Abstract
The nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) proteins are a family of ubiquitously expressed transcription factors that regulate the response to cellular stress. They mediate innate and adaptive immunity through the initiation of an inflammatory response to pro-inflammatory signals. The role of persistent inflammation in aiding tumor development has led to the NF-κB family of transcription factors being strongly implicated in promoting cancer. However, recent studies have now revealed that NF-κB can also function as a tumor suppressor through the induction of cellular senescence. Cellular senescence is a stable cell cycle arrest that normal cells undergo in response to a variety of intrinsic and extrinsic stimuli including: progressive telomere shortening, changes in telomeric structure, or other forms of genotoxic stress. Senescence can compromise tissue repair and regeneration, contributing to tissue and organismal aging via the accumulation of senescent cells, depletion of stem/progenitor cells and secretion of an array of inflammatory cytokines, chemokines, and matrix metalloproteinases. Senescence can also lead to the removal of potentially cancerous cells, thereby acting as a potent tumor suppressor mechanism. Herein, we review the evidence indicating a role for NF-κB in tumor suppression via cellular senescence and suggest that depending upon the subunit expressed, the biological context, and the type and intensity of the signal, NF-κB can indeed promote senescence growth arrest.
Keywords:
Introduction to NF-κB
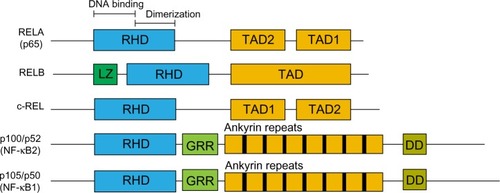
The nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) family of transcription factors have been extensively studied over the last 25 years since they were discovered by binding to the immunoglobulin κ light chain gene enhancer (iκE).Citation1 They comprise five family members: RELA (p65), RELB, c-REL, p105/p50 (NF-κB1), and p100/p52 (NF-κB2), which all share the Rel homology domain, permitting their dimerization and translocation to the nucleus.Citation2,Citation3 RELA, RELB, and c-REL also have a transactivation domain, whilst p105/p50 and p100/p52 contain ankyrin repeat domains (). The primary mediator of NF-κB transcriptional activity in response to activators such as inflammatory cytokines and Toll-like receptor (TLR) signaling, is the RELA:p50 heterodimer, whereas other subunits have a more primary role in other contexts. The RELA:p50 heterodimer is the most readily detectable form of this complex.Citation2,Citation3
Figure 1 NF-κB protein family.
Abbreviations: DD, death domain; DNA, deoxyribonucleic acid; GRR, glycine-rich region; LZ, leucine zipper; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; RHD, Rel homology domain; TAD, transactivation domains.
The mechanism of action underlying the activation of NF-κB, falls into two broad categories: a canonical pathway and a non-canonical pathway (). In the canonical pathway, NF-κB homo-and heterodimers are bound to inhibitors of NF-κB (IκB) in unstimulated cells, leading to them being retained in the cytosol.Citation2 A variety of stimuli can induce the phosphorylation of IκB by the multi-subunit IκB kinase complex (IKKα, IKKβ, and IKKγ/NEMO), leading to ubiquitination and subsequent degradation of IκB by the 26S proteasome.Citation2,Citation4 This unmasks a nuclear localization signal, resulting in the nuclear translocation of NF-κB dimers and activation of a plethora of target genes. The canonical pathway is vital for the activation of innate immunity and inflammation.Citation2
Figure 2 Canonical and non-canonical pathways of NF-κB activation.
Abbreviations: NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; REL, reticuloendotheliosis virus; RIP, receptor-interacting serine/threonine protein kinase 1; TAB, transforming growth factor-beta activated kinase binding protein; TAK, transforming growth factor-beta activated kinase; Ub, ubiquitin.
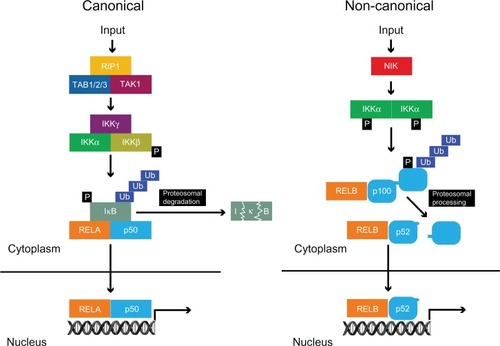
In response to stimuli such as lymphotoxin β, an alternative non-canonical pathway is activated. This involves the NF-κB inducing kinase (NIK), which phosphorylates and activates IKKα, leading to the proteosomal processing of p100 with preferential nuclear translocation of RELB:p52 dimers.Citation5,Citation6 Although the canonical and non-canonical pathways have separate regulatory mechanisms, recent evidence suggests that crosstalk between these pathways is vital for NF-κB activation, as synthesis of RELB is controlled by RELA signaling.Citation7,Citation8
Physiological role of NF-κB
NF-κB mediates the cellular response to stimuli such as cytokines, free radicals, deoxyribonucleic acid (DNA) damage, oxidized low density lipoproteins, and bacterial or viral antigens, most of which are induced upon intrinsic or extrinsic cellular stress.Citation9,Citation10 It does this by binding to specific DNA sequences within target genes in the nucleus and can act as a transcriptional activator or repressor.Citation11 This family of transcription factors (TFs) also has an important role in the development of the skeletal system and epithelium.Citation12,Citation13 They are required for proper organogenesis of several epithelial tissues such as the mammary gland; blockage of NF-κB activity in the mammary gland of mice leads to a severe lactation deficiency.Citation14 Similarly, activation of NF-κB in axon-associated Schwann cells is essential for progression to a myelinating phenotype.Citation15 However, as NF-κB proteins were first identified as factors that regulate B-lymphocyte-specific transcription, the role of NF-κB in the development, specialization, and maintenance of the immune system is more thoroughly documented.Citation16–Citation18 Hemopoiesis is characterized by apoptosis, differentiation, and proliferation, which are key processes controlled by the action of NF-κB. During hemopoiesis, cells of the lymphoid and myeloid lineages such as T cells, B cells, monocytes, macrophages, dendritic cells, natural killer cells, basophils, eosinophils, neutrophils, and mast cells are all formed. NF-κB contributes to hemopoiesis by promoting the survival and differentiation of precursors to a particular cell fate, throughout all stages of this process.Citation19 These cells are important for both innate and adaptive immunity and can initiate an inflammatory response to pro-inflammatory signals such as cytokines and chemokines, many of which are transcriptionally activated by NF-κB.Citation20
NF-κB also has an important role in normal B- cell maturation as purified B cells from p50/NF-kappa B knockout (p50−/−) mice have a reduced ability to mature to Ig secretion, germ-line CH gene activation, and undergo Ig class switching, as well as mitogenesis.Citation18
As a result, deregulation or dysfunction of NF-κB is detrimental to organisms, and can induce pathologies such as chronic inflammation and autoimmune diseases, cardiovascular disease, neurodegenerative diseases, and type II diabetes.Citation21 Persistent inflammation is also an important underlying condition that aids tumor development, thus suggesting a potential role for NF-κB in tumorigenesis.Citation22,Citation23 Accordingly, the NF-κB family of proteins is frequently implicated in cancer particularly since the Rel homology domain within these TFs is homologous to the viral oncogene, v-Rel, from the avian reticuloendotheliosis virus.Citation22–Citation24
The role of NF-κB in cancer
NF-κB proteins mediate proliferation, growth, and apoptosis. Mutations leading to aberrant activation of NF-κB have thus been implicated in various cancers.Citation3,Citation22–Citation25 Indeed, many cancers, in particular melanomas, multiple myeloma, various types of leukemia, and B and T-cell lymphomas, are associated with the aberrant activation of NF-κB.Citation25,Citation26 This is in part due to the characteristic NF-κB pro-inflammatory response, but inhibition of apoptosis and promotion of metastasis and cell proliferation also play vital roles. NF-κB acts to inhibit apoptosis, a key hallmark of cancer, downstream of growth factor signaling such as epidermal growth factor (EGF).Citation27 Here, activation of epidermal growth factor-receptor (EGFR) recruits phosphatidylinositide 3-kinase (PI3K) to the plasma membrane which in turn induces the de novo synthesis of phosphatidylinositol [3, 4, 5]-triphosphate; (PI[3,4,5]P3). This activates protein kinase B (AKT/PKB) with the help of phosphoinositide-dependent kinase-1 (PDK1) and mammalian target of rapamycin complex 2 (mTORC2).Citation28 AKT has been suggested to activate NF-κB via IKK, resulting in the transcription of pro-survival genes, which prevent the death of cancer cells, particularly when EGFR or mTOR are constitutively activated due to mutation.Citation26,Citation29,Citation30 Mutations leading to the activation of K-ras in the context of INK4a/ARF deficiency in mice lead to pancreatic cancer by persistent activation of the Notch and NF-κB signaling pathways.Citation31 NF-κB can also be activated by hypoxia, which has been implicated in the promotion of angiogenesis for sustaining the growth of cancer cells.Citation32 Moreover, NF-κB has been shown to contribute to initiation, promotion, and progression of tumorigenesis through its role in apoptosis, inflammation, and regulating matrix metalloproteinase-9 as well as inducing the epithelial to mesenchymal transition (EMT) via TWIST1 and SNAI1.Citation33–Citation37
Although it had been postulated that NF-κB can also function as a tumor suppressor for a number of years, through for example, its pro-apoptotic activities,Citation38 recent studies have now revealed a new facet of this behavior through the induction of a stable cell growth arrest, otherwise known as cellular senescence.
Introduction to cellular senescence, aging, and cancer
Somatic cells typically undergo a finite number of divisions before they irreversibly exit the cell cycle; this replicative limit is known as the Hayflick limit.Citation39 Cellular senescence occurs in response to a variety of intrinsic and extrinsic stimuli, some of which include: progressive telomere shortening/uncapping, oncogene activation, aberrant DNA damage, oxidative stress, and other forms of genotoxic and non-genotoxic stresses.Citation40–Citation42 Senescence results in cellular hypertrophy and a pro-inflammatory and hyper-secretory phenotype. Senescent cells show resistance to apoptosis, are metabolically active, and remain viable for long periods of time.Citation43 They also exhibit dramatic changes in morphology whereby cells become enlarged and flattened, making the senescent phenotype unique, easy to distinguish, and thus easy to detect.Citation44 Senescent cells may also be distinguished by the upregulation or increased activity of various biomarkers such as senescence-associated-β-galactosidase, plasminogen activator inhibitor (PAI)-1, fibronectin, p53, and the cyclin dependent kinase inhibitors p21CIP1/WAF1/SDI1 and p16INK4a.Citation45
Although there are different categories of cellular senescence, such as replicative senescence or premature senescence, both trigger a DNA-damage response, resulting in activation of the p53 and the retinoblastoma protein (pRB) tumor suppressors.Citation46 P53 initiates senescence by activating the expression of the cyclin dependent kinase inhibitor, p21CIP1/WAF1/SDI1, which in turn inhibits the cyclin E/cyclin dependent kinase 2 (CDK2) complex of the cell cycle. This prevents the phosphorylation and deactivation of the pRB family of pocket proteins permitting hypophosphorylated pRB to complex with the E2F family of heterodimeric TFs.Citation47 In turn, pRB recruits histone deacetylases and remodeling factors to E2F responsive promoters, thereby inhibiting E2F-dependent S-phase gene expression.Citation40,Citation48 In response to non-genotoxic stress, the pRB pathway is activated independently of p53 via the upregulation of p16INK4A, which acts to inhibit cyclinD-CDK4/6 complexes from phosphorylating pRB.Citation44 Equally, p53 may also induce senescence by alternative pathways, as it is a master TF that regulates a plethora of target genes affecting physiological pathways important for senescence such as E2F7, which promotes the repression of several E2F target genes.Citation49 However, many of the pathways downstream of p53 still remain poorly defined ().
Figure 3 Schematic illustration of the pathways linking NF-κB to cellular senescence, cancer, and aging.
Abbreviations: ATM, ataxia telangiectasia mutated kinase; CIP, cyclin dependent kinase interacting protein; CDK, cyclin dependent kinase; CycD, cyclin D; DNA, deoxyribonucleic acid; EGFR, epidermal growth factor receptor; FOXO, forkhead box; IGF1R, insulin like growth factor 1 receptor; HMGB1, high mobility group protein B1; IKK, IkB kinase; IL, interleukin; IR, insulin receptor; MAPK, mitogen activated protein kinase; miRNA, micro ribonucleic acid; NEMO, NF-kappa B essential modulator, also known as inhibitor of nuclear factor kappa B kinase subunit gamma; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; Pi3K, phosphatidylinositol 3-kinase; PIP, phosphatidylinositol phosphate; pRB, retinoblastoma protein; SASP, senescence associated secretory phenotype; TAB, transforming growth factor-beta activated kinase binding protein; TAK, transforming growth factor-beta activated kinase; TGF, transforming growth factor; TNF, tumor necrosis factor; TRAFS, TNF receptor associated factors.
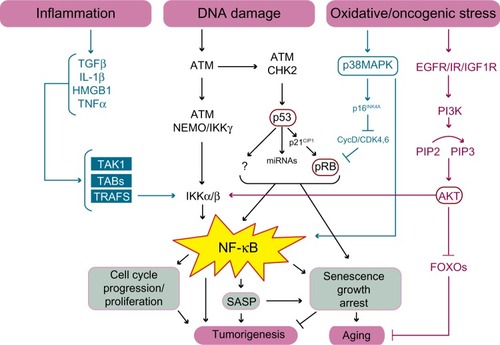
Senescence can lead to organismal aging by compromising tissue renewal, repair, and regeneration via the accumulation of non-dividing cells and secretion of an array of inflammatory cytokines, chemokines, and matrix metalloproteinases.Citation43,Citation50 Accumulation of post-mitotic cells also leads to the depletion of stem and progenitor cell populations via the disruption of stem-cell homeostasis, thereby preventing the replenishment of old or damaged cells.Citation51 Removal of senescent cells attenuates age-related tissue dysfunction and extends healthy lifespan. However, senescence can also act as a potent tumor suppressor mechanism through the removal of genetically unstable and pre-cancerous cells from the proliferative pool.Citation43
Cancer is considered to be a disease of the cell cycle, as cancer cells lose the stringent controls normally present during cell cycle progression. This is often the result of mutations via DNA damage; such damage can also act as a signal for senescence and inhibition of the cell cycle, preventing tumor growth. Thus senescence may act as a quality control mechanism for eliminating less healthy cells from the population, thereby maintaining genome integrity and appropriate cellular function.Citation52 Indeed, the induction of premature senescence in vivo has been shown to suppress tumor formation via the activation of key senescence pathways.Citation53 Several studies suggest that senescence must first be overcome for the initiation of cancer or maintaining the growth of cancer cells, which can then proliferate indefinitely; a key hallmark of cancer.Citation54–Citation56 Interestingly, chemotherapy using antimitotic drugs has been shown to induce cellular senescence in cancer cells that have evaded apoptosis.Citation57 The radiotherapy response of lung cancer cells has been shown to be mediated through both apoptosis and senescence.Citation58 Hence, there has been a recent push in cancer therapeutics to target cellular senescence as a way of treating cancer, but the downstream effectors of cellular senescence are currently poorly defined. Moreover, vital questions remain to be answered about what makes this process of cell cycle arrest essentially irreversible and what are the players that underlie this stable arrest.
Role of NF-κB in promoting cellular senescence
The role of NF-κB in reinforcing cellular senescence is currently emerging and is rapidly being recognized to have a causal role in this growth arrest.Citation59 Bernard and colleagues provided the first evidence indicating that NF-κB may inhibit growth by transiently overexpressing c-REL in HeLa cells; this led to a cell cycle arrest in all stages of the cell cycle, although the arrest was not demonstrated to be irreversible.Citation60,Citation61 Later studies by Hardy et al suggested a novel role for NF-κB in senescence growth arrest.Citation62 They used cDNA microarrays to identify genes that were differentially expressed when conditionally immortal human mammary (HMF3A) fibroblasts underwent senescence, followed by in silico promoter analysis of the differential genes and electrophoretic mobility shift assays to show that NF-κB was activated upon senescence. Shortly after, Adler et al showed that NF-κB was strongly associated with and was required to enforce pathological aging in mice.Citation63
Rovillain et alCitation64 extended the Hardy et al study by using genome-wide expression profiling, in conjunction with inactivation of the p16-pRB and p53-p21 tumor suppressor pathways in the conditionally immortal HMF3A fibroblasts, and found that 91 NF-κB downstream targets were up regulated upon senescence arrest; many of these targets are associated with the senescence associated secretory phenotype (SASP). Moreover, suppression of NF-κB by short hairpin ribonucleic acid (shRNA) silencing, or by ectopic expression of the I-κB super-repressor of NF-κB, abrogated senescence arrest in these conditionally immortal fibroblasts.Citation64
Interestingly, silencing of the bona-fide oncoprotein DEK leads to upregulation of NF-κB and its associated proteins resulting in a G2 cell cycle block and cellular senescence in CaSki cervical carcinoma cells.Citation65 Senescence is thought to be reinforced by the activation of cell surface bound cytokine interleukin (IL)-1α, which in turn activates NF-κB, as the expression of IL-1α in culture is one of the earliest events to occur after growth arrest.Citation66,Citation67 Freund et al have proposed that p38 mitogen activated protein kinase (MAPK) induces senescence via NF-κB activation.Citation68 Collectively, these, as well as other studies, have suggested a causal role for NF-κB in promoting cellular senescence.
NF-κB, DNA damage response, and senescence
Although it is not known how NF-κB activates senescence, links have been made between the DNA damage response, the best-known activator of cellular senescence, and NF-κB activation. Inhibition of NF-κB by depletion of one allele of p65, or pharmacological inhibition of IKK, has been reported to reduce oxidative damage and stress whilst delaying cellular senescence in progeroid mice.Citation69 Further studies, in two different mouse models of accelerated aging, have shown that accumulation of prelamin A, at the nuclear lamina, triggers an ataxia telangiectasia mutated (ATM)- and NEMO- signaling pathway leading to activation of NF-κB and secretion of proinflammatory cytokines.Citation70 Moreover, inhibition of NF-κB increases longevity of these mice by abrogating senescence.Citation70 Lifespan extension in mice has also been recently achieved by preventing aging-related hypothalamic IKKβ and NF-κB activation, which normally inhibit gonadotropin-releasing hormone (GnRH).Citation71 It is currently thought that genotoxic stress activates the NF-κB pathway, in mice, by stimulating Poly [ADP-ribose] polymerase 1 (PARP-1) and ATM, which induces the synthesis of poly adenosine diphosphate (ADP) ribose and the subsequent assembly of ATM and an IKKγ/PIASγ complex that activates NF-κB.Citation72 Furthermore, studies in human dermal fibroblasts have revealed crosstalk between p53 and NF-κB to induce cellular senescence through the repression of cyclin D1 as a response to ultraviolet (UV) light-induced DNA damage.Citation73
Since DNA-damage can also lead to other cell fates such as apoptosis or quiescence through activation of the p53 and pRB pathways, it remains to be elucidated how cellular senescence prevails over these cell fates in a particular context. As NF-κB has been shown in vitro and in vivo to block apoptosis in cancer cells, as well as normal cells, in response to DNA damage,Citation74,Citation75 it can be hypothesized that NF-κB has a role in committing the cell to cellular senescence by minimizing the choice of cell fates. Therefore, activation of ATM leads to a cell cycle arrest and NF-κB activation in response to DNA double strand breaks,Citation75 whereas inhibition of NF-κB sensitizes transformed cells to apoptosis induced by ionizing radiation or chemotherapeutic drugs.Citation76,Citation77
Furthermore, inhibition of aurora kinases with MLN8054/MLN8237 in mice with metastatic melanoma tumors led to suppression of tumor growth by induction of polyploidy and the ATM/CHK2 DNA damage response, which mediated senescence and a NF-κB related SASP.Citation78 However, blockade of IKKβ/NF-κB leads to reversal of MLN8237-induced senescence and SASP.Citation78 This suggests that targeting cellular senescence through NF-κB could provide a potential anticancer therapy.
Senescence associated secretory phenotype and NF-κB
NF-κB activation has been coupled to the SASP.Citation79 Senescent cells secrete a variety of inflammatory cytokines, proteases and growth factors such as: tumor necrosis factor (TNF) α and IL-6, monocyte chemoattractant protein-1, matrix metalloproteinases, and EGFs [44]. Campisi and colleagues have shown that a variety of senescent-inducing stimuli activate p38MAPK, which in turn induces SASP, by upregulating transcriptional activity of NF-κB.Citation68 This is also sufficient for SASP induction in melanoma cells.Citation79 Moreover, Chien et al recently observed that NF-κB signaling controlled the appearance of SASP in H-RasV12-induced senescence in human skin fibroblasts, and that RELA was significantly enriched in the chromatin of senescent fibroblasts.Citation80
NF-κB is thought to promote SASP by activating the expression of IL-6 and IL-8, which also contribute to reinforcing senescence growth arrest. Some studies suggest that IL-1α is also required for this activation, as silencing of IL-1α significantly decreased the senescence associated secretion of IL-6/8 by reducing the DNA binding activity of NF-κB and CCAAT-enhancer-binding protein beta (c/EBPβ).Citation81 Cells undergoing oncogene induced senescence also secrete multiple CXCR2-binding chemokines, in a NF-κB and c/EBPβ dependent manner.Citation82 However, the occurrence of SASP in vivo, in response to NF-κB, remains to be elucidated and the role of SASP in senescence growth arrest is controversial, as SASP can also promote cancer.
SASP and cancer
Adding a further layer of complexity to the NF-κB friend/foe dilemma, senescence also has a role in tumor promotion through the secretion of inflammatory, growth promoting and remodeling factors that senescent cells secrete as SASP.Citation67 Such factors promote the EMT, metastasis, and proliferation of neighboring cells. Metalloproteases, secreted as SASP, lead to the degradation of the basement membrane surrounding tissues, further promoting tumor metastasis. Hence, SASP creates a microenvironment that favors malignancy in neighboring cells when it is persistent, as is the case in aging cells.Citation83 Interestingly, it has recently been shown that cultured medium, derived from senescent cells, is sufficient to induce the growth of cancer cells, but this was inhibited by the anti-diabetic drug, metformin, which inhibits SASP and downregulates NF-κB induced transcription.Citation84 However, when SASP was inhibited by low levels of metformin, growth arrest still occurred, suggesting that other downstream targets are also important for the growth arrest. Interestingly, drug induced senescent cells have been shown to increase the proliferation of bystander cells in vitro but did not significantly alter tumor growth in vivo.Citation85
SASP can also reinforce growth arrest and protect against cancer by activating the immune response, inducing the clearance of damaged and pre-cancerous cells.Citation86 Accordingly, senescence may have evolved as an example of antagonistic pleiotrophy, since it provides beneficial traits during the reproductive age of an individual (tumor suppression via growth arrest), but causes deleterious effects later on in life (aging and cancer via SASP).Citation43 We thus hypothesize that the dual role of NF-κB in tumor promotion and suppression may, in part, be due to its promotion of SASP (summarized in ).
Conclusion
The NF-κB family of transcription factors has gained considerable attention due to their roles in various stress-induced pathways. Central to this is the role of NF-κB in both promoting cancer and acting as a tumor suppressor via the induction of cellular senescence. We have summarized the evidence indicating a role of NF-κB in both of these pathologies and suggest that depending upon the biological context, type, and intensity of the signal, NF-κB can indeed promote senescence arrest in somatic cells. We propose that these opposing decisions are determined by a combination of differential NF-κB subunit expression and modification in combination with crosstalk from other signaling pathways such as p53. Future studies, aimed at providing a greater understanding of the preferential expression of different NF-κB subunits in a particular context, and their engagement with different signaling pathways, will shed light on the opposing roles of NF-κB in cancer.
Acknowledgments
We thank A Munro Neville and Ralph Andre for their critique. We are indebted to Richard Newton for graphics. SNM is a Natural Sciences MSci student, University College, London, UK.
Disclosure
The authors report no conflicts of interest in this work.
References
- SenRBaltimoreDMultiple nuclear factors interact with the immunoglobulin enhancer sequencesCell1986467057163091258 J Immunol2006177117485749617114415
- HaydenMSGhoshSNF-κB, the first quarter-century: remarkable progress and outstanding questionsGenes Dev201226320323422302935
- PerkinsNDThe diverse and complex roles of NF-κB subunits in cancerNat Rev Cancer201212212113222257950
- MonacoCAndreakosEKiriakidisSCanonical pathway of nuclear factor kappa B activation selectively regulates proinflammatory and prothrombotic responses in human atherosclerosisProc Natl Acad Sci U S A2004101155634563915064395
- BasakSKimHKearnsJDA fourth IkappaB protein within the NF-kappaB signaling moduleCell2007128236938117254973
- ChenJChenZJRegulation of NF-κB by ubiquitinationCurr Opin Immunol201325141223312890
- BasakSShihVFHoffmannAGeneration and activation of multiple dimeric transcription factors within the NF-kappaB signaling systemMol Cell Biol200828103139315018299388
- YuMQiXMorenoJLFarberDLKeeganADNF-κB signaling participates in both RANKL- and IL-4-induced macrophage fusion: receptor cross-talk leads to alterations in NF-κB pathwaysJ Immunol201118741797180621734075
- TianBNowakDEJamaluddinMWangSBrasierARIdentification of direct genomic targets downstream of the nuclear factor-kappaB transcription factor mediating tumor necrosis factor signalingJ Biol Chem200528017174351744815722553
- GilmoreTDIntroduction to NF-kappaB: players, pathways, perspectivesOncogene200625516680668417072321
- BasakSShihVFHoffmannAGeneration and activation of multiple dimeric transcription factors within the NF-kappaB signaling systemMol Cell Biol200828103139315018299388
- NovackDVRole of NF-κB in the skeletonCell Res201121116918221079651
- SchauerIGZhangJXingZInterleukin-1β promotes ovarian tumorigenesis through a p53/NF-κB-mediated inflammatory response in stromal fibroblastsNeoplasia201315440942023555186
- CaoYKarinMNF-kappaB in mammary gland development and breast cancerJ Mammary Gland Biol Neoplasia20038221522314635796
- NickolsJCValentineWKanwalSCarterBDActivation of the transcription factor NF-kappaB in Schwann cells is required for peripheral myelin formationNat Neurosci20036216116712514737
- LiYOhmsSJSunCFanJNF-κB controls Il2 and Csf2 expression during T cell development and activation processMol Biol Rep20134021685169223079711
- KailehMSenRNF-κB function in B lymphocytesImmunol Rev2012246125427122435560
- SnapperCMZelazowskiPRosasFRB cells from p50/NF-kappa B knockout mice have selective defects in proliferation, differentiation, germ-line CH transcription, and Ig class switchingJ Immunol199615611831918598461
- GerondakisSBanerjeeAGrigoriadisGNF-κB subunit specificity in hemopoiesisImmunol Rev2012246127228522435561
- ThompsonCCloutierABosséYCysLT1 receptor engagement induces activator protein-1- and NF-kappaB-dependent IL-8 expressionAm J Respir Cell Mol Biol200635669770416809637
- XanthouleaSCurfsDMHofkerMHde WintherMPNuclear factor kappa B signaling in macrophage function and atherogenesisCurr Opin Lipidol200516553654216148538
- KarinMNF-kappaB as a critical link between inflammation and cancerCold Spring Harb Perspect Biol200915a00014120066113
- DiDonatoJAMercurioFKarinMNF-κB and the link between inflammation and cancerImmunol Rev2012246137940022435567
- StaudtLMOncogenic activation of NF-kappaBCold Spring Harb Perspect Biol201026a00010920516126
- CarboneCMelisiDNF-κB as a target for pancreatic cancer therapyExpert Opin Ther Targets201216Suppl 2S1S1022443181
- OdqvistLSánchez-BeatoMMontes-MorenoSNIK controls classical and alternative NF-κB activation and is necessary for the survival of human T-cell lymphoma cellsClin Cancer Res20131992319233023536439
- AlbertiCPinciroliPValeriBLigand-dependent EGFR activation induces the co-expression of IL-6 and PAI-1 via the NFkB pathway in advanced-stage epithelial ovarian cancerOncogene201231374139414922158046
- KorkolopoulouPLevidouGTrigkaEAA comprehensive immunohistochemical and molecular approach to the PI3K/AKT/mTOR (phosphoinositide 3-kinase/v-akt murine thymoma viral oncogene/mammalian target of rapamycin) pathway in bladder urothelial carcinomaBJU Int201211011 Pt CE1237E124823107319
- ParajuliBLeeHGKwonSHSalinomycin inhibits Akt/NF-κB and induces apoptosis in cisplatin resistant ovarian cancer cellsCancer Epidemiol201337451251723545383
- HoweENCochraneDRCittellyDMRicherJKmiR-200c targets a NF-κB up-regulated TrkB/NTF3 autocrine signaling loop to enhance anoikis sensitivity in triple negative breast cancerPLoS ONE2012711e4998723185507
- WangZBanerjeeSAhmadAActivated K-ras and INK4a/Arf deficiency cooperate during the development of pancreatic cancer by activation of Notch and NF-κB signaling pathwaysPLoS ONE201166e2053721673986
- YuHMohanSNatarajanMRadiation-Triggered NF-κB Activation is Responsible for the Angiogenic Signaling Pathway and Neovascularization for Breast Cancer Cell Proliferation and GrowthBreast Cancer (Auckl)2012612513522872788
- PikarskyEPoratRMSteinINF-kappaB functions as a tumour promoter in inflammation-associated cancerNature2004431700746146615329734
- WangFHeWFanghuiPWangLFanQNF-κBP65 promotes invasion and metastasis of oesophageal squamous cell cancer by regulating matrix metalloproteinase-9 and epithelial-to-mesenchymal transitionCell Biol Int201337878078823504993
- KirillovaIChaissonMFaustoNTumor necrosis factor induces DNA replication in hepatic cells through nuclear factor kappaB activationCell Growth Differ1999101281982810616907
- MeiraLBBugniJMGreenSLDNA damage induced by chronic inflammation contributes to colon carcinogenesis in miceJ Clin Invest200811872516252518521188
- LinEYNguyenAVRussellRGPollardJWColony-stimulating factor 1 promotes progression of mammary tumors to malignancyJ Exp Med2001193672774011257139
- PerkinsNDGilmoreTDGood cop, bad cop: the different faces of NF-kappaBCell Death Differ200613575977216410803
- HayflickLThe limited in vitro lifetime of human diploid cell strainsExp Cell Res19653761463614315085
- Ben-PorathIWeinbergRAThe signals and pathways activating cellular senescenceInt J Biochem Cell Biol200537596197615743671
- MalletteFAGaumont-LeclercMFFerbeyreGThe DNA damage signaling pathway is a critical mediator of oncogene-induced senescenceGenes Dev2007211434817210786
- AdamsPDHealing and hurting: molecular mechanisms, functions, and pathologies of cellular senescenceMol Cell200936121419818705
- CampisiJSenescent cells, tumor suppression, and organismal aging: good citizens, bad neighborsCell2005120451352215734683
- Ben-PorathIWeinbergRAWhen cells get stressed: an integrative view of cellular senescenceJ Clin Invest2004113181314702100
- KuilmanTMichaloglouCMooiWJPeeperDSThe essence of senescenceGenes Dev201024222463247921078816
- RyanKMPhillipsACVousdenKHRegulation and function of the p53 tumor suppressor proteinCurr Opin Cell Biol200113333233711343904
- LoweSWSherrCJTumor suppression by Ink4a-Arf: progress and puzzlesCurr Opin Genet Dev2003131778312573439
- Di MiccoRCicaleseAFumagalliMDNA damage response activation in mouse embryonic fibroblasts undergoing replicative senescence and following spontaneous immortalizationCell Cycle20087223601360619001874
- RufiniATucciPCelardoIMelinoGSenescence and aging: the critical roles of p53Oncogene2013
- DolesJStorerMCozzutoLRomaGKeyesWMAge-associated inflammation inhibits epidermal stem cell functionGenes Dev201226192144215322972935
- ChoudheryMSKhanMMahmoodRMehmoodAKhanSNRiazuddinSBone marrow derived mesenchymal stem cells from aged mice have reduced wound healing, angiogenesis, proliferation and anti-apoptosis capabilitiesCell Biol Int201236874775322352320
- VivarelliSWagstaffLPiddiniECell wars: regulation of cell survival and proliferation by cell competitionEssays Biochem201253698222928509
- XueWZenderLMiethingCSenescence and tumour clearance is triggered by p53 restoration in murine liver carcinomasNature2007445712865666017251933
- KrieglLNeumannJViethMUp and downregulation of p16(Ink4a) expression in BRAF-mutated polyps/adenomas indicates a senescence barrier in the serrated route to colon cancerMod Pathol20112471015102221423154
- FavaroEBensaadKChongMGGlucose utilization via glycogen phosphorylase sustains proliferation and prevents premature senescence in cancer cellsCell Metab201216675176423177934
- YangDLiLLiuHInduction of autophagy and senescence by knockdown of ROC1 E3 ubiquitin ligase to suppress the growth of liver cancer cellsCell Death Differ201320223524722935614
- BiggersJWNguyenTDiXAutophagy, cell death and sustained senescence arrest in B16/F10 melanoma cells and HCT-116 colon carcinoma cells in response to the novel microtubule poison, JG-03-14Cancer Chemother Pharmacol201371244145523178952
- SalimHAkbarNSZongDmiRNA-214 modulates radiotherapy response of non-small cell lung cancer cells through regulation of p38MAPK, apoptosis and senescenceBr J Cancer201210781361137322929890
- VaughanSJatPSDeciphering the role of nuclear factor-κB in cellular senescenceAging (Albany NY)201131091391921990145
- BernardDQuatannensBBegueAVandenbunderBAbbadieCAntiproliferative and antiapoptotic effects of crel may occur within the same cells via the up-regulation of manganese superoxide dismutaseCancer Res20016162656266411289144
- BernardDSlomiannyCVandenbunderBAbbadieCcRel induces mitochondrial alterations in correlation with proliferation arrestFree Radic Biol Med200131894395311595379
- HardyKMansfieldLMackayATranscriptional networks and cellular senescence in human mammary fibroblastsMol Biol Cell200516294395315574883
- AdlerASSinhaSKawaharaTLZhangJYSegalEChangHYMotif module map reveals enforcement of aging by continual NF-kappaB activityGenes Dev200721243244325718055696
- RovillainEMansfieldLCaetanoCActivation of nuclear factor-kappa B signalling promotes cellular senescenceOncogene201130202356236621242976
- LiuKFengTLiuJZhongMZhangSSilencing of the DEK gene induces apoptosis and senescence in CaSki cervical carcinoma cells via the up-regulation of NF-κB p65Biosci Rep201232332333222390170
- CampisiJAndersenJKKapahiPMelovSCellular senescence: a link between cancer and age-related degenerative disease?Semin Cancer Biol201121635435921925603
- RodierFCampisiJFour faces of cellular senescenceJ Cell Biol2011192454755621321098
- FreundAPatilCKCampisiJp38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotypeEMBO J20113081536154821399611
- TilstraJSRobinsonARWangJNF-κB inhibition delays DNA damage-induced senescence and aging in miceJ Clin Invest201212272601261222706308
- OsorioFGBárcenaCSoria-VallesCNuclear lamina defects cause ATM-dependent NF-κB activation and link accelerated aging to a systemic inflammatory responseGenes Dev201226202311232423019125
- ZhangGLiJPurkayasthaSHypothalamic programming of systemic ageing involving IKK-β, NF-κB and GnRHNature2013497744821121623636330
- HinzMStilmannMArslanSÇKhannaKKDittmarGScheidereitCA cytoplasmic ATM-TRAF6-cIAP1 module links nuclear DNA damage signaling to ubiquitin-mediated NF-κB activationMol Cell2010401637420932475
- LeeYKChaHJHongMYoonYLeeHAnSRole of NF-κB-p53 crosstalk in ultraviolet A-induced cell death and G1 arrest in human dermal fibroblastsArch Dermatol Res20123041737921947322
- PoltzRNaumannMDynamics of p53 and NF-κB regulation in response to DNA damage and identification of target proteins suitable for therapeutic interventionBMC Syst Biol2012612522979979
- BoldersonERichardDJZhouBBKhannaKKRecent advances in cancer therapy targeting proteins involved in DNA double-strand break repairClin Cancer Res200915206314632019808869
- DeorukhkarAKrishnanSTargeting inflammatory pathways for tumor radiosensitizationBiochem Pharmacol201080121904191420599771
- CusackJCLiuRBaldwinASInducible chemoresistance to 7-ethyl-10-[4-(1-piperidino)-1-piperidino]-carbonyloxycamptothe cin (CPT-11) in colorectal cancer cells and a xenograft model is overcome by inhibition of nuclear factor-kappaB activationCancer Res20006092323233010811101
- LiuYHawkinsOESuYTargeting aurora kinases limits tumour growth through DNA damage-mediated senescence and blockade of NF-κB impairs this drug-induced senescenceEMBO Mol Med20135114916623180582
- OhannaMGiulianoSBonetCSenescent cells develop a PARP-1 and nuclear factor-{kappa}B-associated secretome (PNAS)Genes Dev201125121245126121646373
- ChienYScuoppoCWangXControl of the senescence-associated secretory phenotype by NF-κB promotes senescence and enhances chemosensitivityGenes Dev201125202125213621979375
- OrjaloAVBhaumikDGenglerBKScottGKCampisiJCell surface-bound IL-1alpha is an upstream regulator of the senescence-associated IL-6/IL-8 cytokine networkProc Natl Acad Sci U S A200910640170311703619805069
- AcostaJCO’LoghlenABanitoAChemokine signaling via the CXCR2 receptor reinforces senescenceCell200813361006101818555777
- TchkoniaTZhuYvan DeursenJCampisiJKirklandJLCellular senescence and the senescent secretory phenotype: therapeutic opportunitiesJ Clin Invest2013123396697223454759
- MoiseevaODeschênes-SimardXSt-GermainEMetformin inhibits the senescence-associated secretory phenotype by interfering with IKK/NF-κB activationAging Cell201312348949823521863
- EwaldJDesotelleJAlmassiNJarrardDDrug-induced senescence bystander proliferation in prostate cancer cells in vitro and in vivoBr J Cancer20089871244124918349844
- KuilmanTMichaloglouCVredeveldLCOncogene-induced senescence relayed by an interleukin-dependent inflammatory networkCell200813361019103118555778