Abstract
Notch signaling is an evolutionarily conserved pathway involved in cell fate control during development, stem cell self-renewal, and postnatal tissue differentiation. Roles for Notch in carcinogenesis, the biology of cancer stem cells, tumor angiogenesis, and epithelial-to-mesenchymal transition (EMT) have been reported. This review describes the role of Notch in the “stemness” program in cancer cells and in metastases, together with a brief update on the Notch inhibitors currently under investigation in oncology. These agents may be useful in targeting cancer stem cells and to reverse the EMT process.
Introduction
The Notch pathway is one of the most intensively studied candidate therapeutic targets in cancer stem-like cells (CSCs), and several investigational Notch inhibitors are being developed. Notch signaling has been reported to promote the self-renewal of CSC in several malignancies and to participate in tumor–stroma and tumor–endothelium interactions in CSC niches in primary and metastatic tumors.Citation1,Citation2 However, successful targeting of Notch signaling in CSCs will require a clear understanding of Notch regulation and the context-dependent interactions between Notch and other therapeutically relevant pathways. Understanding these interactions will increase our ability to design rational combination regimens that are more likely to prove safe and effective for primary and metastatic tumors. Additionally, to determine which patients are most likely to benefit from treatment with Notch-targeting therapeutics, reliable biomarkers to measure Notch pathway activity in CSCs from specific tumors will have to be identified and validated.
Notch receptors and ligands
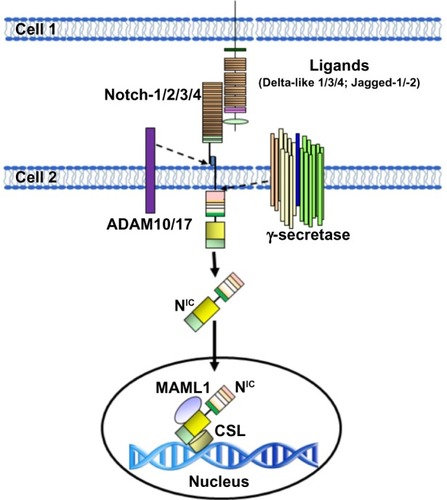
Mammals express four transmembrane Notch receptors (Notch-1, Notch-2, Notch-3, and Notch-4)Citation3 and five canonical transmembrane ligands (Delta-like [DLL] 1, DLL 3, DLL 4, Jagged-1, and Jagged-2) ().Citation4–Citation7 Delta family ligands differ from Jagged family ligands because their smaller extracellular domains can mediate Notch activation in trans (from cell to cell) and Notch inhibition in cis (on the same cell). The relative affinity of Notch receptors for Delta and Jagged family ligands is controlled by receptor glycosylation, and specifically by the addition of Fuc-GlcNac (fucose-N-acetylglucosamine) moieties by a fucosyltransferase and Fringe family N-acetyl-glucosaminidyl-transferases. Cell-to-cell contact is generally necessary for the activation of Notch signaling. This usually results in coordinated modulation of genes involved in cell fate determination, such as proliferation, survival, or differentiation.Citation7 Notch receptors undergo three proteolytic cleavages. First, Notch precursor proteins are processed in the trans-Golgi apparatus. A single polypeptide precursor is cleaved (S1) by a furin-like convertase to produce the mature Notch receptor, which is a heterodimer consisting of Notch extracellular (NEC) and Notch transmembrane (N™) subunits. Mature receptors are trafficked to the plasma membrane, where they await engagement with membrane-associated ligands. Upon ligand–receptor engagement, NEC is dissociated from N™ to be endocytosed with the ligand into the ligand-expressing cell. Subunit separation allows a second cleavage (S2) by a disintegrin and metalloproteinase domain-containing protein 10 or 17 (ADAM10 or ADAM17).Citation8 ADAM10 is thought to cleave Notch in ligand-dependent activation, while ADAM17 may participate in the less clearly understood process of ligand-independent activation. The S2 cleavage releases a short extracellular peptide and generates a short-lived intermediate that is cleaved again (S3) by the γ-secretase complex. The S3 cleavage releases the intracellular portion of Notch (NIC).Citation9 NIC translocates to the nucleus and binds to the CBF-1-Suppressor of Hairless/Lag1 ([CSL] also known as RBP-jκ), a constitutive transcriptional repressor, displacing corepressors and recruiting coactivators such as Mastermind-like (MAML) proteins, homologous to Drosophila Mastermind. The Notch–CSL–MAML complex in turn recruits multiple transcriptional regulators forming the “Notch transcriptional complex” (NTC).Citation10,Citation11 Notch activates many genes associated with differentiation and/or survival, including the Hairy/Enhancer of Split (HES) family and Hairy/enhancer-of-split related with YRPW motif-like protein (Hey) family of basic helix-loop-helix transcription factors,Citation12 cyclin D1,Citation13 and c-Myc ().Citation14 The genomic sites at which Notch activates transcription vary from cell to cell, and quite likely among different Notch paralogs. Other transcriptional regulators influence transcriptional regulation by Notch-1.Citation15–Citation17 The close-range cell–cell interaction necessary for Notch activation may be one of the signals whereby intercellular signals trigger epithelial-to-mesenchymal transition (EMT) in the tumor microenvironment.
Figure 1 Schematic representation of Notch receptors (A) and Notch ligands (B) in mammals.
Abbreviations: ANK, ankyrin repeats; CR, cysteine-rich domain; DLL, Delta-like; DOS, Delta and OSM-11-like protein domain; DSL, Delta, Serrata, and LAG-2 domain; EGF, epidermal growth factor; LNR, cysteine-rich Lin 12-Notch repeats; P, PEST domain; R, RAM domain; TAD, transactivation domain; NIC, Notch intracellular domain; NEC, Notch extracellular domain; N™, Notch transmembrane domain.
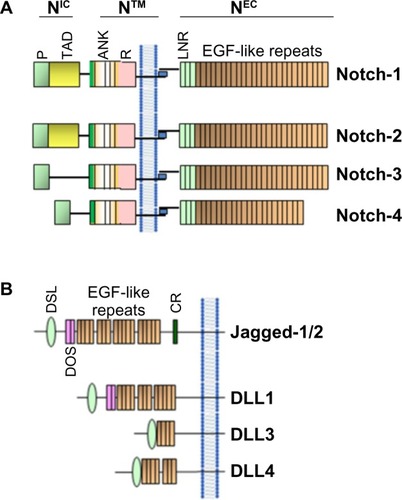
Figure 2 Schematic representation of the activation of Notch in mammal cells.
Abbreviations: ADAM, A disintegrin and metalloprotease; CSL, CBF-1-Suppressor of Hairless/Lag1 (also known as RBP-jκ); NIC, Notch intracellular domain.
Notch signaling, EMT, and cancer stem cells
Many human cancers are thought to contain populations of cells that display stem cell-like properties. These properties include self-renewal, which drives tumorigenesis; resistance to cell death, which drives tumor progression; and differentiation, which contributes to cancer cell heterogeneity. There is increasing evidence that these CSCs mediate tumor metastasis and, by virtue of their relative resistance to chemotherapy and radiation therapy, may contribute to treatment failures and relapses following therapy.Citation18
Self-renewal and cell fate determination of normal stem cells are regulated by both cell-autonomous (intrinsic) and non-cell-autonomous (extrinsic) pathways. The dysregulation of these pathways resulting in stem cell expansion may be a key event initiating carcinogenesis. Developmental pathways such as Notch play an important role in normal stem cell functions and are frequently deranged in cancers.Citation19–Citation22 Deregulated expression of Notch proteins, ligands, and targets, including overexpression and activation of Notch, has been described in a multitude of solid tumors, including cervical,Citation23 head and neck,Citation24 endometrial,Citation25 renal,Citation26 lung,Citation27 pancreatic,Citation28 ovarian,Citation29 prostate,Citation30 esophageal,Citation31 oral,Citation32 hepatocellular,Citation33 and gastricCitation34 carcinomas; osteosarcoma mesothelioma;Citation35 melanoma;Citation36 gliomas;Citation37 and medulloblastomas.Citation38 Dysregulation of Notch signaling has been reported in some hematological malignancies other than T-ALL. These include Hodgkin lymphomas, anaplastic large-cell non-Hodgkin lymphomas,Citation39 some acute myeloid leukemias (AMLs),Citation40 B-cell chronic lymphoid leukemias (B-CLLs),Citation41 and multiple myeloma (MM)Citation42,Citation43 (for a recent review, see Pancewicz and NicotCitation44). In most cases, inappropriate activation of Notch signaling is oncogenic. In some cases, however, loss of function of Notch-1 has oncogenic effects. This has been demonstrated in the epidermisCitation45,Citation46 and, more recently, in a subset of head and neck squamous carcinomas. Notch signaling is essential to the orderly differentiation of squamous epithelia, and loss of Notch-1 causes loss of barrier in such epithelia.Citation47 This in turn triggers an inflammatory response and cytokine cascade that may favor transformation. However, in the case of CSC, the literature supports a role of several Notch paralogs in the maintenance and survival of these cells.Citation2 Extrinsic signals that regulate stem cell behavior originate in the stem cell microenvironment. Although there is still relatively little detailed information on the composition and function of cancer stem cell microenvironments in different malignancies, it is clear that tumor growth and metastasis are highly dependent on the tumor microenvironment. This microenvironment is comprised of tumor-associated fibroblasts, endothelial cells, adipocytes, and several types of immune cells, all of which have been demonstrated to play roles in tumor growth and metastasis.Citation48 Several studies have demonstrated that loss of epithelial phenotype through EMT can promote the acquisition of a stem-like phenotype and drug resistance.Citation49 Notch signaling regulates both the formation of CSCs and the acquisition of the EMT phenotype, which are associated with drug resistance.Citation50,Citation51 An epithelial gene signature has been associated with sensitivity to the epidermal growth factor receptor inhibitor erlotinib in lung cancer cells.Citation52 Similar results have been reported in head and neck squamous cell carcinoma and hepatocellular carcinoma with gefitinib and cetuximab.Citation53,Citation54 Conversely, EMT has also been shown to promote resistance to conventional therapeutics, including paclitaxel, vincristine, and oxaliplatin.Citation55 Recent studies have shown links between EMT and gemcitabine-resistant pancreatic cancer, oxaliplatin-resistant colorectal cancer, lapatinib-resistant breast cancer, and paclitaxel-resistant ovarian carcinoma.Citation56–Citation59 Therefore, elucidating mechanisms that govern the acquisition of EMT in cancer cells would likely be useful for devising targeted therapeutic approaches to overcome or prevent resistance to conventional cancer therapeutics.
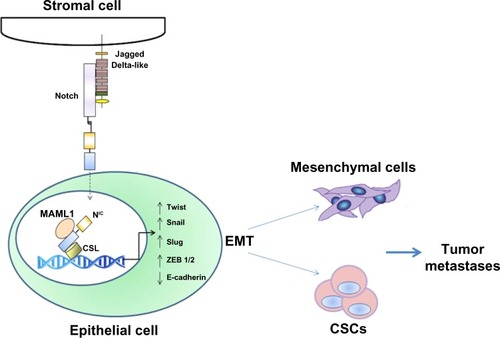
Notch activation triggers mesenchymal transformation not only in epithelial but also in endothelial cells. These changes include downregulation of endothelial markers (vascular endothelial-cadherin, tyrosine kinase with immunoglobulin-like and epidermal growth factor-like domain [Tie]1, Tie2, platelet-endothelial cell adhesion molecule-1, and endothelial nitric oxide synthase) and upregulation of mesenchymal markers (α-SMA, fibronectin, and platelet-derived growth factor receptors).Citation60 Jagged-1-mediated stimulation of endothelial cells induces phenotypic and functional changes consistent with EMT.Citation60 Notch also cross-talks with several transcription and growth factors relevant to EMT, including Snail, Slug, and transforming growth factor (TGF)-β. Notch promotes EMT through the regulation of Snail. Overexpression of Notch-1 in immortalized endothelial cells in vitro induces Snail,Citation61 which is thought to bind to E-boxes in the human E-cadherin promoter and repress E-cadherin gene expression.Citation62 In addition, Notch could induce EMT by stabilizing Snail-1 protein under hypoxic condition.Citation63 It has been reported that Slug is a direct target of Notch and that the Notch directly stimulates the Slug promoter, resulting in the upregulation of Slug and initiation of EMT.Citation64 Slug was found to be essential for Notch-mediated repression of E-cadherin, resulting in β-catenin activation and EMT.Citation65 It has been reported that TGF-β can induce the expression of Notch ligandsCitation66 and that TGF-β-induced EMT could be blocked by Hey-1 or Jagged-1 knockdown or by pharmacological inactivation of Notch.Citation67 Notch-2 and Jagged-1 are highly upregulated in gemcitabine-resistant pancreatic cancer cells, which show acquisition of an EMT phenotype.Citation68 Recently, EMT has been mechanistically linked with stem-like signatures in prostate cancer cells,Citation69 with stem-like cells characterized by increased expression of Notch-1, Sox2, Nanog, Oct4, and Lin28B.Citation69 An independent report has recently confirmed the importance of Notch and Hedgehog signaling in prostate CSCs.Citation70
Epithelial cells from a primary prostate tumor can undergo EMT with activation of embryonic programs of epithelial plasticity, including Notch, and switch from a sessile, epithelial phenotype to a motile, mesenchymal phenotype.Citation71 Growth factors and molecular alterations that contribute to EMT induction in primary tumors have been identified as important stimulators of skeletal metastasis formation.Citation72 Aberrant expression of EMT markers N-cadherin, vimentin, platelet-derived growth factor-D, nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), Notch-1, and zinc finger E-box-binding homeobox (ZEB)1 was observed in primary prostate cancers and bone metastatic lesions. Notch-1 was highly expressed in bone metastases compared to primary prostate cancers, suggesting that Notch-1 could play a role in prostate cancer bone metastasis through the induction of an EMT phenotype ().Citation73 Recent data from Zhu et al support this model, showing that Jagged-1 expression increases dramatically in high-grade and metastatic prostate cancers compared to primary lesions.Citation74 Furthermore, Notch signaling is often and aberrantly activated by hypoxia, which induces EMT during tumor progression. Bone is one of the most frequently targeted organs for breast cancer metastasis, and regions of the bone are known to be hypoxic. This hypoxic niche in bone microenvironment is believed to promote self-renewal of hematopoietic stem cells. Xing et al have also shown that Jagged-2 was upregulated in bone marrow stroma under hypoxia, which significantly promoted EMT and self-renewal of breast CSCs,Citation75 suggesting a critical role of Notch-induced EMT in tumor progression and metastasis.
Figure 3 Role of Notch in tumor metastasis as an inducer of EMT.
Abbreviations: CSC, cancer stem-like cell; EMT, epithelial-to-mesenchymal transition; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; ZEB, zinc finger E-box-binding homeobox; NIC, Notch intracellular domain; MAML1, Mastermind-like protein 1; CSL, CBF-1-Suppressor of Hairless/Lag1 (also known as RBP-jκ).
Notch signaling and mesenchymal stem cells
Mesenchymal stem cells (MSCs) are multipotent cells with non-hematopoietic origin that constitute a minor population (0.01%) of nucleated cells in bone marrow.Citation76 MSCs are a potential source of stem cells for cellular and genetic therapy, and can differentiate into multiple lineages such as chondrocytes, osteocytes, adipocytes, myocytes, and astrocytes. Recently, MSCs were found to play an important role in the tumor-supporting stroma.Citation77,Citation78 MSCs are known for their active mobilization from bone marrow and migration to sites of injury.Citation79–Citation81 Reports have suggested that bone marrow-derived MSCs are preferentially recruited to tumor stromaCitation80 when compared to normal stroma,Citation82 mainly by inflammatory factors in the tumor microenvironment. These reports increased interest in understanding the potential role of MSCs in tumor progression. MSCs are recruited to the tumor microenvironment in response to various cytokines, which are secreted by tumor cells and their associated stromaCitation83–Citation87 and act as precursors for pericytes and carcinoma cancer-associated fibroblasts.Citation77,Citation88,Citation89 MSCs promote tumor cell proliferation indirectly through their immunosuppressive properties and directly through cancer cell supportive properties.Citation90,Citation91 An earlier study from Sanchez et al suggest that, under nutrient-deprived conditions, the MSCs associated with tumor stroma undergo autophagy, secreting antiapoptotic factors that protect breast cancer cells embedded in the stroma.Citation78 These studies suggest that targeting tumor associated stromal cells along with tumor cells may provide more effective treatment strategies for breast cancer.Citation92,Citation93 Recent evidence also suggests that MSCs participate in tumor growth and metastasis and are the most prominent cell type within the stroma of many cancers. Subcutaneously implanted human mammary carcinomas coinjected with MSCs acquire an increased metastatic potential.Citation94 An important factor in the function of tumor microenvironment is the cell–cell communication between stromal cells and cancer cells. The role of gap junctions in the transport of cellular communicatorsCitation95 and juxtacrine regulation based on direct communication is well documented.Citation96,Citation97
The role of MSCs in inducing EMT in tumor cells has been the focus of a number of recent studies. Several possible mechanisms through which MSCs play a role in tumor microenvironment have been proposed. One such mechanism is exosomes secreted by MSCs, which promote EMT in gastric cancer cells.Citation98 EMT processes endow epithelial tumor cells with properties that may facilitate CSC generation and survival, ie, increased invasive ability, increased resistance to apoptotic signals, and increased ability to potentiate angiogenesis. In vivo models of EMT-derived cells in primary tumors have enhanced metastatic potential.Citation99 Inflammatory cells and cytokines, hypoxia-induced increase of reactive oxygen species in mitochondria, and MSC can all effectively drive the EMT of tumor cells.Citation100 Passage by neoplastic epithelial cells through an EMT event allows these cells to approach a stem cell-like state. EMT programs are known to be induced by heterotypic signals that epithelial cells receive from their microenvironment. In response to stimulation by carcinoma cells, MSCs express greatly elevated levels of prostaglandin E2 (PGE2). The resulting PGE2, together with cytokines also induced in the MSCs, contributes to the entrance of nearby carcinoma cells into a stem cell-like state.Citation101 An in vitro study using coculture models of MSCs and breast cancer cells showed that EMT is stimulated by increased expression of oncogenes and other genes associated with invasion, angiogenesis, and antiapoptosis.Citation88,Citation94 However, the nature of these heterotypic signals and the identities of the stromal cells that release them remain poorly understood.
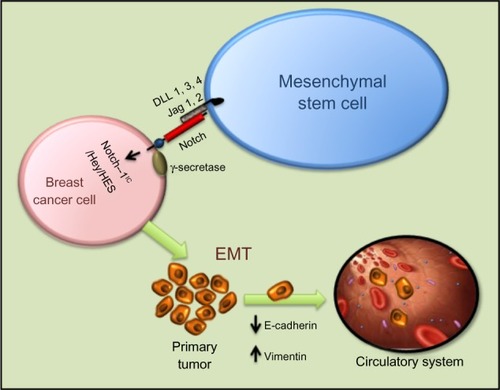
Notch signaling is important for MSC differentiation and related to its role in EMT. MSC modified with miR-126 release proangiogenic factors and induce expression of proangiogenic Notch ligand DLL 4, enhancing angiogenesis.Citation102 Moreover, Notch signaling regulates the expression of CXCR4 in MSCs, modulating their migration.Citation103 Notch-1 has also been reported to mediate the induction of Tregs by MSCs.Citation104 Tregs are thought to promote tumorigenesis by dampening antitumor immune responses. As with BMP and Wnt signaling in osteogenesis, Runx2 function is also influenced by Notch signaling. Notch-1IC can interact directly with Runx2 protein to repress terminal osteoblastic differentiation in vitro ().Citation105
Figure 4 Intercellular interaction between mesenchymal stem cells and breast cancer cells through Notch can activate EMT through NIC, Hey, HES, and other activators. Mesenchymal stem cells modified with miR-126 release proangiogenic factors and induce expression of proangiogenic Notch ligand DLL 1, 3, 4 and Jagged-1/2 enhancing angiogenesis. Moreover, Notch signaling regulates the expression of CXCR4 in mesenchymal stem cells, modulating their migration.
Notch signaling and tumor metastasis
Recent insights have linked Notch signaling to cancer metastasis.Citation106
It is now well recognized that cancer progression not only requires deregulated signaling pathways and accumulated genetic alterations in cancer cells, but also relies on the support from tumor microenvironment.Citation107,Citation108 For example, in the case of breast cancer, tumor cells frequently metastasize to the bone and brain, where tissue microenvironment enhances the metastatic growth of cancer cells by providing growth factors and ligands that activate multiple metastasis-related pathways including Notch, Wnt, and Hedgehog.Citation109–Citation111
Elevated expression of Jagged-1 has been associated with an increased incidence of triple-negative breast cancer (TNBC) bone metastasis and tumor cells that overexpress Jagged-1-generated severe osteolytic lesions in mouse tibiae, suggesting a potential role of tumor-derived Jagged-1 in promoting bone metastasis.Citation106 Interestingly, the same paper also showed that breast cancer cells with a high level of Jagged-1 promoted bone metastasis by activating Notch signaling in the osteoblasts, which in turn directly enhanced osteoclast differentiation by secreting interleukin (IL)-6. These results indicate a new paradigm for Notch signaling in breast cancer metastasis in which bone stromal cells respond to tumor-derived ligands and promote osteolysis in a paracrine manner. Furthermore, it has been shown that Notch signaling is activated in endothelial cells, promoting angiogenesis after interaction with cancer cells in head and neck squamous cell carcinomas.Citation112 These studies indicate that reciprocal interactions of tumor and untransformed stromal cells in the microenvironment play critical roles in the activation of Notch signaling. Tumor microenvironment includes not only various types of stromal and immune cells but also several homeostatic factors, such as pH and oxygen concentration.Citation113,Citation114 Hypoxic breast cancer cells have been shown to enhance the capillary-like tube formation of endothelial cells in a Jagged-2-dependent manner. Knockdown of Jagged-2 by siRNA in cancer cells blocks angiogenesis of endothelial cells in coculture experiments.Citation115
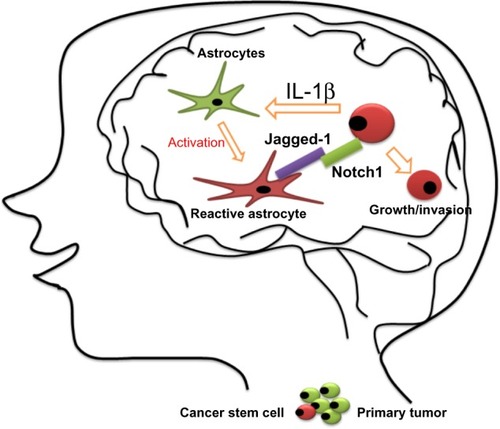
The brain is a frequent metastatic site for several types of tumors, including melanoma and lung and breast cancers, and metastatic tumor cells need to adapt to this totally different microenvironment. It has been demonstrated that brain metastatic TNBC cells excessively expressed IL-1β, which stimulates the surrounding astrocytes to express Jagged-1.Citation116 Direct interaction of the reactivated astrocytes with CSCs resulted in significantly upregulated Notch signaling in CSCs. This in turn further enhanced the self-renewal of CSC, suggesting that there is a vicious circle paracrine loop of IL-1β and Notch signaling inducing one another through direct interaction between CSCs and astrocytes. This vicious circle promotes the growth of metastatic CSCs in the brain. The blood–brain barrier-permeable Notch inhibitor γ-secretase inhibitor (GSI) Compound E can significantly suppress brain metastasis in vivo.Citation113 These results represent a novel paradigm for the understanding of how metastatic breast CSCs re-establish a niche for their self-renewal in a glial microenvironment entirely different from their tissue of origin, opening a new avenue by which to identify a novel and specific target for the brain metastatic disease ().
Figure 5 Proposed model for the growth of breast cancer stem cells in the brain.
Targeting Notch signaling to reverse EMT and stemness in CSCs
Several classes of investigational Notch inhibitors have been developed. These include monoclonal antibodies against Notch receptors or ligands;Citation117–Citation123 decoys (soluble forms of the extracellular domain of Notch receptor or Notch ligands);Citation124–Citation127 blocking peptides;Citation128 GSIs;Citation129–Citation135 or natural compounds.Citation136–Citation140 To date, GSIs are the most extensively explored. GSIs are less specific than biologics, but have the potential advantages of favorable biodistribution and pan-Notch inhibition. While γ-secretase has numerous substrates besides Notch receptors, the pharmacologic activity and toxicity of GSIs in vivo appears to be due largely to Notch inhibition.Citation141,Citation142 GSIs have been administered to patients in Phase I clinical trials, either as single agents or in combination with standard of care.Citation143 As is the case for most stem cell pathway inhibitors, the development of Notch inhibitors will need to be guided by biology. Biomarkers indicative of Notch activity (and of its inhibition by investigational drugs) will have to be identified and validated in each indication. Additionally, mechanism-based combinations will have to be developed. This implies that standard clinical trial designs with single-agent investigational drug and tumor volume as the primary end point may not be the most appropriate strategy for clinical trials of Notch-targeting agents, or, for that matter, for other CSC-targeted drugs. For example, in Her2/Neu positive BT474 xenografts, the combination of two chemically different GSIs with trastuzumab dramatically inhibited tumor recurrence, producing complete cures in most animals treated with one drug and all animals treated with another.Citation132 GSIs given as single agents had virtually no effect on tumor volume in this experimental model, nor did they enhance tumor volume regression induced by trastuzumab. Thus, these agents prevented tumor regression with no significant effect on tumor volume. This effect is most likely due to CSC blockade, and suggests that survival-based end points may be needed in the clinic, at least for some indications. Recurrence-free survival and/or good surrogate end points predictive of survival (eg, circulating tumor cells, tumor-sphere-forming cells) are likely to be more informative. These challenges do not diminish the tremendous therapeutic opportunity offered by a pathway that is essential for EMT and CSC maintenance, angiogenesis, and, in many cases, proliferation and survival of cancer cells.
The Notch pathway has tremendous potential as a new target in cancer therapy. Importantly, Notch may be a particularly powerful target for CSCs, which are resistant to standard treatments such as chemotherapy and radiation but seem especially sensitive to inhibition of stem cell pathways such as Notch. Although several Notch inhibitors are currently at the clinic,Citation143 Notch inhibitors used as single agents do not always yield major responses based on tumor volume in all models. Several issues remain to be addressed:
GSIs can affect bulk tumor cells, CSCs, stroma, and angiogenesis. The effects observed in vivo depend on the relative importance of these cellular targets in each tumor and tumor model.
GSIs are not pharmacologically equivalent: they have different pharmacokinetics, potencies, and off-target effects, and should not be considered equivalent.
At least one Notch paralog, Notch-4, has been shown to be resistant to some GSIs.Citation144 Whether this is true of all GSIs is unclear, but Notch-4-driven tumors may be resistant to some GSIs.
GSIs generally have gastrointestinal toxicity, which is Notch-mediated and results from goblet cell metaplasia of intestinal stem cells. This precludes long-term, sustained administration of these drugs. Intermittent administration schedules have been used in the clinic and in preclinical models. These regimens do dramatically decrease toxicity. However, it is unknown whether intermittent inhibition of Notch signaling is sufficient to achieve an anti-CSC effect. Tumor-selective delivery systems may be necessary to achieve sustained Notch inhibition within the tumor microenvironment.
Combination treatments, ideally based on mechanistic information, are likely to prove more successful than single-agent regiments. For instance, glucocorticoids decrease the intestinal side effects of GSIs in T-ALL models.Citation145 In estrogen receptor alpha (ERα)-positive breast cancer, combinations of Notch inhibitors with endocrine therapy have shown promise in preclinical modelsCitation146 and in two early-phase clinical trials.Citation147,Citation148 In a presurgical window study, the addition of GSI MK0752 to tamoxifen or letrozole decreased Ki67 in 17/20 patients compared to endocrine therapy alone.Citation147 In the metastatic setting, a combination of exemestane and GSI RO4929097 yielded clinical responses in seven out of 14 patients.Citation148 In both cases, no diarrhea was observed. This may be due to the fact that endocrine therapy ameliorates the gastrointestinal toxicity of GSIs.Citation149 Similarly, in TNBC, combinations of GSIs and taxanes have shown synergistic efficacy.Citation150
Non-GSI strategies to inhibit Notch signaling, including stapled peptides, decoys, monoclonal antibodies to Notch ligands or receptors, or inhibitors of downstream mediators may prove useful in some indications. Several classes of non-GSI Notch inhibitors are currently being developed. As our understanding of this group of targets and agents increases, it becomes clear that these issues are surmountable and there is growing optimism that Notch inhibition will become an exciting new approach to cancer.
Conclusion
Deregulation of Notch signaling has been associated with mobilization and spread of primary tumor cells to distant locations. EMT and mesenchymal–epithelial transition play important roles during tumor invasion, metastasis, and therapeutic resistance. EMT is also linked with the acquisition of stem cell-like characteristics. The concept of EMT inducing a CSC phenotype provides a possible mechanistic basis for metastasis, chemoresistance, tumor dormancy, and delayed recurrence. Notch signaling is one of a handful of embryonic pathways that control the generation and self-renewal of CSCs, at least in part through EMT. Significant efforts are underway to develop pharmacologic inhibitors of Notch signaling that can inhibit EMT and/or eradicate CSCs in common human malignancies.
Acknowledgments
This work was financially support by grants from the National Institute of Health (NIH): R01CA151851 (RP), R01CA124650 (KW), R01CA129000 (KW), and P01 AG2553101 (LM).
Disclosure
The authors report no conflicts of interest in this work.
References
- GuJWRizzoPPannutiAGoldeTOsborneBMieleLNotch signals in the endothelium and cancer “stem-like” cells: opportunities for cancer therapyVasc Cell20124722487493
- PannutiAForemanKRizzoPTargeting Notch to target cancer stem cellsClin Cancer Res201016123141315220530696
- BlaumuellerCMQiHZagourasPArtavanis-TsakonasSIntracellular cleavage of Notch leads to a heterodimeric receptor on the plasma membraneCell19979022812919244302
- DunwoodieSLHenriqueDHarrisonSMBeddingtonRSMouse Dll3: a novel divergent Delta gene which may complement the function of other Delta homologues during early pattern formation in the mouse embryoDevelopment19971246306530769272948
- LindsellCEShawberCJBoulterJWeinmasterGJagged: a mammalian ligand that activates Notch1Cell19958069099177697721
- ShawberCBoulterJLindsellCEWeinmasterGJagged2: a serrate-like gene expressed during rat embryogenesisDev Biol199618013703768948600
- CallahanRRaafatANotch signaling in mammary gland tumorigenesisJ Mammary Gland Biol Neoplasia200161233611467450
- BrouCLogeatFGuptaNA novel proteolytic cleavage involved in Notch signaling: the role of the disintegrin-metalloprotease TACEMol Cell20005220721610882063
- SaxenaMTSchroeterEHMummJSKopanRMurine notch homologs (N1–4) undergo presenilin-dependent proteolysisJ Biol Chem200127643402684027311518718
- HsiehJJZhouSChenLYoungDBHaywardSDCIR, a corepressor linking the DNA binding factor CBF1 to the histone deacetylase complexProc Natl Acad Sci U S A199996123289874765
- WuLAsterJCBlacklowSCLakeRArtavanis-TsakonasSGriffinJDMAML1, a human homologue of Drosophila mastermind, is a transcriptional co-activator for NOTCH receptorsNat Genet200026448448911101851
- MaierMMGesslerMComparative analysis of the human and mouse Hey1 promoter: Hey genes are new Notch target genesBiochem Biophys Res Commun2000275265266010964718
- RonchiniCCapobiancoAJInduction of cyclin D1 transcription and CDK2 activity by Notch(ic): implication for cell cycle disruption in transformation by Notch(ic)Mol Cell Biol200121175925593411486031
- WengAPMillhollandJMYashiro-OhtaniYc-Myc is an important direct target of Notch1 in T-cell acute lymphoblastic leukemia/lymphomaGenes Dev200620152096210916847353
- GermarKDoseMKonstantinouTT-cell factor 1 is a gatekeeper for T-cell specification in response to Notch signalingProc Natl Acad Sci U S A201110850200602006522109558
- CalderwoodMALeeSHolthausAMBlacklowSCKieffEJohannsenEEpstein-Barr virus nuclear protein 3C binds to the N-terminal (NTD) and beta trefoil domains (BTD) of RBP/CSL; only the NTD interaction is essential for lymphoblastoid cell growthVirology20114141192521440926
- MieleLTranscription factor RBPJ/CSL: a genome-wide look at transcriptional regulationProc Natl Acad Sci U S A201110836147151471621873209
- KakaralaMWichaMSImplications of the cancer stem-cell hypothesis for breast cancer prevention and therapyJ Clin Oncol200826172813282018539959
- DontuGJacksonKWMcNicholasEKawamuraMJAbdallahWMWichaMSRole of Notch signaling in cell-fate determination of human mammary stem/progenitor cellsBreast Cancer Res200466R605R61515535842
- LiuSDontuGMantleIDHedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cellsCancer Res200666126063607116778178
- LiuSDontuGWichaMSMammary stem cells, self-renewal pathways, and carcinogenesisBreast Cancer Res200573869515987436
- ReyaTCleversHWnt signalling in stem cells and cancerNature2005434703584385015829953
- ZagourasPStifaniSBlaumuellerCMCarcangiuMLArtavanis-TsakonasSAlterations in Notch signaling in neoplastic lesions of the human cervixProc Natl Acad Sci U S A19959214641464187604005
- LeethanakulCPatelVGillespieJDistinct pattern of expression of differentiation and growth-related genes in squamous cell carcinomas of the head and neck revealed by the use of laser capture microdissection and cDNA arraysOncogene200019283220322410918578
- SuzukiTAokiDSusumuNUdagawaYNozawaSImbalanced expression of TAN-1 and human Notch4 in endometrial cancersInt J Oncol20001761131113911078798
- RaeFKStephensonSANicolDLClementsJANovel association of a diverse range of genes with renal cell carcinoma as identified by differential displayInt J Cancer200088572673211072240
- DangTPGazdarAFVirmaniAKChromosome 19 translocation, overexpression of Notch3, and human lung cancerJ Natl Cancer Inst200092161355135710944559
- MiyamotoYMaitraAGhoshBNotch mediates TGF alpha-induced changes in epithelial differentiation during pancreatic tumorigenesisCancer Cell20033656557612842085
- HopferOZwahlenDFeyMFAebiSThe Notch pathway in ovarian carcinomas and adenomasBr J Cancer200593670971816136053
- SantagataSDemichelisFRivaAJAGGED1 expression is associated with prostate cancer metastasis and recurrenceCancer Res200464196854685715466172
- SubramaniamDPonnurangamSRamamoorthyPCurcumin induces cell death in esophageal cancer cells through modulating Notch signalingPloS One201272e3059022363450
- LiaoSXiaJChenZWangZInhibitory effect of curcumin on oral carcinoma CAL-27 cells via suppression of Notch-1 and NF-kappaB signaling pathwaysJ Cell Biochem201111241055106521308734
- WangCQiRLiNNotch1 signaling sensitizes tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in human hepatocellular carcinoma cells by inhibiting Akt/Hdm2-mediated p53 degradation and up-regulating p53-dependent DR5 expressionJ Biol Chem200928424161831619019376776
- YehITReddickRLKumarAPMalignancy arising in seminal vesicles in the transgenic adenocarcinoma of mouse prostate (TRAMP) modelProstate200969775576019170049
- BocchettaMMieleLPassHICarboneMNotch-1 induction, a novel activity of SV40 required for growth of SV40-transformed human mesothelial cellsOncogene2003221818912527910
- BalintKXiaoMPinnixCCActivation of Notch1 signaling is required for beta-catenin-mediated human primary melanoma progressionJ Clin Invest2005115113166317616239965
- PurowBWHaqueRMNoelMWExpression of Notch-1 and its ligands, Delta-like-1 and Jagged-1, is critical for glioma cell survival and proliferationCancer Res20056562353236315781650
- FanXMikolaenkoIElhassanINotch1 and notch2 have opposite effects on embryonal brain tumor growthCancer Res200464217787779315520184
- JundtFAnagnostopoulosIForsterRMathasSSteinHDorkenBActivated Notch1 signaling promotes tumor cell proliferation and survival in Hodgkin and anaplastic large cell lymphomaBlood20029993398340311964309
- TohdaSNaraNExpression of Notch1 and Jagged1 proteins in acute myeloid leukemia cellsLeuk Lymphoma200142346747211699411
- HubmannRSchwarzmeierJDShehataMNotch2 is involved in the overexpression of CD23 in B-cell chronic lymphocytic leukemiaBlood200299103742374711986231
- HoudeCLiYSongLOverexpression of the NOTCH ligand JAG2 in malignant plasma cells from multiple myeloma patients and cell linesBlood2004104123697370415292061
- JundtFProbstingKSAnagnostopoulosIJagged1-induced Notch signaling drives proliferation of multiple myeloma cellsBlood200410393511351514726396
- PancewiczJNicotCCurrent views on the role of Notch signaling and the pathogenesis of human leukemiaBMC Cancer20111150222128846
- RestivoGNguyenBCDziunyczPIRF6 is a mediator of Notch pro-differentiation and tumour suppressive function in keratinocytesEMBO J201130224571458521909072
- NicolasMWolferARajKNotch1 functions as a tumor suppressor in mouse skinNat Genet200333341642112590261
- DumortierADurhamADDi PiazzaMAtopic dermatitis-like disease and associated lethal myeloproliferative disorder arise from loss of Notch signaling in the murine skinPloS One201052e925820174635
- AlbiniASpornMBThe tumour microenvironment as a target for chemopreventionNat Rev Cancer2007213914717218951
- KongDLiYWangZSarkarFHCancer stem cells and epithelial-mesenchymal transition (EMT)-phenotypic cells: are they cousins or twins?Cancers (Basel)20113171672921643534
- WangZLiYBanerjeeSSarkarFHEmerging role of Notch in stem cells and cancerCancer Lett2009279181219022563
- WangZLiYKongDAhmadABanerjeeSSarkarFHCross-talk between miRNA and Notch signaling pathways in tumor development and progressionCancer Lett2010292214114820022691
- YauchRLJanuarioTEberhardDAEpithelial versus mesenchymal phenotype determines in vitro sensitivity and predicts clinical activity of erlotinib in lung cancer patientsClin Cancer Res20051124 Pt 18686869816361555
- VoulgariAPintzasAEpithelial-mesenchymal transition in cancer metastasis: mechanisms, markers and strategies to overcome drug resistance in the clinicBiochim Biophys Acta200917962759019306912
- FrederickBAHelfrichBAColdrenCDEpithelial to mesenchymal transition predicts gefitinib resistance in cell lines of head and neck squamous cell carcinoma and non-small cell lung carcinomaMol Cancer Ther2007661683169117541031
- SabbahMEmamiSRedeuilhGMolecular signature and therapeutic perspective of the epithelial-to-mesenchymal transitions in epithelial cancersDrug Resist Updat2008114–512315118718806
- KajiyamaHShibataKTerauchiMChemoresistance to paclitaxel induces epithelial-mesenchymal transition and enhances metastatic potential for epithelial ovarian carcinoma cellsInt J Oncol200731227728317611683
- KonecnyGEVenkatesanNYangGActivity of lapatinib a novel HER2 and EGFR dual kinase inhibitor in human endometrial cancer cellsBr J Cancer20089861076108418334972
- ShahANSummyJMZhangJParkSIParikhNUGallickGEDevelopment and characterization of gemcitabine-resistant pancreatic tumor cellsAnn Surg Oncol200714123629363717909916
- YangADFanFCampERChronic oxaliplatin resistance induces epithelial-to-mesenchymal transition in colorectal cancer cell linesClin Cancer Res20061214 Pt 14147415316857785
- NosedaMMcLeanGNiessenKNotch activation results in phenotypic and functional changes consistent with endothelial-to-mesenchymal transformationCirc Res200494791091714988227
- TimmermanLAGrego-BessaJRayaANotch promotes epithelial-mesenchymal transition during cardiac development and oncogenic transformationGenes Dev20041819911514701881
- BeckerKFRosivatzEBlechschmidtKKremmerESarbiaMHoflerHAnalysis of the E-cadherin repressor Snail in primary human cancersCells Tissues Organs20071851–320421217587826
- SahlgrenCGustafssonMVJinSPoellingerLLendahlUNotch signaling mediates hypoxia-induced tumor cell migration and invasionProc Natl Acad Sci U S A2008105176392639718427106
- NiessenKFuYChangLHoodlessPAMcFaddenDKarsanASlug is a direct Notch target required for initiation of cardiac cushion cellularizationJ Cell Biol2008182231532518663143
- LeongKGNiessenKKulicIJagged1-mediated Notch activation induces epithelial-to-mesenchymal transition through Slug-induced repression of E-cadherinJ Exp Med2007204122935294817984306
- NiimiHPardaliKVanlandewijckMHeldinCHMoustakasANotch signaling is necessary for epithelial growth arrest by TGF-betaJ Cell Biol2007176569570717325209
- ZavadilJCermakLSoto-NievesNBottingerEPIntegration of TGF-beta/Smad and Jagged1/Notch signalling in epithelial-to-mesenchymal transitionEMBO J20042351155116514976548
- WangZLiYKongDAcquisition of epithelial-mesenchymal transition phenotype of gemcitabine-resistant pancreatic cancer cells is linked with activation of the notch signaling pathwayCancer Res20096962400240719276344
- KongDBanerjeeSAhmadAEpithelial to mesenchymal transition is mechanistically linked with stem cell signatures in prostate cancer cellsPloS One201058e1244520805998
- Domingo-DomenechJVidalSJRodriguez-BravoVSuppression of acquired docetaxel resistance in prostate cancer through depletion of notch- and hedgehog-dependent tumor-initiating cellsCancer Cell201222337338822975379
- van der PluijmGEpithelial plasticity, cancer stem cells and bone metastasis formationBone201148374320670698
- PapachristouDJBasdraEKPapavassiliouAGBone metastases: molecular mechanisms and novel therapeutic interventionsMed Res Rev201232361163620818675
- SethiSMacoskaJChenWSarkarFHMolecular signature of epithelial-mesenchymal transition (EMT) in human prostate cancer bone metastasisAm J Transl Res201031909921139809
- ZhuHZhouXRedfeldSLewinJMieleLElevated Jagged-1 and Notch-1 expression in high grade and metastatic prostate cancersAm J Transl Res20135336837823634247
- XingFOkudaHWatabeMHypoxia-induced Jagged2 promotes breast cancer metastasis and self-renewal of cancer stem-like cellsOncogene201130394075408621499308
- PittengerMFMackayAMBeckSCMultilineage potential of adult human mesenchymal stem cellsScience1999284541114314710102814
- SpaethELDembinskiJLSasserAKMesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progressionPloS One2009174e499219352430
- SanchezCGPenfornisPOskowitzAZActivation of autophagy in mesenchymal stem cells provides tumor stromal supportCarcinogenesis201132796497221317300
- ProckopDJRepair of tissues by adult stem/progenitor cells (MSCs): controversies, myths, and changing paradigmsMol Ther200917693994619337235
- HallBAndreeffMMariniFThe participation of mesenchymal stem cells in tumor stroma formation and their application as targeted-gene delivery vehiclesHandb Exp Pharmacol200718026328317554513
- VallabhaneniKCTkachukSKiyanYUrokinase receptor mediates mobilization, migration, and differentiation of mesenchymal stem cellsCardiovasc Res201190111312121088115
- StudenyMMariniFCChamplinREZompettaCFidlerIJAndreeffMBone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumorsCancer Res200262133603360812097260
- SchichorCBirnbaumTEtminanNVascular endothelial growth factor A contributes to glioma-induced migration of human marrow stromal cells (hMSC)Exp Neurol2006199230131016574102
- BirnbaumTRoiderJSchankinCJMalignant gliomas actively recruit bone marrow stromal cells by secreting angiogenic cytokinesJ Neurooncol200783324124717570034
- FengBChenLReview of mesenchymal stem cells and tumors: executioner or coconspirator?Cancer Biother Radiopharm200924671772120025552
- SpaethEKloppADembinskiJAndreeffMMariniFInflammation and tumor microenvironments: defining the migratory itinerary of mesenchymal stem cellsGene Ther2008151073073818401438
- DwyerRMPotter-BeirneSMHarringtonKAMonocyte chemotactic protein-1 secreted by primary breast tumors stimulates migration of mesenchymal stem cellsClin Cancer Res200713175020502717785552
- KiddSSpaethEKloppAAndreeffMHallBMariniFCThe (in) auspicious role of mesenchymal stromal cells in cancer: be it friend or foeCytotherapy200810765766718985472
- HoganNMDwyerRMJoyceMRKerinMJMesenchymal stem cells in the colorectal tumor microenvironment: recent progress and implicationsInt J Cancer201213111722290082
- ZhuWXuWJiangRMesenchymal stem cells derived from bone marrow favor tumor cell growth in vivoExp Mol Pathol200680326727416214129
- DjouadFPlencePBonyCImmunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animalsBlood2003102103837384412881305
- MaoYKellerETGarfeldDHShenKWangJStromal cells in tumor microenvironment and breast cancerCancer Metastasis Rev2013321–230331523114846
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG); DaviesCGodwinJRelevance of breast cancer hormone receptors and other factors to the effiacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trialsLancet2011378979377178421802721
- MartinFTDwyerRMKellyJPotential role of mesenchymal stem cells (MSCs) in the breast tumour microenvironment: stimulation of epithelial to mesenchymal transition (EMT)Breast Cancer Res Treat2010124231732620087650
- KandouzMBatistGGap junctions and connexins as therapeutic targets in cancerExpert Opin Ther Targets201014768169220446866
- MroueRMEl-SabbanMETalhoukRSConnexins and the gap in contextIntegr Biol (Camb)20113425526621437329
- GilleronJCaretteDChevallierDSegretainDPointisGMolecular connexin partner remodeling orchestrates connexin traffic: from physiology to pathophysiologyCrit Rev Biochem Mol Biol201247540742322551357
- GuJQianHShenLGastric cancer exosomes trigger differentiation of umbilical cord derived mesenchymal stem cells to carcinoma-associated fibroblasts through TGF-beta/Smad pathwayPloS One2012712e5246523285052
- BonnometABrysseATachsidisAEpithelial-to-mesenchymal transitions and circulating tumor cellsJ Mammary Gland Biol Neoplasia201015226127320449641
- JingYHanZZhangSLiuYWeiLEpithelial-mesenchymal transition in tumor microenvironmentCell Biosci201112921880137
- LiHJReinhardtFHerschmanHRWeinbergRACancer-stimulated mesenchymal stem cells create a carcinoma stem cell niche via prostaglandin E2 signalingCancer Discov20122984085522763855
- HuangFZhuXHuXQMesenchymal stem cells modified with miR-126 release angiogenic factors and activate Notch ligand Delta-like-4, enhancing ischemic angiogenesis and cell survivalInt J Mol Med201331248449223229021
- XieJWangWSiJWNotch signaling regulates CXCR4 expression and the migration of mesenchymal stem cellsCell Immunol20132811687523474530
- Del PapaBSportolettiPCecchiniDNotch1 modulates mesenchymal stem cells mediated regulatory T-cell inductionEur J Immunol201343118218723161436
- EnginFYaoZYangTDimorphic effects of Notch signaling in bone homeostasisNat Med200814329930518297084
- SethiNDaiXWinterCGKangYTumor-derived JAGGED1 promotes osteolytic bone metastasis of breast cancer by engaging notch signaling in bone cellsCancer Cell201119219220521295524
- WhitesideTLThe tumor microenvironment and its role in promoting tumor growthOncogene200827455904591218836471
- JoyceJAPollardJWMicroenvironmental regulation of metastasisNat Rev Cancer20099423925219279573
- McGowanPMSimedreaCRibotEJNotch1 inhibition alters the CD44hi/CD24lo population and reduces the formation of brain metastases from breast cancerMol Cancer Res20119783484421665937
- RohnerASpilkerMELamJLEffective targeting of Hedgehog signaling in a medulloblastoma model with PF-5274857, a potent and selective Smoothened antagonist that penetrates the blood-brain barrierMol Cancer Ther2012111576522084163
- BleckmannASiamLKlemmFNuclear LEF1/TCF4 correlate with poor prognosis but not with nuclear β-catenin in cerebral metastasis of lung adenocarcinomasClin Exp Metastasis201230447148223224985
- ZengQLiSChepehaDBCrosstalk between tumor and endothelial cells promotes tumor angiogenesis by MAPK activation of Notch signalingCancer Cell200581132316023595
- PolicastroLLIbañezILNotcovichCDuranHAPodhajcerOLThe tumor microenvironment: characterization, redox considerations, and novel approaches for reactive oxygen species-targeted gene therapyAntioxid Redox Signal Epub1022012
- FiaschiTChiarugiPOxidative stress, tumor microenvironment, and metabolic reprogramming: a diabolic liaisonInt J Cell Biol2012201276282522666258
- PietrasAvon StedingkKLindgrenDPahlmanSAxelsonHJAG2 induction in hypoxic tumor cells alters Notch signaling and enhances endothelial cell tube formationMol Cancer Res20119562663621402725
- XingFKobayashiAOkudaHReactive astrocytes promote the metastatic growth of breast cancer stem-like cells by activating Notch signalling in brainEMBO Mol Med20135338439623495140
- RidgwayJZhangGWuYInhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesisNature200644471221083108717183323
- Noguera-TroiseIDalyCPapadopoulosNJBlockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesisNature200644471221032103717183313
- ScehnetJSJiangWKumarSRInhibition of Dll4-mediated signaling induces proliferation of immature vessels and results in poor tissue perfusionBlood2007109114753476017311993
- ThurstonGNoguera-TroiseIYancopoulosGDThe Delta paradox: DLL4 blockade leads to more tumour vessels but less tumour growthNat Rev Cancer20077532733117457300
- YanMPlowmanGDDelta-like 4/Notch signaling and its therapeutic implicationsClin Cancer Res200713247243724618094402
- ReynoldsNDLukacsNWLongNKarpusWJDelta-like ligand 4 regulates central nervous system T cell accumulation during experimental autoimmune encephalomyelitisJ Immunol201118752803281321788444
- HayashiITakatoriSUranoYNeutralization of the gamma-secretase activity by monoclonal antibody against extracellular domain of nicastrinOncogene201231678779821725355
- FunahashiYHernandezSLDasIA notch1 ectodomain construct inhibits endothelial notch signaling, tumor growth, and angiogenesisCancer Res200868124727473518559519
- Varnum-FinneyBWuLYuMImmobilization of Notch ligand, Delta-1, is required for induction of notch signalingJ Cell Sci2000113Pt 234313431811069775
- SmallDKovalenkoDKacerDSoluble Jagged 1 represses the function of its transmembrane form to induce the formation of the Src-dependent chord-like phenotypeJ Biol Chem200127634320223203011427524
- SmasCMChenLSulHSCleavage of membrane-associated pref-1 generates a soluble inhibitor of adipocyte differentiationMol Cell Biol19971729779889001251
- MoelleringRECornejoMDavisTNDirect inhibition of the NOTCH transcription factor complexNature2009462727018218819907488
- TammamJWareCEffersonCDown-regulation of the Notch pathway mediated by a gamma-secretase inhibitor induces anti-tumour effects in mouse models of T-cell leukaemiaBr J Pharmacol200915851183119519775282
- WeiPWallsMQiuMEvaluation of selective gamma-secretase inhibitor PF-03084014 for its antitumor efficacy and gastrointestinal safety to guide optimal clinical trial designMol Cancer Ther2010961618162820530712
- FouladiMStewartCFOlsonJPhase I trial of MK-0752 in children with refractory CNS malignancies: a pediatric brain tumor consortium studyJ Clin Oncol201129263529353421825264
- PandyaKMeekeKClementzAGTargeting both Notch and ErbB-2 signalling pathways is required for prevention of ErbB-2-positive breast tumour recurrenceBr J Cancer2011105679680621847123
- MacyMESawczynKKGarringtonTPGrahamDKGoreLPediatric developmental therapies: interesting new drugs now in early-stage clinical trialsCurr Oncol Rep200810647749018928662
- Zweidler-McKayPANotch signaling in pediatric malignanciesCurr Oncol Rep200810645946818928660
- ZhouBBZhangHDamelinMGelesKGGrindleyJCDirksPBTumour-initiating cells: challenges and opportunities for anticancer drug discoveryNat Rev Drug Discov200981080682319794444
- KallifatidisGLabschSRauschVSulforaphane increases drug-mediated cytotoxicity toward cancer stem-like cells of pancreas and prostateMol Ther201119118819520940707
- KawaharaTKawaguchi-IharaNOkuhashiYItohMNaraNTohdaSCyclopamine and quercetin suppress the growth of leukemia and lymphoma cellsAnticancer Res200929114629463220032413
- OkuhashiYItohMNaraNTohdaSEffects of combination of notch inhibitor plus hedgehog inhibitor or Wnt inhibitor on growth of leukemia cellsAnticancer Res201131389389621498710
- ZhouWKallifatidisGBaumannBDietary polyphenol quercetin targets pancreatic cancer stem cellsInt J Oncol201037355156120664924
- WangZZhangYBanerjeeSLiYSarkarFHNotch-1 down-regulation by curcumin is associated with the inhibition of cell growth and the induction of apoptosis in pancreatic cancer cellsCancer2006106112503251316628653
- LuistroLHeWSmithMPreclinical profile of a potent gamma-secretase inhibitor targeting notch signaling with in vivo efficacy and pharmacodynamic propertiesCancer Res200969197672768019773430
- RaoSSO’NeilJLiberatorCDInhibition of NOTCH signaling by gamma secretase inhibitor engages the RB pathway and elicits cell cycle exit in T-cell acute lymphoblastic leukemia cellsCancer Res20096973060306819318552
- EspinozaIMieleLNotch inhibitors for cancer treatmentPharmacol Ther201313929511023458608
- HarrisonHFarnieGHowellSJRegulation of breast cancer stem cell activity by signaling through the Notch4 receptorCancer Res201070270971820068161
- SamonJBCastillo-MartinMHadlerMPreclinical analysis of the gamma-secretase inhibitor PF-03084014 in combination with glucocorticoids in T-cell acute lymphoblastic leukemiaMol Cancer Ther20121171565157522504949
- RizzoPMiaoHD’SouzaGCross-talk between notch and the estrogen receptor in breast cancer suggests novel therapeutic approachesCancer Res200868135226523518593923
- AlbainKCzerlanisCZlobinAModulation of cancer stem cell biomarkers by the Notch inhibitor MK-0752 added to endocrine therapy for early stage ER+ breast cancerCancer Res20117197s
- Means-PowellJAMintonSEMayerIAA Phase Ib dose escalation trial of RO4929097 (a γ-secretase inhibitor) in combination with exemestane in patients with ER + metastatic breast cancerCancer Res201272280s
- JieunYunPannutiAPEspinozaICrosstalk between PKCα and Notch-4 in endocrine-resistant breast cancer cellsOncogenesis2013
- SchottAFLandisMDDontuGPreclinical and clinical studies of gamma secretase inhibitors with docetaxel on human breast tumorsClin Cancer Res20131961512152423340294