Abstract
Primary carcinoid tumor of the kidney is an extremely rare well-differentiated neuroendocrine tumor, which is generally a low-grade malignant cancer with a good prognosis. Carcinoid tumors are rarely found in the urinary system. Here, we report a 34-year-old woman with primary renal well-differentiated neuroendocrine tumor who underwent nephron sparing surgery and no evidence of recurrence or distant metastasis was found during routine follow-up. We searched the case of renal carcinoid with the search phrase “carcinoid [title] and kidney [title]” and “carcinoid [title] and renal [title]” using the PubMed and restricted the search to articles published in English since 2013. The clinical manifestations, age, sex, tumor size, location, gross pathology, light microscopy and immunohistochemistry were analyzed. A total of 28 cases of renal carcinoid were retrieved from PubMed. Higher proportion of positive labeling of CgA, Syn, NSE and CD56 are most valuable in the diagnosis of primary renal well-differentiated neuroendocrine tumor. At present, radical nephrectomy remains the gold standard in the curative-intent therapy for well-differentiated neuroendocrine carcinoma of kidney, in metastatic renal carcinoid, long-term use of octreotide may be an effective adjuvant therapy.
Introduction
Carcinoid tumors are low-grade malignant neoplasms which arise from neuroendocrine cells. This type of tumor was mostly located in the digestive tract and respiratory system, and rarely found in the urinary system. Carcinoid tumor of renal is extremely rare. Since the first carcinoid tumor case was reported by Resnick et alCitation1 in 1966, so far in 2020, there have been around 100 cases reported in the English-language literature. In recent decades, due to wide application of electron microscope, immunohistochemistry, octreotide scintigraphyCitation2 and positron emission tomography (PET) scan,Citation3,Citation4 the number of people diagnosed with renal carcinoid has been increasing. However, the incidence rates remained stable all over the world. Here we add one more case of primary renal carcinoid and review the related literature.
Materials and Methods
A 34-year-old woman was found to have a complex renal cyst in her left kidney when she underwent a routine physical examination. The patient denied recent headache, dizziness, flushing, fever, chills, diarrhea, weight loss or night sweats. There was no known history of hypertension, diabetes, cancer, or other relevant family history. The physical examination and biological tests were unremarkable. Enhanced abdominal CT showed a mass of 30 × 40 mm in the upper pole of left renal that was classified as Bosniak category IV (). The complete blood count, erythrocyte sedimentation rate, renal and hepatic function tests and serum C-reactive protein of the patient was completely normal. The patient received retroperitoneal laparoscopic nephron sparing surgery (NSS). The macroscopic examination of the surgical specimen revealed a 38×30×30 mm single cyst. Postoperative pathology showed left renal carcinoid tumor (neuroendocrine tumor) ().
Figure 1 Computed tomographic imaging: The enhanced computed tomographic (CT) scan revealed a mass of 30×40 mm in the upper pole of left renal that was classified as Bosniak category IV.
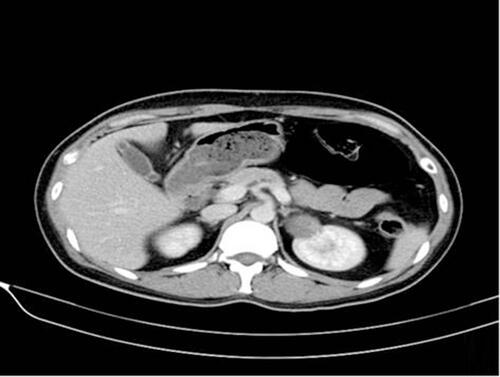
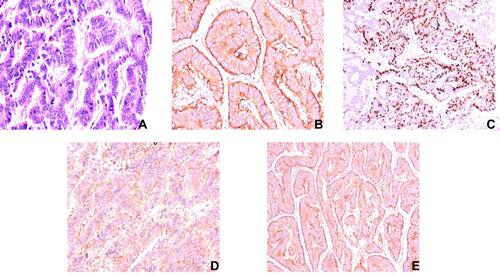
Figure 2 Pathological features of the renal carcinoid: (A) H&E (×100) showing nests and cords of neuroendocrine cells, which were arranged in a rosette like structure; (B) Positive immunostaining in tumor cells with synaptophysin (×200); (C) Intense positive immunostaining for chromogranin in tumor cells (×100); (D) Positive immunostaining in tumor cells with CD56 (×200); (E) Positive immunostaining in tumor cells with neuron specific enolase (×100).
We searched the case of renal carcinoid with the search phrase “carcinoid [title] and kidney [title]” and “carcinoid [title] and renal [title]” using the PubMed and restricted the search to articles published in English. The clinical manifestations, age, sex, tumor size, location, gross pathology, light microscopy and immunohistochemistry of primary renal carcinoid were analyzed. The trial was conducted in accordance with the principles of the Declaration of Helsinki. Written informed consent for the publication of this report was obtained from the patient, and ethical approval was given by the Fifth Affiliated Hospital of Zunyi Medical University (Grant No.07BTQ048).
Results
In our case, the postoperative period was uneventful and the patient was discharged five days after surgery. During the follow-up, the patient had no symptoms of carcinoid syndrome such as flushing, abdominal pain, wheezing and diarrhea. We found no evidence of recurrence or distant metastasis on abdominal computed tomography (CT) scan in the patient 12 months after the operation, routine blood indexes and biochemical indexes were within the range of normal values.
In a recent comprehensive review of the English-language literature, Romero et alCitation5 summarized 56 cases of renal carcinoid up to 2006, and Korkmaz et alCitation6 reviewed the literature and found 26 cases of primary renal carcinoid from 2006 to 2012. We reviewed the literature published in English after these reports and found 28 cases had been reported since 2013, including 1 unpublished case from our institute. We found that there is no significant difference in median age between male and female, distant metastasis of kidney well-differentiated neuroendocrine carcinoma often occured in the early stage. The well-differentiated neuroendocrine tumor of kidney was frequently associated with horseshoe kidney and renal teratoma. Interestingly, although the malignancy of renal well-differentiated neuroendocrine tumor is low, it was prone to distant metastasis, local invasion and lymph node in the early stage of the disease. In terms of diagnosis, chromogranin A, synaptophysin, neuron-specific enolase and CD56 are the most valuable markers in the diagnosis of kidney well-differentiated neuroendocrine tumor. At present, surgery is still the main treatment for renal neuroendocrine tumor.
Clinical features of renal carcinoid are shown in . Pathological features and pathological stages are shown in . Immunohistochemical results of these cases are shown in .
Table 1 Clinical Features of the Patients
Table 2 Pathologic Features of the Patients
Table 3 Immunohistochemical Features of the Patients
Discussion
Pathogenesis
Primary renal carcinoid is an extremely rare neoplasm, and the vast majority of reports refer to sporadic cases, therefore, there are no detailed epidemiological features and etiological characteristics about primary carcinoid tumor of kidney. A total of 159 of primary renal carcinoid cases have been reported worldwide so far. In our study, a total number of 29 cases were analyzed to further clarify the clinical and pathological characteristics of primary renal carcinoid. According to the latest classification of endocrine and neuroendocrine tumors from WHO in 2022, this type of tumor is defined as well-differentiated neuroendocrine tumors (NETs).Citation29 And our case also belongs to the renal well-differentiated neuroendocrine tumors based on the clinical symptoms and pathological examination.
Neuroendocrine tumors originate from cells that are capable of amine precursor (such as dopa and 5-hydroxytryptophan) uptake and decarboxylation (APUD cells). They are rare in the genitourinary tract, but have been identified in the prostate, bladder trigone, and the renal collecting system. Such tumors may secrete a number of other substances: like kinin, dopamine, histamine, and prostaglandin, causing signs and symptoms such as facial flushing due to widened blood vessels on the face, abdominal pain, diarrhea, bronchoconstriction, and carcinoid heart disease, which often occurred in gastrointestinal tract and respiratory system.Citation24,Citation30,Citation31 However, only 3 cases of carcinoid syndrome were reported in more than 100 patients with renal carcinoid.Citation1,Citation32,Citation33 In addition, the pathogenesis of renal carcinoid is unclear, although neuroendocrine cells may be developed by metaplasia from tissue of intermediate mesoderm, no argyrophilic cells have been found in normal renal parenchyma.Citation34 It has been reported that well-differentiated neuroendocrine tumor of renal with teratomatous lesions may originate from bronchial endocrine (Kulchitsky) cells present among the basal parts of the bronchial and bronchiolar epithelium,Citation35 and primary well-differentiated neuroendocrine tumor of kidney is often associated with renal teratoma.Citation17,Citation19,Citation35–Citation40 In a study conducted by Azzopardi et al,Citation41 they found that renal primordia could transform into argyrophilic cells in the tumor environment. The current research found intestinal metaplasia as a sequelae of chronic infection of renal pelvis, which may be an etiological factor in the occurrence of neuroendocrine cell hyperplasia and carcinoid tumor in the renal pelvicaliceal system.Citation10,Citation42–Citation46
The diagnosis of well-differentiated NET of kidney should be also noted that the possibility of poorly differentiated neuroendocrine carcinomas (NECs) was strictly excluded by extensive clinical examinations. Although the poorly differentiated NEC is even rarer than well-differentiated NET, only 2 cases were reported in the English literature.Citation28,Citation47 It is very important to identify the poorly differentiated NEC and well-differentiated NET, because there is no significant difference in histomorphology and immunohistochemical staining between them. Therefore, the diagnosis of neuroendocrine carcinomas should be combined with patient history, serum 5-HT, urine 5-HIAA,Citation48,Citation49 abdominal CT, MRI, octreotide scintigraphyCitation2 and positron emission tomography (PET),Citation3,Citation4 and so on.
The well-differentiated NET of kidney was frequently associated with horseshoe kidney (18–26%) and renal teratoma (15%). In this study, seven of the previously reported renal carcinoid tumors occurred in horseshoe kidneys (24.1%). This may be due to the presence of more abnormal epithelial cells in these types of kidney diseases.Citation50,Citation51 In the study of Krishnan et al,Citation51 it was found that the high relative risk of renal carcinoid tumor in a horseshoe kidney versus a non-horseshoe kidney is approximately 62. The study suggested that the tumor originated from goblet cells with dilated cystic lesion, and its histomorphology showed that the proliferation of neuroendocrine cells and the immunohistochemical characteristics were the same as those of carcinoid cells. In this study, one patient with well-differentiated NET of renal was complicated with mature cystic teratoma and horseshoe kidney (3.4%).Citation17
Recent genetic studies have found that three of four primary renal carcinoid tumors revealed monosomy of chromosome 3 (D3Z1) in the fluorescence in situ hybridization study, one patient showed loss of heterozygosity microsatellite markers at D3 S1300 and D3S1768 by PCR amplification and fragment analysis, and one patient manifested monosomy of chromosome 13 (D13S319/13q34). It is suggested that chromosomal abnormalities, especially chromosome 3, are related to the pathogenesis of renal carcinoid.Citation52
Clinical Features
Well-differentiated NET of kidney lacks the characteristic clinical manifestation, patients with carcinoid tumor can be completely asymptomatic, which is most often found during a routine physical examination (48.3%), some patients may have abdominal pain (17.2%), low back pain (20.7%), hematuria (6.9%), metastases as the first symptom (3.4%). Carcinoid syndrome occurs to very few people with carcinoid tumors (3.4%), Therefore, it often leads to misdiagnosis before operation, which is often found by postoperative pathological examination. Although renal carcinoid is a special type of carcinoid, the incidence of carcinoid syndrome is much lower than that of carcinoid tumors of gastrointestinal and respiratory tract, and there were only three cases had carcinoid syndrome in the current literature. In this study, only one patient presented carcinoid syndrome, the reason for this difference is not clear, we speculate that it is most likely related to inactivation of the vasoactive substances by the liver.
Unlike renal clear cell carcinoma, well-differentiated NET of kidney seemed to occur earlier than renal cell carcinoma, and most of the cases were diagnosed at the age about 49 years, and there was no gender difference in the incidence of well-differentiated NET of kidney between men and women.Citation12,Citation53 The median age at onset was 45 years (range: 21 to 78 years), which was consistent with the literature report; in terms of gender differences, there were 16 cases of female and 13 cases of male.
Well-differentiated NET of renal is a relatively low-grade malignancy, but it often occurs local invasion, lymph node and distant metastasis. In our study, seven cases (24.1%, 7/29) had local renal sinus,Citation16,Citation20,Citation21 perirenal fat and nerve invasion; eight cases (27.6%, 8/29) had lymph nodes metastasis;Citation6,Citation16,Citation17,Citation21,Citation27 and nine cases (31%, 9/29) had distant metastasis,Citation6,Citation7,Citation13,Citation14,Citation16,Citation21,Citation22,Citation25,Citation26 including five (17.2%, 5/29) cases of liver metastasisCitation6,Citation7,Citation14,Citation16,Citation26 and three cases (10.3%, 3/29) of lung metastasis.Citation6,Citation21,Citation25 In addition, the tumor can metastasize to bone,Citation6 contralateral kidney and adrenal,Citation13 eye,Citation14 pancreas,Citation21,Citation25 thyroid, skin,Citation13 renal vein and inferior vena cava.Citation22 Among the cases of distant metastasis, 20.7% of patients with metastasis of renal carcinoid are at initial diagnosis. Moreover, it should be noted that distant metastasis of poorly differentiated NEC could happen even 9 years after radical nephrectomy. In our case, no local recurrence or distant metastasis was found during routine follow-up. In view of the unclear biological characteristics of NET of renal, regular long-term follow-up plays an important role in the prognosis of patients.
Pathological Features
Romero et alCitation5 reviewed 56 case reports of renal carcinoid till 2006, the carcinoid tumor of kidney was found in the right-side renal (60.9%) more frequently than in the left. However, Korkmaz et alCitation6 reviewed the literature from 2006 to 2012 and found 26 cases, and the incidence rate of right kidney was 46%. In our study, there were 12 cases of left kidney (41.4%) and 17 cases of right kidney (58.6%), this is similar to the study of Romero.Citation5 According to statistics, the average diameter of well-differentiated NET of kidney was 5.9cm (1.8–22cm). In those cases, 59.3% of the cases with tumor diameter was greater than 4cm at the time of diagnosis, and 56.3% of the tumors with diameter > 4cm had metastasized at the initial diagnosis. In most case, gross examination of well-differentiated NET of kidney showed solid and cystic component, the cut surfaces were yellow or yellowish gray. Cysts of various sizes, with internal hemorrhage and rare necrosis, were observed. Along with microscopic feature, capsule pseudocapsule can be seen.
At present, there is no standard staging system for renal NET, the 8th edition of the AJCC staging system for renal cell carcinoma was adopted to evaluate renal carcinoid. The results of the statistical tests showed that T1, T2, T3 and T4 tumors accounted for 57.1%, 10.7%, 25% and 7.1% of the cases, respectively, and the percentages of stage I, stage II, stage III, and stage IV were 42.9%, 7.1%, 28.6% and 21.4%, respectively. Only one patient with stage IV died on the 11th month of follow-up. It is suggested that NET of kidney was indolent and prone to early distant metastasis, and they were associated with prolonged survival. The occurrence of this phenomenon may be related to the short follow-up time, and current TNM staging of renal cell carcinoma is not suitable for well-differentiated NET of kidney. In view of this, some scholars used the classification of pulmonary neuroendocrine tumors (WHO 1999) and confirmed its clinical relevance and prognostic significance, which seemed to be suitable for predicting the biological behavior of well-differentiated NET of kidney.Citation54,Citation55 Yakemchuk et alCitation4 reported that 18F-Dopa positron emission tomography (PET)/CT was proved to be more valuable for the assessment of NET of kidney by itself than MRI. In addition, somatostatin receptor positron emission tomography (SSTR-PET) is helpful for patient with CT undetermined renal NET as well as in the postoperative monitoring.Citation53,Citation56
Examination under a light microscope revealed that the tumors were composed of a single population of small, round to oval shape, bland-appearing cells with a distinctive “salt and pepper” chromatin pattern. Inconspicuous nucleoli and scant cytoplasm characterize carcinoid of the mediastinum and of any site. Tumor cells were arranged in cords, flakes and even chrysanthemum like structure with less necrosis (11.1%). The mitotic count was important to differentiate typical from atypical carcinoid tumors. In our study, the mitotic figures were no more than 2/10 HPF (96.3%), which indicated that well-differentiated NET of kidney was a low-grade malignant tumor.
Considering the NET of kidney is extremely rare, and lack of characteristic clinical and imaging manifestations, meanwhile, immunohistochemistry also has great uncertainty. Therefore, it is difficult to differentiate it in clinical diagnosis. However, scanning electron microscopy (SEM) is a magnetic domain observation method. Under electron microscope, there are a few neuroendocrine membrane-bound granules in the cytoplasm of carcinoid cells, usually showing a polar distribution. In general, SEM established the diagnosis of a primary neuroendocrine tumor.Citation15,Citation57
Immunohistochemical Features
The results of typical immunohistochemistry play a significant role in the pathological diagnosis of renal NET. The confirmation of kidney NET mainly depended on the typical immune markers and other exclusive immune indexes, which widely distributed in the nervous system. Chromogranin A (CgA), synaptophysin (Syn), neuron-specific enolase (NSE) and CD56 are the most valuable markers in the diagnosis of primary carcinoid of kidney.Citation8,Citation58 Well-differentiated NET of kidney samples stained positive for Syn significantly more than those of CgA, CD56 and NSE.Citation5 In the study of Romoero et alCitation5 the sensitivity of Syn in renal carcinoid was 100% (15/15), and the sensitivity of CgA, NSE, CK and Vim were 97.2%, 93.5%, 88.2% and 40%, respectively. In this study, the sensitivity of Syn is also 100% (28/28), and the sensitivity of CgA, NSE and Vim were 92.3% (24/26), 100% (6/6) and 40% (2/5), respectively. In our case, the surgical specimen was also immunohistochemically positive for Syn, NSE and CD56. Because of the rarity of NET of renal, and the lack of specific marker of immunohistochemistry, it is important to exclude other tumors and metastatic tumors of kidney. TTF-1 is a tissue-specific transcription factor, which is mainly expressed in the thyroid ACs, lung and high-grade neuroendocrine carcinomas, TTF-1 is rarely expressed in tumor of extrapulmonary tissues, therefore, TTF-1 could be used to distinguish renal NET from other sources that have metastasized to the kidney.Citation59 In our study, the expression of TTF-1 is negative. Cytokeratins 7 and 20 are some of useful immunohistochemical markers used in most patients who presented with primary and metastatic adenocarcinomas, we found that expression of CK7 and CK20 were negative in renal NET. And Ki-67 is a cancer antigen that is strictly associated with cell proliferation,Citation60 in this study, low grade: 46.2% (6/13), intermediate grade: 53.8% (7/13), and high grade: 0.
Treatment
Although there is no standard treatment for well-differentiated NET of kidney, radical nephrectomy has traditionally been considered the main treatment of choice for localized the carcinoid tumor of kidney. Partial nephrectomy is also a good alterative regarding diameter and location of the tumor, however, there is no evidence showing that radical nephrectomy is better than partial nephrectomy in patients with renal NET. A study of 56 cases of well-differentiated NET of kidney showed that the cure and survival rate of patients 3 years after undergoing surgery were 86% and 96%, respectively.Citation5 In addition, radiotherapy and chemotherapy are not an effective treatment for well-differentiated NET of kidney. For patients with regional lymph node metastasis, the study showed that patients with radical nephrectomy combined with lymph node resection had a disease-free survival rate of 47% at 43 months.Citation5 Even for patients with inferior vena cava tumor thrombus, radical nephrectomy and removal of the tumor thrombus can ensure long-term survival of the patient.Citation61
Octreotide, a nonspecific synthetic octapeptide analogue of somatostatin, and has been widely used to treat esophageal variceal bleeding and a varieties of NETs. Rinke et alCitation62 reported that octreotide had a good antiproliferative effect on metastatic neuroendocrine tumors. In a Phase II study, the combination of octreotide and everolimus have shown additive antitumor efficacy in patients with NETs. In metastatic carcinoid tumor of kidney, long-term use of octreotide was reported, and octreotide showed a good clinical effect.Citation7,Citation14,Citation16
Conclusions
Primary carcinoid tumor of the kidney is an extremely rare well-differentiated neuroendocrine tumor, which is generally a low-grade malignant cancer with a good prognosis. CgA, Syn, NSE and CD56 are the most valuable markers in the diagnosis of renal NET. Surgical treatment remains the standard curative therapy for localized well-differentiated NET, and octreotide may be an effective adjuvant therapy in metastatic renal carcinoid.
Consent for Publication
Written informed consent was obtained from the patient for publication.
Acknowledgments
The authors would like to thank our patient for allowing for his case to be presented.
Disclosure
The authors declare that they have no competing interests.
Additional information
Funding
References
- Resnick ME, Unterberger H, McLoughlin PT. Renal carcinoid producing the carcinoid syndrome. Med Times. 1966;94(8):895–896.
- Bukowczan J, Lois KB, Skinner J, Petrides G, James RA, Perros P. Metastatic midgut carcinoid in the myocardium. Clin Nucl Med. 2015;40(9):e446–e447. doi:10.1097/RLU.0000000000000884
- Nataf V, Balard M, de Beco V, et al. Safety of 18F-DOPA injection for PET of carcinoid tumor. J Nucl Med. 2006;47(10):1732.
- Yakemchuk VN, Jager PL, Chirakal R, Reid R, Major P, Gulenchyn KY. PET/CT using 18F-FDOPA provides improved staging of carcinoid tumor patients in a Canadian setting. Nucl Med Commun. 2012;33(3):322–330. doi:10.1097/MNM.0b013e32834f2603
- Romero FR, Rais-Bahrami S, Permpongkosol S, Fine SW, Kohanim S, Jarrett TW. Primary carcinoid tumors of the kidney. J Urol. 2006;176(6 Pt 1):2359–2366. doi:10.1016/j.juro.2006.07.129
- Korkmaz T, Seber S, Yavuzer D, Gumus M, Turhal NS. Primary renal carcinoid: treatment and prognosis. Crit Rev Oncol Hematol. 2013;87(3):256–264. doi:10.1016/j.critrevonc.2013.02.003
- Ouyang B, Ma X, Yan H, He J, Xia C, Yu H. Renal carcinoid tumor with liver metastasis followed up postoperatively for 9 years. Diagn Pathol. 2015;10:182. doi:10.1186/s13000-015-0417-7
- Li B, Cui T, Ban Z, Luo L, Sun L. Primary renal carcinoid tumor: case report and review of the literature. Onco Targets Ther. 2016;9:741–743. doi:10.2147/OTT.S88730
- Lamb L, Shaban W. Primary renal carcinoid tumor: a radiologic review. Radiol Case Rep. 2014;9(2):923. doi:10.2484/rcr.v9i2.923
- Kuba MG, Wasserman A, Vnencak-Jones CL, et al. Primary carcinoid tumor of the renal pelvis arising from intestinal metaplasia: an unusual histogenetic pathway? Appl Immunohistochem Mol Morphol. 2017;25(7):e49–e57. doi:10.1097/PAI.0000000000000445
- Lin C, Wu J, Gao Z, Qu G, Wang W, Yu G. Primary carcinoid tumor of the kidney with estrogen and progesterone receptor expression. Oncol Lett. 2015;10(1):449–452. doi:10.3892/ol.2015.3167
- Seker KG, Sam E, Sahin S, et al. Partial nephrectomy in horseshoe kidney: primary carcinoid tumor. Archivio italiano di urologia andrologia. 2017;89(4):316–318. doi:10.4081/aiua.2017.4.316
- Hartman MS, Mittal P, Lewis M. Multifocal renal carcinoid tumor arising in horseshoe kidney with metastases to the thyroid. Radiol Case Rep. 2006;1(3):108–111. doi:10.2484/rcr.v1i3.31
- Kiratli H, Uzun S, Tarlan B, Ateş D, Baydar DE, Söylemezoğlu F. Renal carcinoid tumor metastatic to the uvea, medial rectus muscle, and the contralateral lacrimal gland. Ophthal Plast Reconstr Surg. 2015;31(4):e91–e93. doi:10.1097/IOP.0000000000000112
- Gu X, Cheng M, Herrera GA. Kidney carcinoid tumor: histological, immunohistochemical and ultrastructural features. Ultrastruct Pathol. 2018;42(1):18–22. doi:10.1080/01913123.2017.1388321
- Rosenberg JE, Albersheim JA, Sathianathen NJ, Murugan P, Weight CJ. Five new cases of primary renal carcinoid tumor: case reports and literature review. Pathol Oncol Res. 2020;26(1):341–346. doi:10.1007/s12253-018-0481-x
- Sun K, You Q, Zhao M, Yao H, Xiang H, Wang L. Concurrent primary carcinoid tumor arising within mature teratoma and clear cell renal cell carcinoma in the horseshoe kidney: report of a rare case and review of the literature. Int J Clin Exp Pathol. 2013;6(11):2578–2584.
- Chen CT, Hsieh SW, Hsieh TS. Case report: a case of primary renal carcinoid tumor. Urol Case Rep. 2018;21:14–16. doi:10.1016/j.eucr.2018.07.020
- Kim J, Suh K. Primary carcinoid tumor in a mature teratoma of the kidney: ultrasonographic and computed tomographic findings. J Med Ultrasound. 2004;23(3):433–437. doi:10.7863/jum.2004.23.3.433
- Anderson DA, Tretiakova MS. Primary renal carcinoid with bilateral multiple clear cell papillary renal cell carcinomas. Case Rep Pathol. 2017;2017:9672368. doi:10.1155/2017/9672368
- Daboul N, Monga D, Bunker M. Primary renal carcinoid tumour with lung metastasis misdiagnosed as renal cell carcinoma. BMJ Case Rep. 2016;2016:bcr2015213432.
- Kanodia KV, Vanikar AV, Patel RD, et al. Primary renal carcinoid tumor. Saudi J Kidney Dis Transpl. 2013;24(5):988–990. doi:10.4103/1319-2442.118095
- Jabaji R, Kern T, Shen D, Chu W, Merchant M. Primary renal carcinoid tumor: report of two cases. Perm J. 2020;24. doi:10.7759/cureus.13907
- Yoon JY, Sigel K, Martin J, et al. Evaluation of the prognostic significance of TNM staging guidelines in lung carcinoid tumors. J Thorac Oncol. 2019;14(2):184–192. doi:10.1016/j.jtho.2018.10.166
- Arshad H, Rali P, Malik K. Metastatic renal carcinoid: to skin, lungs, and pancreas. Lung India. 2017;34(4):383–385. doi:10.4103/lungindia.lungindia_291_16
- Salehipour M, Mostaghni AA, Geramizadeh B, Makarem A, Rezvani A. Constipation, the sole presentation of primary renal carcinoid tumor: a case report. Rare Tumors. 2019;11:2036361319878915. doi:10.1177/2036361319878915
- Linke CS, Shie S. An asymptomatic primary renal carcinoid tumor: a case report. Urol Case Rep. 2016;7:70–71. doi:10.1016/j.eucr.2016.04.018
- Kato Y, Nakamura K, Yamada Y, et al. A rare case of metastatic renal carcinoid. BMC Urol. 2010;10(1):22. doi:10.1186/1471-2490-10-22
- Rindi G, Mete O, Uccella S, et al. Overview of the 2022 WHO classification of neuroendocrine neoplasms. Endocr Pathol. 2022;33(1):115–154. doi:10.1007/s12022-022-09708-2
- Mallick B, Nath P, Praharaj DL, Biswal SK, Panigrahi SC, Anand AC. Gastrointestinal: an unusual cause of lower gastrointestinal bleed: ileal carcinoid tumor. J Gastroenterol Hepatol. 2020;35(3):359. doi:10.1111/jgh.14883
- Nandy N, Hanson JA, Strickland RG, McCarthy DM. Solitary gastric carcinoid tumor associated with long-term use of omeprazole: a case report and review of the literature. Dig Dis Sci. 2016;61(3):708–712. doi:10.1007/s10620-015-4014-0
- Couttenye MM, Verpooten GA, Daelemans RA, De Broe ME. Functional acute renal failure in a patient with carcinoid syndrome. Nephron. 1987;47(2):131–133. doi:10.1159/000184475
- Parry RG, Glover S, Dudley CR. Acute renal failure associated with carcinoid crisis. Nephrol Dial Transplant. 1996;11(12):2489–2490. doi:10.1093/oxfordjournals.ndt.a027221
- Acconcia A, Miracco C, Mattei FM, deSanti MM, Del Vecchio MT, Luzi P. Primary carcinoid tumor of kidney. Light and electron microscopy, and immunohistochemical study. Urology. 1988;31(6):517–520. doi:10.1016/0090-4295(88)90221-X
- Kojiro M, Ohishi H, Isobe H. Carcinoid tumor occurring in cystic teratoma of the kidney: a case report. Cancer. 1976;38(4):1636–1640. doi:10.1002/1097-0142(197610)38:4<1636::AID-CNCR2820380432>3.0.CO;2-N
- Higgins A, Eisa W, Walton J, Baber J, Zhu S, Williams H. Metastatic mucinous adenocarcinoma and carcinoid tumor arising from a mature cystic teratoma of a horseshoe kidney. Urol Case Rep. 2017;11:39–41. doi:10.1016/j.eucr.2016.11.017
- Armah HB, Parwani AV, Perepletchikov AM. Synchronous primary carcinoid tumor and primary adenocarcinoma arising within mature cystic teratoma of horseshoe kidney: a unique case report and review of the literature. Diagn Pathol. 2009;4(1):17. doi:10.1186/1746-1596-4-17
- Armah HB, Parwani AV. Primary carcinoid tumor arising within mature teratoma of the kidney: report of a rare entity and review of the literature. Diagn Pathol. 2007;2(1):15. doi:10.1186/1746-1596-2-15
- Kurzer E, Leveillee RJ, Morillo G. Rare case of carcinoid tumor arising within teratoma in kidney. Urology. 2005;66(3):658. doi:10.1016/j.urology.2005.03.029
- Yoo J, Park S, Jung Lee H, Jin Kang S, Kee Kim B. Primary carcinoid tumor arising in a mature teratoma of the kidney: a case report and review of the literature. Arch Pathol Lab Med. 2002;126(8):979–981. doi:10.5858/2002-126-0979-PCTAIA
- Azzopardi JG, Hou LT. Intestinal metaplasia with argentaffin cells in cervical adenocarcinoma. J Pathol Bacteriol. 1965;90(2):686–690. doi:10.1002/path.1700900245
- Gordon A. Intestinal metaplasia of the urinary tract epithelium. J Pathol Bacteriol. 1963;85(2):441–444. doi:10.1002/path.1700850224
- Kim SS, Choi C, Kang TW, Choi YD. Carcinoid tumor associated with adjacent dysplastic columnar epithelium in the renal pelvis: a case report and literature review. Pathol Int. 2016;66(1):42–46. doi:10.1111/pin.12367
- Kuroda N, Katto K, Tamura M, et al. Carcinoid tumor of the renal pelvis: consideration on the histogenesis. Pathol Int. 2008;58(1):51–54. doi:10.1111/j.1440-1827.2007.02188.x
- Rudrick B, Nguyen GK, Lakey WH. Carcinoid tumor of the renal pelvis: report of a case with positive urine cytology. Diagn Cytopathol. 1995;12(4):360–363. doi:10.1002/dc.2840120416
- Ji X, Li W. Primary carcinoid of the renal pelvis. J Environ Pathol Toxicol Oncol. 1994;13(4):269–271.
- Barton JC, Barton JC, Bertoli LF. Recurrent acute kidney injury associated with metastatic bronchial carcinoid. Am J Med Sci. 2012;343(1):106–108. doi:10.1097/MAJ.0b013e31823183c9
- Soga J, Yakuwa Y, Osaka M. Carcinoid syndrome: a statistical evaluation of 748 reported cases. J Exp Clin Cancer Res. 1999;18(2):133–141.
- Kema IP, de Vries EG, Slooff MJ, Biesma B, Muskiet FA. Serotonin, catecholamines, histamine, and their metabolites in urine, platelets, and tumor tissue of patients with carcinoid tumors. Clin Chem. 1994;40(1):86–95. doi:10.1093/clinchem/40.1.86
- Rodríguez-Covarrubias F, Gómez X, Valerio JC, Lome-Maldonado C, Gabilondo F. Carcinoid tumor arising in a horseshoe kidney. Int Urol Nephrol. 2007;39(2):373–376. doi:10.1007/s11255-006-9031-7
- Krishnan B, Truong LD, Saleh G, Sirbasku DM, Slawin KM. Horseshoe kidney is associated with an increased relative risk of primary renal carcinoid tumor. J Urol. 1997;157(6):2059–2066. doi:10.1016/S0022-5347(01)64674-3
- Kuroda N, Alvarado-Cabrero I, Sima R, et al. Renal carcinoid tumor: an immunohistochemical and molecular genetic study of four cases. Oncol Lett. 2010;1(1):87–90. doi:10.3892/ol_00000015
- Canacci AM, MacLennan GT. Carcinoid tumor of the kidney. J Urol. 2008;180(5):2193. doi:10.1016/j.juro.2008.08.011
- Quinchon JF, Aubert S, Biserte J, Lemaitre L, Gosselin B, Leroy X. Primary atypical carcinoid of the kidney: a classification is needed. Pathology. 2003;35(4):353–355. doi:10.1080/0031302031000152900
- Gibbs AR, Thunnissen FB. Histological typing of lung and pleural tumours: third edition. J Clin Pathol. 2001;54(7):498–499. doi:10.1136/jcp.54.7.498
- James C, Starks M, MacGillivray DC, White J. The use of imaging studies in the diagnosis and management of thyroid cancer and hyperparathyroidism. Surg Oncol Clin N Am. 1999;8(1):145–169. doi:10.1016/S1055-3207(18)30230-8
- Tamaki Y, Hashimoto T, Kamai T, et al. Oncocytic carcinoid of the kidney positively detected by I-131 MIBG scintigraphy. Clin Nucl Med. 2011;36(11):1025–1028. doi:10.1097/RLU.0b013e3182291a44
- Pivovarcikova K, Agaimy A, Martinek P, et al. Primary renal well-differentiated neuroendocrine tumour (carcinoid): next-generation sequencing study of 11 cases. Histopathology. 2019;75(1):104–117. doi:10.1111/his.13856
- Du EZ, Goldstraw P, Zacharias J, et al. TTF-1 expression is specific for lung primary in typical and atypical carcinoids: TTF-1-positive carcinoids are predominantly in peripheral location. Hum Pathol. 2004;35(7):825–831. doi:10.1016/j.humpath.2004.02.016
- Gill AJ. Why did they change that? Practical implications of the evolving classification of neuroendocrine tumours of the gastrointestinal tract. Histopathology. 2021;78(1):162–170. doi:10.1111/his.14172
- Szymanski KM, Baazeem A, Sircar K, Tanguay S, Kassouf W. primary renal carcinoid tumor with inferior vena caval tumour thrombus. Can Urol Assoc J. 2009;3(3):E7–E9. doi:10.5489/cuaj.1091
- Rinke A, Müller HH, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27(28):4656–4663. doi:10.1200/JCO.2009.22.8510
