumor cells overexpressing EGFR to radiation activates proliferation mechanisms through stimulated PI3K and MAPK signaling.Citation71 Thus, in combination with radiotherapy, EGFR inhibitors would be expected to enhance sensitivity of tumor cells to ionizing radiation.
Akashi et al have previously reported the synergistic potential of nimotuzumab to increase the antitumor activity of radiation in NSCLC cell lines of differing EGFR status.Citation45 In this study, nimotuzumab inhibited the EGF induced phosphorylation of EGFR in H292 and Ma-1 cells, with high and moderate levels of EGFR expression, respectively, consistent with the mode of action of this antibody. In contrast, nimotuzumab did not block EGFR phosphorylation in H460, H1299, or H1975 cells with low levels of EGFR expression.Citation45 These observations suggest that the inhibition of the EGFR signaling by nimotuzumab may be related to the surface expression level of EGFR. Moreover, despite irradiation of tumor cells that was shown to activate EGFR, possibly accounting for radiation induced acceleration of tumor cell repopulation and radioresistance,Citation48 such activation might increase the ability of nimotuzumab to effectively blockade the EGFR downstream signaling in tumors. Similar findings were documented when nimotuzumab was administered concomitant with radiation in U87MG xenografts.Citation37 In the GBM model, combination therapy significantly increased the capacity of nimotuzumab to inhibit both EGFR phosphorylation and extracellular signal-regulated kinase (ERK) 1/2 induced activation when compared with nimotuzumab alone.Citation37 Altogether, these findings support the notion that inhibition of EGFR signaling by nimotuzumab is responsible, at least in part, for the enhancement of the cytotoxic effect of radiation by this antibody. Such radiation induced activation of EGFR dependent processes may represent a rationale for treatment combination.
Nimotuzumab: an EGFR inhibitor with a unique toxicity profile
In contrast to other EGFR inhibitors, nimotuzumab has a very low toxicity profile and its use in combination with cytotoxic therapies does not exacerbate the toxicity inherent with such therapies. These observations have been largely documented in HGG patients, but they are not restricted to brain tumors. Evidence gathered from more than 20,000 patients treated with the antibody in clinical trials and in open populations with advanced tumors, including HNSCC,Citation32,Citation33,Citation72 NSCLC,Citation73,Citation74 and gastrointestinal cancer among others, support the therapeutic efficacy of nimotuzumab.Citation36,Citation75–Citation77 The clinical benefit of nimotuzumab was equivalent or superior to those of other anti-EGFR monoclonal antibodies with a very low incidence of adverse related events (especially skin rash, which accounts for less than 10% of treated patients) making this antibody an appropriate agent that may be efficaciously administered under long-term schedules and in combination with standard cytotoxic therapies.Citation78 Accumulated clinical experience in HGG patients, especially in the pediatric population, has provided evidence for the feasibility to prolong nimotuzumab therapy with a significant survival benefit.Citation79
An explanation for nimotuzumab’s unique toxicity profile has emerged from several pieces of experimental and modeling data generated by separate groups. In 2004, Crombet and coworkers proposed the existence of an optimal affinity window for antibodies with intermediate affinity for EGFR, based on a mathematical model.Citation32 The hypothesis predicted that antibodies with an intermediate affinity would have a higher ratio of accumulation in tissues with higher EGFR expression levels (tumors) when compared to low density EGFR tissues (healthy tissues) than high affinity antibodies. In contrast to nimotuzumab, higher affinity antibodies would induce a rapid uptake by normal tissues reducing the therapeutic index of these agents. This might be particularly relevant in brain tumors, located in an anatomical area difficult to access for high molecular weight compounds, such as antibodies.Citation80 A subsequent pharmacodynamic trial conducted in HNSSC patients demonstrated that even though nimotuzumab produces downstream inhibition of the EGFR signaling pathway in normal skin cells, the characteristic lymphocytic infiltrates, folliculitis, or perifolliculitis induced by other EGFR inhibitors is not observed in nimotuzumab treated skin patient samples.Citation81 These findings may help to explain the lack of skin rash in patients treated with nimotuzumab.
A more recent study published in Cancer Biology and Therapy has given further support to this hypothesis.Citation82 In this study, the authors evaluated the binding properties of nimotuzumab and cetuximab, and their corresponding Fab fragments, in several cell lines with different levels of surface EGFR expression. Experimental observations demonstrated that the binding properties of nimotuzumab were strongly dependent on the EGFR surface density in tumor cells, whereas cetuximab bound to EGFR expressed in tumor cells irrespective of receptor density. Moreover, the binding properties of the Fab component of nimotuzumab were significantly reduced, even in cell lines with high EGFR expression, while remaining unaffected with cetuximab, even in cell lines with lower levels of EGFR expression. These results strongly support that nimotuzumab receptor attachment is reliant on the antibody’s bivalent binding (two arms of the antibody binds simultaneously to EGFR molecules) and therefore, it is only possible when the EGFR surface density is above a certain threshold (a condition favored in tumors with high EGFR expression).
These observations may have important clinical implications. Firstly, from these findings we could predict a preferential uptake of the antibody in EGFR overexpressing tumors, suggesting a greater benefit from nimotuzumab treatment in those patients with tumors that overexpress the receptor. An increasing interest has arisen over the last few years in identifying molecular traits in HGG that can help predict a response, so that a group of patients who are likely to benefit from a selected treatment might be prospectively selected.Citation83,Citation84 Whether EGFR levels in tumors may have a prognostic value as a predictive marker for nimotuzumab efficacy or not needs to be confirmed in definitive Phase III clinical trials, but recently emerging findings from studies in different tumor indications may support this idea. Two separate Phase II and Phase II/III clinical trials published in 2010 evaluated the efficacy of nimotuzumab in combination with standard cytotoxic therapies in 106 and 92 HNSCC patients, respectively.Citation33,Citation85 In both studies, the EGFR detection by immunohistochemistry showed a significant survival improvement for nimotuzumab treated patients with tumors that overexpressed EGFR. In addition, a new study performed in 68 nonresectable esophageal cancer patients compared the advantage of adding nimotuzumab to a standard regimen of radiation and chemotherapy.Citation36 For nimotuzumab treated patients that overexpress EGFR, the objective response and disease control rate was 60% and 80%, which compares very favorably with the response and disease control rate seen in the control group. Finally, in the above mentioned Phase III study conducted in newly diagnosed GBM patients who received nimotuzumab in addition to temozolomide and radiation, patients with EGFR gene amplification had a trend to benefit in OS when compared to control group ().Citation86 In contrast, OS in patients with no amplifications in the EGFR gene was 16.2 months in nimotuzumab treated patients compared with 21.0 months in the control group.
Altogether, these observations suggest a potential synergy of nimotuzumab with agents that increase EGFR expression, such as radiation containing regimens. The mutual benefit expected from concomitant use of nimotuzumab with cytotoxic therapies, together with a low toxicity profile, makes nimotuzumab a useful candidate for combining with standard cytotoxic agents such radiation in the management of patients with HGG.
Concluding remarks
The current cytotoxic standard therapies have yielded limited efficacy in the treatment of patients with HGG. Unfortunately, despite the aggressive nature of new approaches, modest and transient clinical responses are observed due to the appearance of resistance that develops to these therapies. Thus, novel treatments that can overcome or avoid such limitations are urgently needed to improve patient survival. Nimotuzumab has shown a radiosensitizing capacity and its use concomitant to radiation or chemoradiation may exert synergistic effects with DNA damaging cytotoxic agents, without increasing the toxicity of these standard treatments. The studies presented here summarize published data on the activity of nimotuzumab in combination with radiation containing regimens in HGG. Unusual response rates and longer survival time of HGG patients seen in controlled Phase II and III studies are promising. Moreover, the possibility of using nimotuzumab under long-term schedules due to its low toxicity profile opens new avenues in the therapeutic management of HGG. Based on currently encouraging results, a better understanding of underlying mechanisms of radiosensitization should guide the future optimal implementation of nimotuzumab radiosensitization approaches in HGG.
Acknowledgments
The authors are indebted to Dr Thomas P Quinn for his excellent review of the manuscript for language corrections prior to its publication.
Disclosure
The authors report no conflicts of interest in this work.
References
- LouisDNOhgakiHWiestlerODThe 2007 WHO classification of tumours of the central nervous systemActa Neuropathol20071149710917618441
- LaperriereNZurawLCairncrossGRadiotherapy for newly diagnosed malignant glioma in adults: a systematic reviewRadiother Oncol20026425927312242114
- HartMGGrantRGarsideRRogersGSomervilleMSteinKTemozolomide for high grade glioma [review]Cochrane Database Syst Rev2008CD00741518843749
- KoshyMVillanoJLDolecekTAImproved survival time trends for glioblastoma using the SEER 17 population-based registriesJ Neurooncol201210720721221984115
- NazzaroJMNeuweltEAThe role of surgery in the management of supratentorial intermediate and high-grade astrocytomas in adultsJ Neurosurg1990733313442166779
- LorenzoniJTorricoAVillanuevaPGederliniATorrealbaGSurgery for high-grade gliomas in a developing country: survival estimation using a simple stratification systemSurg Neurol200870591597 discussion 7.18440602
- WolffJEDrieverPHErdlenbruchBIntensive chemotherapy improves survival in pediatric high-grade glioma after gross total resection: results of the HIT-GBM-C protocolCancer201011670571219957326
- OszvaldAGuresirESetzerMGlioblastoma therapy in the elderly and the importance of the extent of resection regardless of ageJ Neurosurg201211635736421942727
- Wild-BodeCWellerMRimnerADichgansJWickWSublethal irradiation promotes migration and invasiveness of glioma cells: implications for radiotherapy of human glioblastomaCancer Res2001612744275011289157
- GardenASMaorMHYungWKOutcome and patterns of failure following limited-volume irradiation for malignant astrocytomasRadiother Oncol199120991101851573
- BaoSWuQMcLendonREGlioma stem cells promote radioresistance by preferential activation of the DNA damage responseNature200644475676017051156
- CiardielloFTortoraGEGFR antagonists in cancer treatmentN Engl J Med20083581160117418337605
- HennequinCQueroLFavaudonVDeterminants and predictive factors of tumour radiosensitivityCancer Radiother20081231318187356
- StuppRMasonWPvan den BentMJEuropean Organization for Research and Treatment of Cancer Brain Tumor and Radiotherapy GroupsNational Cancer Institute of Canada Clinical Trials GroupRadiotherapy plus concomitant and adjuvant temozolomide for glioblastomaN Engl J Med200535298799615758009
- WellerMStuppRReifenbergerGMGMT promoter methylation in malignant gliomas: ready for personalized medicine?Nat Rev Neurol20106395119997073
- YabroffKRHarlanLZerutoCAbramsJMannBPatterns of care and survival for patients with glioblastoma multiforme diagnosed during 2006Neuro Oncol20121435135922241797
- ZawlikIVaccarellaSKitaDMittelbronnMFranceschiSOhgakiHPromoter methylation and polymorphisms of the MGMT gene in glioblastomas: a population-based studyNeuroepidemiology200932212918997474
- StuppRMayerMKannRNeoadjuvant chemotherapy and radiotherapy followed by surgery in selected patients with stage IIIB non-small-cell lung cancer: a multicentre phase II trialLancet Oncol20091078579319604722
- SartorCIMechanisms of disease: Radiosensitization by epidermal growth factor receptor inhibitorsNat Clin Pract Oncol20041808716264825
- Viana-PereiraMLopesJMLittleSAnalysis of EGFR overexpression, EGFR gene amplification and the EGFRvIII mutation in Portuguese high-grade gliomasAnticancer Res20082891392018507036
- JarvelaSHelinHHaapasaloJAmplification of the epidermal growth factor receptor in astrocytic tumours by chromogenic in situ hybridization: association with clinicopathological features and patient survivalNeuropathol Appl Neurobiol20063244145016866989
- BredelMPollackIFHamiltonRLJamesCDEpidermal growth factor receptor expression and gene amplification in high-grade non-brainstem gliomas of childhoodClin Cancer Res199951786179210430083
- MellinghoffIKWangMYVivancoIMolecular determinants of the response of glioblastomas to EGFR kinase inhibitorsN Engl J Med20053532012202416282176
- FedrigoCAGrivicichISchunemannDPRadioresistance of human glioma spheroids and expression of HSP70, p53 and EGFrRadiat Oncol2011615622077956
- GilbertsonRJHillDAHernanRERBB1 is amplified and overexpressed in high-grade diffusely infiltrative pediatric brain stem gliomaClin Cancer Res200393620362414506149
- YardenYPinesGThe ERBB network: at last, cancer therapy meets systems biologyNat Rev Cancer20121255356322785351
- MateoCMorenoEAmourKLombarderoJHumanization of a mouse monoclonal antibody that blocks the epidermal growth factor receptor: recovery of antagonistic activityImmunotechnology1997371819154469
- Diaz MiqueliABlancoRGarciaBBiological activity in vitro of anti-epidermal growth factor receptor monoclonal antibodies with different affinitiesHybridoma (Larchmt)20072642343118158788
- SpicerJHarperPTargeted therapies for non-small cell lung cancerInt J Clin Pract2005591055106216115182
- Crombet-RamosTRakJPerezRViloria-PetitAAntiproliferative, antiangiogenic and proapoptotic activity of h-R3: A humanized anti-EGFR antibodyInt J Cancer200210156757512237899
- Viloria-PetitACrombetTJothySAcquired resistance to the antitumor effect of epidermal growth factor receptor-blocking antibodies in vivo: a role for altered tumor angiogenesisCancer Res2001615090510111431346
- CrombetTOsorioMCruzTUse of the humanized anti-epidermal growth factor receptor monoclonal antibody h-R3 in combination with radiotherapy in the treatment of locally advanced head and neck cancer patientsJ Clin Oncol2004221646165415117987
- RodriguezMORiveroTCdel Castillo BahiRNimotuzumab plus radiotherapy for unresectable squamous-cell carcinoma of the head and neckCancer Biol Ther2010934334920448462
- RamosTCFigueredoJCatalaMTreatment of high-grade glioma patients with the humanized anti-epidermal growth factor receptor (EGFR) antibody h-R3: report from a phase I/II trialCancer Biol Ther2006537537916575203
- MassiminoMBodeUBiassoniVFleischhackGNimotuzumab for pediatric diffuse intrinsic pontine gliomasExpert Opin Biol Ther20111124725621171927
- Ramos-SuzarteMLorenzo-LuacesPLazoNGTreatment of malignant, non-resectable, epithelial origin esophageal tumours with the humanized anti-epidermal growth factor antibody nimotuzumab combined with radiation therapy and chemotherapyCancer Biol Ther20121360060522555809
- Diaz MiqueliARolffJLemmMFichtnerIPerezRMonteroERadiosensitisation of U87MG brain tumours by anti-epidermal growth factor receptor monoclonal antibodiesBr J Cancer200910095095819293809
- Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events v3.0 (CTCAE) Available from : http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdfAccessed July 5, 2013
- SolomonMTSelvaJCFigueredoJRadiotherapy plus the anti-EGFR mAb nimotuzumab or placebo for the treatment of high-grade glioma patients. ASCO Annual Meeting; June 1–5, 2012; Chicago, IL, USAJ Clin Oncol30SupplAbstr 2515
- HongJPengYLiaoYNimotuzumab prolongs survival in patients with malignant gliomas: A phase I/II clinical study of concomitant radiochemotherapy with or without nimotuzumabExp Ther Med2012415115723060940
- WestphalMBachFFinal results of a randomized phase III trial of nimotuzumab for the treatment of newly diagnosed glioblastoma in addition to standard radiation and chemotherapy with temozolomide versus standard radiation and temoziolamide. ASCO Annual Meeting; June 1–5, 2012; Chicago, IL, USAJ Clin Oncol30SupplAbstr 2033
- HegiMEDiserensACGorliaTMGMT gene silencing and benefit from temozolomide in glioblastomaN Engl J Med2005352997100315758010
- FleischhackGSieglerSWarmuth-MetzMFinal results of a phase III study consisting of nimotuzumab and concomitant standard radiotherapy for the treatment of newly diagnosed diffuse intrinsic pontine gliomasPediatr Blood Cancer2009Suppl75519165888
- CrombetTCabanasRAlertJNimotuzumab and radiotherapy in children and adolescents with brain stem glioma: preliminary results from a phase II studyEur J Cancer200972497
- AkashiYOkamotoIIwasaTEnhancement of the antitumor activity of ionising radiation by nimotuzumab, a humanised monoclonal antibody to the epidermal growth factor receptor, in non-small cell lung cancer cell lines of differing epidermal growth factor receptor statusBr J Cancer20089874975518253126
- GonzalezJEBarquineroJFLeeMGarciaOCasacoARadiosensitization induced by the anti-epidermal growth factor receptor monoclonal antibodies cetuximab and nimotuzumab in A431 cellsCancer Biol Ther201213717622231391
- PerezRMorenoEGarridoGCrombetTEGFR-Targeting as a Biological Therapy: Understanding Nimotuzumab’s Clinical EffectsCancers2011320142031
- Schmidt-UllrichRKContessaJNLammeringGAmorinoGLinPSERBB receptor tyrosine kinases and cellular radiation responsesOncogene2003225855586512947392
- HuangSMBockJMHarariPMEpidermal growth factor receptor blockade with C225 modulates proliferation, apoptosis, and radiosensitivity in squamous cell carcinomas of the head and neckCancer Res1999591935194010213503
- DentPReardonDBParkJSRadiation-induced release of transforming growth factor alpha activates the epidermal growth factor receptor and mitogen-activated protein kinase pathway in carcinoma cells, leading to increased proliferation and protection from radiation-induced cell deathMol Biol Cell1999102493250610436007
- ClarkeMFDickJEDirksPBCancer stem cells – perspectives on current status and future directions: AACR Workshop on cancer stem cellsCancer Res2006669339934416990346
- HemmatiHDNakanoILazareffJACancerous stem cells can arise from pediatric brain tumorsProc Natl Acad Sci USA2003100151781518314645703
- SinghSKClarkeIDTerasakiMIdentification of a cancer stem cell in human brain tumorsCancer Res2003635821582814522905
- SinghSKClarkeIDHideTDirksPBCancer stem cells in nervous system tumorsOncogene2004237267727315378086
- BaoSWuQSathornsumeteeSStem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factorCancer Res2006667843784816912155
- EramoARicci-VitianiLZeunerAChemotherapy resistance of glioblastoma stem cellsCell Death Differ2006131238124116456578
- LiuGYuanXZengZAnalysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastomaMol Cancer200656717140455
- MazzoleniSPolitiLSPalaMEpidermal growth factor receptor expression identifies functionally and molecularly distinct tumorinitiating cells in human glioblastoma multiforme and is required for gliomagenesisCancer Res2012707500751320858720
- Ayuso-SacidoAMoliternoJAKratovacSActivated EGFR signaling increases proliferation, survival, and migration and blocks neuronal differentiation in post-natal neural stem cellsJ Neurooncol20109732333719855928
- SatoASunayamaJMatsudaKRegulation of neural stem/progenitor cell maintenance by PI3K and mTORNeurosci Lett201047011512020045038
- CalabreseCPoppletonHKocakMA perivascular niche for brain tumor stem cellsCancer Cell200711698217222791
- ShintaniSLiCMiharaMEnhancement of tumor radioresponse by combined treatment with gefitinib (Iressa, ZD1839), an epidermal growth factor receptor tyrosine kinase inhibitor, is accompanied by inhibition of DNA damage repair and cell growth in oral cancerInt J Cancer20031071030103714601066
- IliakisGWangYGuanJWangHDNA damage checkpoint control in cells exposed to ionizing radiationOncogene2003225834584712947390
- HarariPMHuangSMHead and neck cancer as a clinical model for molecular targeting of therapy: combining EGFR blockade with radiationInt J Radiat Oncol Biol Phys20014942743311173137
- NyatiMKMaheshwariDHanasogeSRadiosensitization by pan ErbB inhibitor CI-1033 in vitro and in vivoClin Cancer Res20041069170014760092
- HerbstRSJohnsonDHMininbergEPhase I/II trial evaluating the anti-vascular endothelial growth factor monoclonal antibody bevacizumab in combination with the HER-1/epidermal growth factor receptor tyrosine kinase inhibitor erlotinib for patients with recurrent non-small-cell lung cancerJ Clin Oncol2005232544255515753462
- TaberneroJThe role of VEGF and EGFR inhibition: implications for combining anti-VEGF and anti-EGFR agentsMol Cancer Res2007520322017374728
- NiuGWrightKLHuangMConstitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesisOncogene2002212000200811960372
- MilasLMasonKHunterNIn vivo enhancement of tumor radioresponse by C225 antiepidermal growth factor receptor antibodyClin Cancer Res2000670170810690556
- Schmidt-UllrichRKMikkelsenRBDentPRadiation-induced proliferation of the human A431 squamous carcinoma cells is dependent on EGFR tyrosine phosphorylationOncogene199715119111979294612
- Schmidt-UllrichRKValerieKFoglemanPBWaltersJRadiation-induced autophosphorylation of epidermal growth factor receptor in human malignant mammary and squamous epithelial cellsRadiat Res199614581858532841
- RamakrishnanMSEswaraiahACrombetTNimotuzumab, a promising therapeutic monoclonal for treatment of tumors of epithelial originMAbs20091414820046573
- BebbGSmithCRorkeSPhase I clinical trial of the anti-EGFR monoclonal antibody nimotuzumab with concurrent external thoracic radiotherapy in Canadian patients diagnosed with stage IIb, III or IV non-small cell lung cancer unsuitable for radical therapyCancer Chemother Pharmacol20116783784520563810
- ChoiHJSohnJHLeeCGA phase I study of nimotuzumab in combination with radiotherapy in stages IIB-IV non-small cell lung cancer unsuitable for radical therapy: Korean resultsLung Cancer201171555920451284
- LingYChenJTaoMChuXZhangXA pilot study of nimotuzumab combined with cisplatin and 5-FU in patients with advanced esophageal squamous cell carcinomaJ Thorac Dis20124586222295168
- KimYHSasakiYLeeKHRandomized phase II study of nimotuzumab, an anti-EGFR antibody, plus irinotecan in patients with 5-fluorouracil-based regimen-refractory advanced or recurrent gastric cancer in Korea and Japan: Preliminary results. 2011 Gastrointestinal Cancers Symposium; January 20–22, 2011; San Francisco, CA, USAJ Clin Oncol201129Suppl 4Abstr 87
- ZhaoKLHuXCWuXHFuXLFanMJiangGLA phase I dose escalation study of Nimotuzumab in combination with concurrent chemoradiation for patients with locally advanced squamous cell carcinoma of esophagusInvest New Drugs2012301585159021901403
- BebbGBolandWMeloskyBDon’t jump to rash conclusionsCancer Biol Ther20111163964121304273
- CabanasRSaurezGRiosMTreatment of children with high grade glioma with nimotuzumab: A 5-y institutional experienceMAbs20135220220723575267
- FortinDThe blood-brain barrier: its influence in the treatment of brain tumors metastasesCurr Cancer Drug Targets20121224725922229251
- RojoFGraciasEVillenaNPharmacodynamic trial of nimotuzumab in unresectable squamous cell carcinoma of the head and neck: a SENDO Foundation studyClin Cancer Res2010162474248220371675
- GarridoGTikhomirovIARabasaABivalent binding by intermediate affinity of nimotuzumab: a contribution to explain antibody clinical profileCancer Biol Ther20111137338221150278
- ZarghooniMBartelsULeeEWhole-genome profiling of pediatric diffuse intrinsic pontine gliomas highlights platelet-derived growth factor receptor alpha and poly (ADP-ribose) polymerase as potential therapeutic targetsJ Clin Oncol2010281337134420142589
- VerhaakRGHoadleyKAPurdomECancer Genome Atlas Research NetworkIntegrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1Cancer Cell2010179811020129251
- BasavarajCSierraPShivuJMelarkodeRMonteroENairPNimotuzumab with chemoradiation confers survival advantage in treatment naive head and neck tumors overexpressing EGFRCancer Biol Ther201010767368120647773
- BodeUMassiminoMBachFNimotuzumab treatment of malignant gliomasExpert Opin Biol Ther201212121649165923043252