Abstract
Nrf2 has gained great attention with respect to its pivotal role in cell and tissue protection. Primarily defending cells against metabolic, xenobiotic and oxidative stress, Nrf2 is essential for maintaining tissue integrity. Owing to these functions, Nrf2 is regarded as a promising drug target in the chemoprevention of diseases, including cancer. However, much evidence has accumulated that the beneficial role of Nrf2 in cancer prevention essentially depends on the tight control of its activity. In fact, the deregulation of Nrf2 is a critical determinant in oncogenesis and found in many types of cancer. Therefore, amplified Nrf2 activity has profound effects on the phenotype of tumor cells, including radio/chemoresistance, apoptosis protection, invasiveness, antisenescence, autophagy deficiency, and angiogenicity. The deregulation of Nrf2 can result from various epigenetic and genetic alterations directly affecting Nrf2 control or from the complex interplay of Nrf2 with numerous oncogenic signaling pathways. Additionally, alterations of the cellular environment, eg, during inflammation, contribute to Nrf2 deregulation and its persistent activation. Therefore, the status of Nrf2 as anti- or protumorigenic is defined by many different modalities. A better understanding of these modalities is essential for the safe use of Nrf2 as an activation target for chemoprevention on the one hand and as an inhibition target in cancer therapy on the other. The present review mainly addresses the conditions that promote the oncogenic function of Nrf2 and the resulting consequences providing the rationale for using Nrf2 as a target structure in cancer therapy.
Introduction
The integrity and function of mammalian cells is under the tight control of numerous homeostatic signaling pathways that provide protection against endogenous and environmental hazards. However, the deregulation and amplification of these pathways can cause devastating diseases, as particularly seen in cancer, where cytoprotective mechanisms are often inadequately engaged, giving rise to highly stress-tolerant cells that escape homeostatic control.
One prominent example is the oxidative and xenobiotic stress-response pathway, under the control of Nrf2, which has an essential role in cytoprotection.Citation1–Citation4 Based on this role, in particular by preventing deoxyribonucleic acid (DNA) damage and mutagenic events, Nrf2 was regarded for a long time as a tumor inhibitor, thus providing the rationale of tumor-prevention strategies using Nrf2 activators.Citation5–Citation8 However, this view had to be modified when it was recognized that deregulated Nrf2 activity can also favor a malignant phenotype and promote carcinogenesis.Citation6,Citation9–Citation12 Ample evidence has accumulated that Nrf2 either acts itself as a proto-oncogene or that Nrf2 supports the transforming potential of other proto-oncogenes. For example, the Nrf2-dependent upregulation of antioxidative and antiapoptotic pathways essentially contributes to the forced proliferation and survival of kRas-transformed cells.Citation13 Other Nrf2 effects that add to a malignant phenotype include an altered metabolism and an enhanced regulated protein turnover by the ubiquitin–proteasome system.
Therefore, under homeostatic conditions, Nrf2-dependent cytoprotective effects avoid excessive cellular damage and dysfunction, but when they lose their tight control, these effects substantially add to the malignant phenotype of cancer cells. Accordingly, enhanced Nrf2 activity has been found in a great number of solid and hematologic tumors resulting either from mutational events, epigenetic effects, or constitutive stress adaptation. This condition of deregulated Nrf2 provides several growth advantages to cancer cells, including antisenescence, proliferation, and antiapoptosis, as well as a profound resistance to drugs and radiotherapy. Therefore, Nrf2 has become of great interest with regard to its use in therapy and the diagnosis of malignant diseases.
The present review primarily discusses the most recent aspects regarding the role of Nrf2 in the initiation and development of cancer. Other findings beyond this issue have been the subject of many excellent reviews on the dual role of Nrf2 in tumorigenesis, published recently.Citation5–Citation12
Oxidative and xenobiotic stress
During evolution, cells became exposed to an oxygen-enriched (aerobic) atmosphere, and thereby acquired essential growth advantages. In particular, an oxidative metabolism along with a maximum of energy yield has favored the development of complex (higher) organisms, but many hazards also arose from oxygen. Deriving from molecular oxygen, various reactive molecules and free radicals (eg, superoxide, hydrogen peroxide, hydroxyl radical, hydroxyl ion, and nitric oxide) can form, which are collectively designated as reactive oxygen species (ROS).Citation14,Citation15 Besides environmental conditions, eg, heat and irradiation, ROS are also produced biologically in aerobic cells. In these cells, most ROS are generated as by-products during mitochondrial electron transport. ROS are also produced as intermediates of enzymatic oxidation reactions, having an important role as intra- and intercellular messengers.Citation16 In addition, ROS – mainly released by phagocytes – are effective in the cellular defense against microbes, thus having a pivotal role in innate immunity.Citation17
Owing to their deleterious effects on cell components (lipids, proteins, and DNA) ROS need to be efficiently neutralized in aerobic cells, and a balance between generation and removal of ROS is an essential prerequisite for cellular survival. This balance is maintained by a number of tightly regulated defense mechanisms, a failure of which leads to a condition termed oxidative stress.Citation18 If left uncompensated, oxidative stress impacts on cellular and tissue integrity, particularly through accumulating DNA damage, giving rise to severe pathologies,Citation19 including cancer.Citation20,Citation21 Representing the major protection system in higher organisms, Nrf2 drives ROS detoxificationCitation22,Citation23 through inducing expression of many antioxidative enzymes, such as glutamate–cysteine ligase catalytic/modifier subunit (GCLC/M) and glutathione S-reductase for glutathione synthesis and recovery, heme oxygenase (HO)-1, peroxiredoxin-1/4, superoxide dismutases, thioredoxin or thioredoxin reductase 1, and aldo-keto reductases (AKRs).Citation24,Citation25
Another environmental hazard for cells emerges from xenobiotics, collectively designating natural or synthetic compounds foreign to the organism’s metabolism and biochemistry.Citation26 Xenobiotics include drugs, food additives, environmental pollutants, and fungal or microbial toxins making up hundreds of thousands of compounds affecting cellular integrity,Citation27 and the exposure to these foreign compounds is known as xenobiotic stress. To cope with this stress condition, all major groups of organisms possess particular metabolic pathways to detoxify potentially hazardous compounds.Citation26 However, intermediates of xenobiotic metabolism may become toxic as well, and sometimes give rise to ROS formation.Citation28
Detoxication of these xenobiotic compounds includes three phases.Citation29 In phase I, xenobiotics are oxidized by enzymes like nicotinamide adenine dinucleotide phosphate quinone oxidoreductase 1 (NQO1), reduced by enzymes like alcohol dehydrogenase, aldehyde dehydrogenases (ALDHs) and AKRs or hydroxylated by cytochrome P450 monooxygenases.Citation30 In phase II, the phase I products are conjugated to hydrophilic compounds, such as glucuronic acid (by uridine 5′-diphospho-glucuronosyltransferases [UGTs]), sulfate (by sulfotransferases), or glutathione (by glutathione S-transferases [GSTs]), thereby generating water-soluble products that can be easily eliminated from the body.Citation31 In phase III, these products are exported from cells by ABC/MRP or ABC/MDR transporters. Notably, many phase I, II, and III enzymes are target genes of Nrf2.Citation32 These include NQO1, AKR1B1, ALDH3A1, GSTA1, GSTN1, UGT1A1, ABCB6, or ABCC15.Citation24,Citation25,Citation32,Citation33
The CNC-bZIP protein family and control of Nrf2 signaling
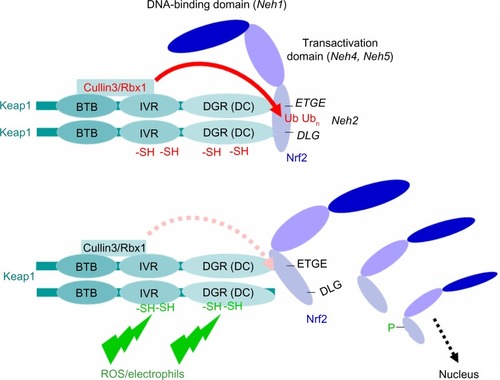
Together with two closely related proteins – Nrf1 and Nrf3 – as well as with NF-E2 and Bach-1, and Bach-2, Nrf2 belongs to a family of transcription factors termed CNC-bZIP proteins.Citation34 Quite unique to Nrf2 is the Neh2 domain near to the amino-terminal end, which serves as interaction domain with the Nrf2 inhibitory protein Keap1.Citation35 This interaction depends on the low-affinity binding of a DLG and the high-affinity binding of an ETGE motif. Under homeostatic conditions, Nrf2 is kept at low levels when bound via DLG and ETGE to a homodimer of Keap1 through the DC domain of either one of the Keap1 subunits, leading to Cullin3/Rbx1-catalyzed polyubiquitination and subsequent proteasomal degradation of Nrf2 ().Citation36,Citation37 During conditions of oxidative/electrophilic or xenobiotic stress, the Cullin3/Rbx1-dependent polyubiquitination of Nrf2 under the assistance of Keap1 is blocked, thereby leading to the accumulation of Nrf2 protein and its subsequent translocation to the nucleus ().Citation36 The derepression of Nrf2 in this way depends on the dissociation of the Nrf2 polyubiquination site from Cullin3/Rbx1 without releasing Nrf2 from the protein complex (according to the hinge–latch model, in which the ETGE motif remains bound to Keap1), hence saturating Keap1 and competing with free Nrf2 that instead can enter the nucleus ().Citation38,Citation39 An alternative mode of induction occurs through the release of Cullin3 from Keap1, thereby directly impeding the polyubiquitination of Nrf2.Citation40 During Nrf2 activation, Keap1 itself can be Lys63 polyubiquitinated,Citation41 which does not target the protein to the proteasome (in contrast to the Lys48 polyubiquitination of Nrf2), but instead seems to destabilize its Cullin3 interaction. Recently, the ubiquitin-specific protease-15 deubiquitinase was identified to deubiquitinate Keap1,Citation42 thereby restoring Cullin3 recruitment and accordingly Nrf2 suppression. This ubiquitination/deubiquitination of Keap1 may function as an additional Nrf2-regulatory mechanism.
Figure 1 (A and B) Principle of controlling Nrf2 by Keap1 and its activation.
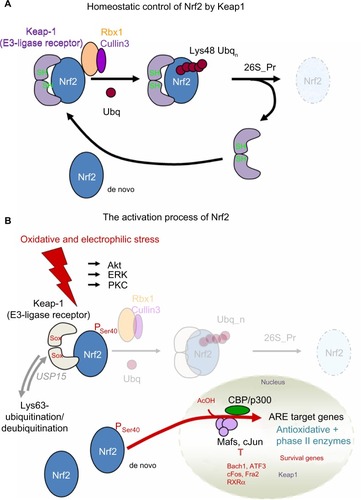
Figure 2 Interaction of Nrf2 and Keap1 according to the hinge–latch model.
Abbreviations: DNA, deoxyribonucleic acid; BTB, bric-a-brac; IVR, intervening region.
Along with its release from Keap1-dependent suppression, Nrf2 undergoes certain posttranslational modificationsCitation43 that support its nuclear translocation and binding to the antioxidant response element (ARE)/electrophile response element (EpRE) serving as a recognition motif in the target gene promoters: 1) PKC-dependent phosphorylation on Ser-40,Citation44 2) phosphorylation through the MAPK/PERK signaling pathway in response to endoplasmic reticulum/unfolded protein stressCitation45 or by casein kinase 2,Citation46 and 3) CBP/p300- or hMOF (males absent on the first)-mediated acetylation of lysyl residues within the Neh1 domain of Nrf2,Citation47,Citation48 facilitating binding to the ARE/EpRE sites.Citation49 The activation of Nrf2 is further promoted by additional signal transduction pathways, eg, ERK, JNK, or PI3K/Akt.Citation38,Citation50,Citation51 Negative control of Nrf2 is exerted by (for example) p38/MAPK, GSK3B, or PTEN.Citation52–Citation54
For DNA binding, Nrf2 further needs to heterodimerize with other bZIP proteins, eg, with c-Jun or with one of seven Maf proteins.Citation55,Citation56 These proteins – in particular the three small Maf proteins Maf-F, -G, and -K (themselves lacking transactivation activity) – associate with extended ARE/EpRE motifs at the promoters, and are essential for binding of Nrf2 to the core ARE/EpRE motif and thereby for Nrf2-dependent transactivation.Citation57
The interaction of Nrf2 with Maf proteins is also under the control of several signal-transduction pathways, eg, ERK, JNK, p38/MAPK, and PI3K/Akt,Citation58–Citation60 and requires additional cofactors, eg, JDP2.Citation61 In contrast to Maf proteins, the two proteinsCitation62 Bach-1 and Bach-2 act rather as transcriptional repressors of Nrf2.Citation63–Citation65
The major principle of Nrf2 activation is the redox-dependent alteration of the Keap1 conformation conferred by the sulfhydryl groups of more than 20 intrinsic cysteine residues. ROS and electrophilic compounds lead to disulfide formation and cysteine conjugates, respectively, similarly affecting the function of Keap1 as an E3-ligase receptor for Nrf2.Citation66–Citation68 There is a restriction of cysteine residues within the Keap1 protein, eg, Cys251, Cys273, and Cys288, with respect to the access of electrophilic compounds defining the activity of Nrf2-inducing substances like tert-butylhydroquinone (tBHQ), diethyl maleate, sulforaphane, 15-deoxy-Δ12,14-prostaglandin J2, 4-hydroxynonena, bardoxolone methyl (CDDO), curcumin, resveratrol, and many more.Citation69,Citation70 Meanwhile, hundreds of natural product-derived compounds have been established as inducers of the Nrf2/ARE pathway, including polyphenols, quinones, organosulfur compounds, polyenes, flavonoids, chalcones, and terpenoids. These agents have been successfully tested in experimental prevention studies with inflammatory bowel and neurodegenerative diseases, diabetes, and metabolic disorders, as well as lung, heart, and kidney diseases.Citation71 All these effectors of the Nrf2/ARE pathway are summarized in a comprehensive review by Kumar et al.Citation72
Representing an alternative mechanism of Nrf2 control, a ternary complex of Keap1 and Nrf2 is tethered to the outer mitochondrial membrane through PGAM5,Citation73 a member of the phosphoglycerate mutase family. Thereby, PGAM5 builds up a molecular framework that provides a direct link between mitochondrial functions and Nrf2-dependent antioxidant gene expression. Intriguingly, Keap1 has been reported to interfere also with the Bcl-xL apoptosis inhibitor in a PGAM5-dependent fashion.Citation74 Another condition of Keap1 deactivation is its interaction with phospho-p62 accumulating during autophagy deficiency.Citation75 Consequently, Keap1 fails to control Nrf2 stabilization if bound to phospho-p62, and autophagy-deficient cells show up with enhanced Nrf2 activation.Citation76 A similar disrupting effect on Keap1-mediated control of Nrf2 has been reported for the cyclin inhibitor p21/Waf1.Citation77 Therefore, the prosurvival effects described for p21 may at least partially rely on increased Nrf2 activation along with antioxidative protection. Besides Keap1, the E3 ligase receptor and F-box protein βTrCP is able to control the stability of the Nrf2 protein when phosphorylated in the Neh6 domain by GSK3B.Citation78,Citation79
The tumor suppressor p53 is also capable of preventing Nrf2 activation,Citation80 an effect involving destabilization of the Nrf2 protein and regarded as another mode of action by which p53 controls apoptosis.Citation81 This is restricted to cytotoxic stress conditions, because p53 also positively affects Nrf2, eg, through its target gene p21/Waf1. Hence, the modulation of Nrf2 by p53 is exerted in two ways, differentially affecting basal and stress-induced Nrf2 activity. Moreover, certain mutations of p53 even result in decreased Nrf2 activation and thereby in the amplification of oxidative stress,Citation82 pointing to the complexity of the cross talk between p53 and Nrf2.Citation81
Furthermore, negative regulation of Nrf2 is exerted by the GSK3B/Src-Fyn tyrosine-kinase pathway.Citation54,Citation83 Upon Fyn-dependent phosphorylation, Nrf2 is redirected to the cytoplasm, where it is bound to Keap1, itself being a target gene of Nrf2.Citation84 Nrf2 activation is also impaired by the NF-κB subunit p65/RelA, which coimports Keap1 into the nucleus,Citation85 and by E-cadherin, which blocks nuclear translocation of Nrf2 in a β-catenin-dependent fashion.Citation86 Other pathways negatively interfering with Nrf2 include those initiated by TFGβ1Citation87 through ATF3,Citation88 by nuclear hormone receptors, eg, estrogen receptor alpha (ERα), estrogen related receptor beta (ERRβ), glucocorticoid receptor (GR),Citation89 and under certain conditions also retinoid receptors (RXRα/RARα).Citation90–Citation93 Since the complex regulation of Nrf2 activation and the structural basis thereof have been the subject of numerous excellent reviews found in the recent literature,Citation9–Citation11,Citation94,Citation95 this issue is not addressed in greater detail here.
The antitumorigenic role of Nrf2
Before the protumorigenic effects exerted by Nrf2 are outlined in detail, some key aspects of its tumor-inhibiting potential should be briefly highlighted here. Over the years, a plethora of studies demonstrated that Nrf2 exerts tumor-preventive activity through its cytoprotective effects. These protect cells from DNA damage and the deterioration of proteins, carbohydrates, and lipids if exposed to oxidative and xenobiotic stress.Citation5,Citation7,Citation8 Accordingly, an impaired function of Nrf2 accounts for many stress-induced pathological disorders,Citation96 which could be most impressively seen in per-se viable Nrf2-knockout mice.Citation97 For instance, greater tissue damage in the lung, kidney, brain, liver, eye, or heart was seen in Nrf2-knockout mice subjected to acute insults like cigarette smoke, hyperoxia, ischemic reperfusion, portal vein embolization, and chemical toxins or when being subject of aging.Citation96,Citation98–Citation102 Similarly, mice treated with the mutagen azoxymethane and/or the colitis-inducing chaotropic agent dextran sulfate sodium became much more sensitive to colonic inflammation and colon cancer formation if the Nrf2 gene had been ablated.Citation103–Citation105 Likewise, nitrosamine-induced carcinogenesis in the bladder, ultraviolet-induced skin carcinogenesis, or aflatoxin-induced formation of human hepatocellular carcinomas (HCCs) was greatly enhanced in Nrf2-deficient mice when compared with Nrf2-proficient mice.Citation106–Citation110 Accordingly, the cancer-preventive effects of Nrf2-inducing agents like oltipraz or CDDO–imidazole were abrogated in such mouse models if Nrf2-dependent gene expression had been shut down.Citation111,Citation112 Other chemoprevention strategies, eg, by curcumin or sulforaphane, were also affected by Nrf2 deficiency in mouse models for such organ sites as the lung, kidney, breast, liver, small intestine, stomach, and skin.Citation96,Citation111,Citation113–Citation116 For instance, development of colon tumors in APCMin mice was efficiently prevented by sulforaphane, but this effect was abrogated in Nrf2-deficient mice. Moreover, mice expressing an aberrant APC protein were per se more sensitive to colon-tumor formation if the Nrf2 gene had been ablated.Citation117 These observations strongly indicate that Nrf2 also protects from deleterious effects initiated by certain genetic alterations, eg, of the APC gene, presumably through the inhibition of further, ROS-dependent genetic lesions and by interfering with metabolic alterations that could favor tumorigenesis. Under certain conditions, Nrf2 can suppress metastasis formation, eg, in pulmonary cancer in mice, which was shown to be related to control of the redox balance in inflammatory cells, including myeloid-derived suppressor cells.Citation118
Additional evidence for the view that Nrf2 exerts antitumor effects, eg, in hormone-sensitive tumors, was provided by the observation that Nrf2 interferes with nuclear hormone receptors, eg, in prostate cancer. A recent study showed that Nrf2 and the related CNC-bZIP protein Nrf1 differentially modulate AR transactivation in prostate cancer.Citation119 While an N-terminally truncated variant of Nrf1 (p65-Nrf1) promoted the expression of androgen-regulated genes and thereby facilitated recurrent growth of castration-resistant prostate cancer cells in the presence of residual trace amounts of androgens, Nrf2 inhibited this effect of Nrf1. Besides a role as transdominant repressor of Nrf2,Citation120 p65-Nrf1 is obviously able directly to form a transactivation complex with the AR, and Nrf2 dampens the formation of p65-Nrf1.Citation119
Altogether, there seems to be no doubt that Nrf2 can efficiently protect from cancer initiation/development, mainly by mitigating genotoxic insults that emerge from exposure to carcinogens and extrinsically or intrinsically generated ROS. This issue has been addressed by numerous, sometimes enthusiastic, articles and reviews published recently.Citation7,Citation121–Citation123 Indeed, as long as the cytoprotective action of Nrf2 is kept under tight control, it could serve as a target in cancer prevention. Based on this idea, many efforts have been undertaken to establish conditions of increased Nrf2 activity and thereby to avoid ROS or carcinogen-induced tissue damage and hence pathologies like cancer. In fact, most of these studies were first carried out in cell-culture settings and then in animal studies – mainly in Nrf2-proficient versus -deficient mice – by using established disease models. It was therefore not surprising that as long as the investigators made use of timely Nrf2 activation close to the damaging and disease-causing insult, a favorable response was always quite well (and mostly) affected by Nrf2 knockout. This included not only cancer but also other severe pathologies, such as diabetes, obesity, pulmonary and cardiovascular diseases, or neurodegenerative disorders.Citation29,Citation70,Citation124–Citation128
Considering Nrf2 in this way as an inducible and cancer-preventive target, several population-based clinical trials have been conducted or are under way, particularly in the People’s Republic of China.Citation129 For example, CDDO, sulforaphane, and oltipraz applications have been shown to be well tolerated in humans, resulting in elevated levels of cytoprotective enzymes.Citation130,Citation131 Moreover, aflatoxin intoxication as a risk factor for HCC was decreased by oltipraz during a 12-week study with a high-risk Chinese population.Citation132 Other trials, however, eg, with CDDO or bardoxolone, have been unsuccessful or resulted in severe side effects.Citation133 Even though most of these trials still await evaluation, the rather positive view regarding Nrf2-based prevention strategies has begun to change. Since the oncogenic activity of Nrf2 has been clearly demonstrated, concerns regarding the safe use of Nrf2 activators, eg, in long-term administration, are increasing. Therefore, more efforts are needed to better understand the modalities by which Nrf2 can be used as a therapeutic target. For example, the use of the recently established conditional Nrf2-knockout mouse,Citation134 allowing ablation of the gene in a cell/tissue-specific and even better in a temporarily controlled fashion (if crossed with the appropriate CreER[T] system), would bring detailed insights into the dual role of Nrf2 in carcinogenesis and by what cellular context this dualism is defined.
The protumorigenic role of Nrf2
Molecular mechanisms of Nrf2 amplification in cancer
It is well established that a great number of tumors frequently exhibit enhanced Nrf2 activity, which may essentially contribute to a malignant phenotype. Nrf2 amplifications range from a frequency of 20%–25% in some tumor types up to 80%–95% in lung, breast, ovarian, endometrial, pancreatic, colorectal, or prostate cancer.Citation6,Citation9–Citation11,Citation95,Citation135,Citation136 This amplified Nrf2 activation manifests in increased nuclear accumulation of Nrf2,Citation137 and even more in its Ser-40 phosphorylated form,Citation138 and by greater expression levels of phase II and antioxidative enzymes, as well as a wide range of other Nrf2-regulated genes. Besides a few exceptions, such as acute myeloid leukemia and myelodysplastic syndrome,Citation139 the presence of activated Nrf2 in tumors generally correlates with a poor clinical outcome, as reported for (for example) pancreatic, cervical, or lung cancer, and indicates poor responsiveness to chemo-and/or radiotherapy. However, the exact modalities by which Nrf2 is deregulated and thereby becomes protumorigenic are quite complex and only partially understood.Citation9,Citation10
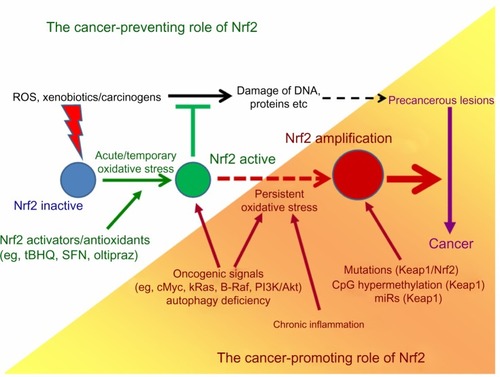
By conferring protection from oxidative stress or carcinogen-induced damage of cellular components – in particular, DNA, thereby avoiding mutational events – Nrf2 primarily acts in a tumor-preventive fashion. However, Nrf2 may become protumorigenic if persistently activated. This can result from certain genetic and epigenetic alterations affecting the Nrf2/Keap1 pathway, from other oncogenic pathways, or from long-term exposure of epithelial cells to persistent oxidative stress. For example, the latter occurs during chronic inflammation (), and represents a condition favoring the early onset of malignant transformation. In fact, precursor lesions of tumors already exhibit elevated Nrf2 activities with considerable frequency,Citation140–Citation143 presumably reflecting a critical stage when Nrf2 is part of the cellular stress adaptation and exerts pro- as well as antitumorigenic effects. At that point, additional events could then direct Nrf2 to act as an oncogene.
Figure 3 The dual role of Nrf2 in cancer.
Abbreviations: ROS, reactive oxygen species; DNA, deoxyribonucleic acid; tBHQ, tert-butylhydroquinone; SFN, sulforaphane.
It is well established that certain somatic genetic alterations affecting the KEAP1 gene (frequencies of up to 30% in gallbladder, ovarian, or lung cancer) or the Nrf2 gene itself (less frequent) directly impact on Nrf2 signaling.Citation95,Citation135,Citation144,Citation145 The most common genetic alterations affect Keap1 in its DC domain, and here mainly within the DLG motif, thereby disrupting Nrf2 binding. In lung cancer, these mutations are found with the greatest frequency. Other tumor entities exhibit mutations in the intervening region (IVR) domain (eg, in prostate cancer) and sometimes in the bric-a-brac (BTB) domain of the KEAP1 gene (eg, in liver and ovary cancer). These mutations impair the interaction of Keap1 with Cullin3 and its homodimerization, thereby affecting polyubiquitination and binding of Nrf2, respectively.Citation95,Citation135 Many of the mutations affect the reactive cysteine residues residing in the IVR and DC domains of Keap1, while others affect additional amino acids, eg, serine at position 104 in the BTB domain, essential for Keap1 homodimerization. Another set of cancer-derived mutations that have been identified include those of glycine (positions 186, 423), arginine (positions 320 or 470), and other amino acids within the BTB, IVR, and DC domains. As shown for lung cancer, these mutations do not impair polyubiquitination of Nrf2, but instead suppress its degradation.Citation146 The functional significance and underlying mechanisms of these so-called superbinder mutations still need to be elucidated.
In contrast to the rather widespread mutations in the KEAP1 gene, gain-of-function-like mutations in the Nrf2 gene are restricted to the DLG and ETGE motifs within the Neh2 domain.Citation147 These mutations are found, eg, in skin, larynx, lung, and squamous esophageal cancers,Citation148,Citation149 but overall are much less frequently found in tumors than KEAP1 mutations.
Moreover, a number of epigenetic alterations affecting Keap1 expression have been described that can lead to disinhibition of Nrf2 in cancer cells as well.Citation95,Citation135,Citation144 In several tumor entities, disruption of Keap1 expression due to CpG DNA hypermethylation has been reported. These modifications are found in 51% of breast, 20% of colorectal, and 12% of lung cancers, accounting for decreased levels of Keap1 and conversely enhanced Nrf2 activation.Citation94,Citation150 Accordingly, 5′-aza-2′-deoxycytidine (5-Aza-dC) treatment could restore Keap1 expression and the control of Nrf2 activity in a number of tumor cell lines.Citation151,Citation152 Interestingly, a recent study revealed that Keap1-promoter hypermethylation is more frequent in preinvasive breast neoplasms than in the breast cancers.Citation141 Furthermore, Nrf2 was identified as a methylation target. During the development of prostate cancer in TRAMP (transgenic adenocarcinoma of the mouse prostate) mice, a modified expression of Nrf2 based on hypermethylation was demonstrated,Citation153 and treatment with 5-Aza-dC restores the expression of Nrf2 and its target genes. Another study showed that curcumin, a DNA hypomethylating agent, reverses the methylation status of the Nrf2 promoter and leads to reexpression of Nrf2/NQO1.Citation154 A DNA methylation-dependent alteration of the inhibitory feedback loop between the integrin adaptor protein p66Shc and Nrf2 has been demonstrated in lung cancer, accounting for tumoral Nrf2 upregulation.Citation155
Also a number of micro-ribonucleic acids (miRNAs) have been reported to attenuate Keap1 expression. miR141 and miR200a, both member of the miR200 family, suppress Keap1 and are overexpressed in ovarian and breast cancers, respectively.Citation156,Citation157 Downregulation of Nrf2-suppressing miRNAs has been described in cancer cells as well. Recent studies identified decreased targeting of Nrf2 by miR28 in breast cancer,Citation158 and four miRNAs are downregulated in esophageal squamous cell carcinomas (miR-507, -634, -450a, and -129-5p),Citation159 the reexpression of which negatively regulate Nrf2 and its oncogenic pathways.
Besides these genetic and epigenetic effects that have been mainly found in solid-tumor entities, metabolic conditions marked by fumarate hydratase deficiency lead to cysteine succinylation of Keap1, hampering its Nrf2 suppressive activity.Citation160,Citation161 Nrf2 activation may be enhanced also in hematologic tumors and/or deregulated in solid tumors through its cross talk with different tumor-associated pathways and proto-oncogenes. This is outlined in the next section.
Cross talk of Nrf2 with oncogenic and tumor-suppressor pathways
Numerous signaling pathways have been shown to cooperate intimately with Nrf2. These pathways are either oncogenic per se and assisted by Nrf2 to induce a transformed phenotype (eg, the kRas or PI3K/Akt pathway), or are Nrf2-dependently modulated to favor malignant transformation (eg, the ubiquitin/proteasome pathway, metabolic pathways). Therefore, inhibition of Nrf2 would either dampen the oncogenic potential of certain pathways or even switch off cellular functions that are essential for cancer cell survival. Moreover, tumor-suppressor pathways can positively and negatively cooperate with Nrf2 (eg, p53), depending on the duration of oxidative and genotoxic stress. Following are several pathways that have been reported to be part of the oncogenic activity of Nrf2.
Nrf2 and kRas, BRaf, and cMyc oncogenes
A number of studies reported a close association of kRas transformation and an increased activity of Nrf2 along with accelerated cell growth and increased cellular survival. In a pancreatic cancer mouse model,Citation13 it was shown that oncogenic kRas activates expression of a series of antioxidant and metabolic genes via Nrf2 that are collectively used as the reducing power for ROS detoxification.Citation162 During kRas-dependent lung carcinogenesis in mice, Nrf2 seems to have two roles: a preventive one during tumor initiation and a promoting one during tumor progression and metastasis formation.Citation163,Citation164 In non-small-cell lung cancer (NSCLC), Nrf2 has been shown to be a downstream mediator of both EGFR and Ras signaling, making Nrf2 an important molecular target for the treatment of NSCLC with mutations in the EGFR and KRAS genes.Citation165 BRaf mutations or mutated H-Ras autonomously driving activation of the ERK pathway similarly enhance Nrf2-dependent cytoprotection.Citation13,Citation166 The involvement of Nrf2 in oncogene-dependent pathways confers protection from ROS and provides reducing power for biosynthesis in proliferating cells. Interestingly and in striking contrast, Nrf2-dependent oxidative stress response is suppressed in H-Ras-transformed mesenchymal stem cells and breast cancer cells through the Ras/Raf/Erk pathway, thus indicating that oncogenic Ras and Nrf2 may also negatively cooperate in certain types of tumors.Citation167
Like kRas, the cMyc oncogene has been linked to Nrf2-dependent cytoprotection. In a transgenic mouse model, cMyc has been shown to directly increase Nrf2 gene expression,Citation13 and a recent study in Drosophila revealed Myc-dependent activation of Nrf2 through an increased expression and phosphorylation of p62 as well as activation of PERK.Citation168 In contrast, cMyc has been reported to interfere negatively with Nrf2 transactivation, causing suppression of phase II enzyme expression.Citation169
Nrf2 and the PI3K/Akt pathway, PTEN, and mTOR
Since activation of Nrf2 is linked to the PI3K/Akt pathway there is a clear association in cancers that frequently are subject to amplification of PI3K/Akt. By inducing cell proliferation, apoptosis, migration, and differentiation, the PI3K/Akt signaling pathway is a major determinant in cancer development and malignant progression.Citation170 In multiple studies with cancer and/or epithelial cell lines, it was shown that Nrf2-mediated defense mechanisms against intracellular ROS are induced by the activated PI3K/Akt signaling pathway.Citation171–Citation173 Accordingly, functional loss of PTEN, an event occurring frequently in cancer, can lead to enhanced Nrf2 activation along with greater cytoprotection.Citation53,Citation174 Moreover, PTEN deficiency might affect the GSK3β-dependent regulation of FynCitation175 or the recruitment of Nrf2 to βTrCP,Citation176 thereby preventing Keap1-independent control of Nrf2 activation.
Beyond that, Nrf2 acts not only downstream of Akt but also synergizes with Akt for providing growth advantages to tumor cells. In an experimental study with breast cancer cells, the transcriptional coactivator AIB1 was able to activate Akt and Nrf2 concurrently. Activated AIB1 results in an enhanced oncogenic effect of Akt through an upregulation of antiapoptotic Bcl-2 as well as an Nrf2-mediated increase of antioxidative enzymes, both leading to enhanced growth and chemoresistance.Citation177
Besides the reports on mTOR controlling Nrf2 activation, eg, through modulation of the Keap1 sequestration by the autophagy protein p62/SQSTM1,Citation178 an upregulation of the mTOR pathway in response to an amplified Nrf2 activation has been demonstrated. As one underlying mechanism, an indirect induction of the small G-protein RagD by activated Nrf2 has been proposed.Citation179 Consequently, tumors exhibiting enhanced Nrf2 activity may serve as an indicator for sensitivity against mTOR inhibitors.
Nrf2 and estrogen/ER
There are different pathways by which estrogen (E2) contributes to cancer development. Besides DNA–E2 adduct formation leading to DNA mutations that are critical for cancer initiation, it is known that oxidative estrogen metabolites generate ROS,Citation180 and the induction of Nrf2-dependent detoxifying enzymes, eg, NQO1, is regarded as an important mechanism to remove these toxic agents.Citation91 Through ERα and ERβ, estrogen differentially affects Nrf2 activation, and hence it has been shown that estrogen inhibits Nrf2 activity, resulting in augmented genotoxicity. This inhibition can result from physical interactions between ERα and Nrf2,Citation181 from competitive binding of ERα and the histone deacetylase SIRT1 to the promoter of Nrf2-inducible genes like NQO1,Citation91 or from interference with p300 recruitment leading to histone acetylation of Nrf2 target genes.Citation182 Moreover, estrogen-dependent decrease of miR-200a in breast cancer cells has been reported, resulting in increased cytoplasmic Keap1 levels and consequently in reduced Nrf2 expression.Citation156 Likewise, modulation of the miR-200 family by ERRαCitation183 may affect the Keap/Nrf2 pathway.
In contrast to ERα-mediated Nrf2 repression, ERβ-dependent Nrf2 induction has been reported. Equol, a specific activator of ERβ, leads to an induction of the PI3K/Akt pathway in endothelial cells, which in turn causes a translocation of activated Nrf2 into the nucleus.Citation184 Similar results were published for breast epithelial cells, and estrogen has been recently shown to induce Nrf2 activity in BRCA1-deficient breast cancer cells through activating the PI3K/Akt pathway.Citation185 Moreover, estrogen-bound ERβ forms a complex with Nrf2, and leads subsequently to an upregulation of ARE-dependent Nrf-2 target genes in MCF7 and MDA-MB-231 breast cancer cells.Citation186
Nrf2 and NF-κB
The cross talk with NF-κB as another important tumor-driving pathway certainly has a crucial role in the shaping of the Nrf2 function toward a protumorigenic one.Citation187 On the one hand, both pathways negatively interfere with each other.Citation188 Therefore, activated NF-κB competes with Nrf2 for CBP coactivators and leads to histone deacetylase 3 binding to the ARE/EpRE and thereby to Nrf2 inactivation.Citation189 Induction of NF-κB target genes, such as COX2, can also lead to Nrf2 suppression.Citation190 Therefore, anti-inflammatory agents, such as resveratrol,Citation191,Citation192 curcumin,Citation193 shogaol derivatives,Citation194 or ethyl 3′,4′,5′-trimethoxythionocinnamate,Citation195 may suppress NF-κB signaling pathways and activate Nrf2 at the same time. Nrf2 also averts cellular damage of inflammation-exposed cells, thereby indirectly preventing sustained NF-κB activation.Citation188 On the other hand, under conditions of NF-κB-driven immune responses, Nrf2 may be activated indirectly by NF-κB as a result of the oxidative stress during inflammation. Moreover, a direct inducing effect of NF-κB on Nrf2 expression has been recently reported in acute myeloid leukemia cells,Citation196 and several common target genes of NF-κB and Nrf2, such as IL8, HO1, GCLC, and Gαi2, have been described.Citation188 Evidence also exists that Nrf2 may trigger the activation of NF-κB. This results from increased proteasome activity, which can potentiate degradation of the NF-κB inhibitor IκBα.Citation197
Nrf2 and the apoptosis regulators Bcl-2 and Bcl-xL
Both Bcl-2 and Bcl-xL represent ARE-controlled target genes of Nrf2.Citation198,Citation199 Accordingly, Nrf2 activation leads to an increased expression of these apoptosis-regulating proteins. Through the upregulation of Bcl-2 and Bcl-xL, amplified Nrf2 activity would therefore directly confer apoptosis protection to tumor cells, a condition essentially adding to the oncogenic effect of Nrf2 and its impact on a malignant phenotype. In addition to the direct transcriptional control by Nrf2, the fate of these two apoptosis regulators has been shown to be influenced by their interaction with Keap1 affecting their protein stability.Citation74 However, the physiological significance of this effect is not clear yet.
Nrf2 and p53
The tumor-suppressor gene p53, which substantially defines the cellular fate by regulating both pro- and antiapoptotic responses, is another cross-talk partner of Nrf2.Citation188,Citation200 When kept at homeostatic low protein levels, p53 controls ROS production and the cellular redox statusCitation201 by modulating antioxidative and metabolic gene expression, eg, GLS2 and C12orf5.Citation202,Citation203 In this way, p53 may exert chemoprotective or tumor-suppressive effects in collaboration with Nrf2.Citation200 Moreover, p53 is stabilized through Nrf2 target genes like NQO1,Citation204 and in turn p53 has been shown to support Nrf2 activity,Citation82 thus pointing to a positive-feedback mechanism between both pathways. By contrast, p53 can also be suppressed through Nrf2-dependent induction of MDM2, representing an ARE-regulated target gene.Citation205 Therefore, the interplay between p53 and Nrf2 is of a complex nature and subject to subtle fine tuning that might be severely impaired in cancer.
Nrf2 and Notch
By controlling cell fate and cell renewal, the Notch pathway has a pivotal role in development and tissue homeostasis. Even though it exerts tumor-suppressive activities, the hyperactivation of Notch signaling has been implicated as oncogenic in several types of cancer.Citation206 Recently, activation of the Notch pathway was reported to control the Nrf2 promoter, leading to increased Nrf2 expression.Citation207 In turn, Nrf2 cooperates with the Notch pathway through inducing the expression of the soluble Notch ligand JAG1.Citation208 Therefore, the interplay between Nrf2 and Notch may represent a condition turning both Nrf2 and Notch protumorigenic, eg, with respect to self-renewal of cancer stem cells.Citation209
Nrf2 and caveolin
Recent studies have demonstrated that direct interaction with the scaffold protein caveolin 1 reduces the cytoprotective effects of Nrf2.Citation210,Citation211 Moreover, in colon cancer cells, loss of caveolin 1 contributes to a transformed phenotype in an Nrf2-dependent fashion.Citation212 When restoring caveolin 1 expression, a p53-dependent senescence pathway is initiated, due to the suppressed oxidative stress response.Citation212 Caveolin 1 has been shown also to modulate TFGβ1-dependent signaling in cancer cells, eg, related to epithelial–mesenchymal transition (EMT),Citation213 providing a possible link between oncogenic TFGβ1 and Nrf2 activation.
Nrf2 and autophagy
Representing the basic degradation machinery of cell components and organelles, selective autophagy is an integral part of the cellular stress response and cell-fate decision.Citation214 Selective autophagic cargos, such as ubiquitinated organelles, assemble together with the scaffold protein p62/SQSTM1 phosphorylated in an mTORC1-dependent manner. Intriguingly, phospho-p62 interferes with the Keap1/Nrf2 pathway,Citation178 and the accumulation of phospho-p62 leads to the persistent activation of Nrf2 in autophagy-deficient cells.Citation75,Citation215 Consequently, autophagy deficiency in cancer cells is accompanied by the cytoprotective and growth-supportive effects of Nrf2 that add, eg, to the growth of HCCs.Citation76,Citation216 The role of the Keap1/Nrf2 pathway in the control of selective autophagy is underscored by the fact that p62 is a target gene of Nrf2.Citation217 Therefore, Nrf2 may compensate for the deficiency of selective autophagy on the one hand and for the resulting cytotoxicity on the other hand. Accordingly, in other tumor entities, an increase of selective autophagy has been demonstrated that may be linked to the enhanced Nrf2 activity resulting from other amplification mechanisms.Citation218,Citation219 By contrast, Nrf2 has been shown to block autophagy induction by temozolomide in glioma or by mitoquinone in breast cancer cells,Citation220,Citation221 and a recent study demonstrated that tBHQ-mediated induction of autophagy is required for full activation of Nrf2 in hepatocytes.Citation222 These findings point to a complex interrelation between autophagy and cellular redox control.
Nrf2 as modulator of HIF1α and angiogenesis
Experimental evidence strongly suggests that Nrf2 also controls the fate of HIF1α and thereby contributes to angiogenesis.Citation223,Citation224 By stabilizing HIF1α through its impact on mitochondrial O2 consumption and ROS formation, Nrf2 promotes VEGF-dependent microvessel formation in tumors, eg, in colon cancer or glioblastoma. Accordingly, Nrf2 inhibition suppresses tumor growth in vitro and in vivo, involving reduced tumor vascularization and invasiveness as a direct result of HIF1α destabilization and downregulation.Citation225,Citation226
Nrf2 as modulator of the ubiquitin/proteasome pathway
The huge battery of Nrf2 target genes includes a cluster of more than 20 genes encoding for proteasomal subunits of both the 20S core and the 19S regulatory particle.Citation227–Citation229 Accordingly, Nrf2 activation confers increased proteasomal activity, probably reflecting the need for renewal of the protein-degradation machinery in cells subjected to ROS-dependent protein damage.Citation230,Citation231 The resulting gain of proteasome activity further enables cells to drive signaling pathways crucial for proliferation and survival more efficiently.Citation232–Citation235 Therefore, in particular, tumor cells acquire substantial growth advantages from this condition, as recently reported for colon and pancreatic cancer.Citation138,Citation197,Citation236 Proteasome inhibitors (PIs) used for the therapy of certain types of tumors, eg, multiple myeloma, are likewise able to induce Nrf2Citation237,Citation238 and thereby a compensatory mechanism by de novo synthesis of proteasomal proteins.Citation239 Therefore, enhanced Nrf2 activation must be regarded as one condition accounting for the failure of PI-based therapies in solid tumors.Citation240 Recent studies have demonstrated that TCF11/Nrf1 has an essential role in maintaining proteasomal homeostasis also, adding to the oxidative stress-associated effects of Nrf2.Citation241
Nrf2 as modulator of EMT and TFGβ1 signaling
EMT is a transdifferentiation process by which epithelial/carcinoma cells can acquire an invasive and metastatic phenotype. Moreover, EMT-associated alterations have been discussed in the context of adaptation to oxidative stress. While inducing effects of ROS on EMT and a role of TFGβ1 through interfering with Nrf2 have been shown recently,Citation87,Citation242,Citation243 the effects of Nrf2 on EMT and TFGβ1 signaling are a matter of considerable debate. On the one hand, several reports indicate that Nrf2 prohibits the fibrotic reaction through TFGβ1, an effect that was attributed to a direct inhibition of Smad3 and thereby of EMT-related gene expression,Citation244,Citation245 and Nrf2 has been shown to decrease the motility of hepatoma cells.Citation246 On the other hand, Nrf2 is able to promote a motile and invasive phenotype of glioma, gallbladder carcinoma, or esophageal squamous carcinoma cells,Citation247,Citation248 eg, through induction of matrix metallopeptidase (MMP)-2/9 expression, and favors tumor invasiveness and metastasis formation in mice.Citation249 Given an involvement in EMT and EMT-associated alterations, Nrf2 would have a particularly great impact on early events in malignant transformation that precede its later effects on tumor progression (eg, chemoresistance) or tumor angiogenesis.Citation223,Citation225
Nrf2 and metabolic reprogramming
Another hallmark of cancer is an altered metabolism (metabolic reprogramming),Citation250 ensuring glucose as a source for biomass production along with cell proliferation and tumor growth. Recent data have indicated that Nrf2 cooperates with the PI3K/Akt pathway in the metabolic reprogramming of cancer cells.Citation66,Citation251,Citation252
A number of key enzymes of the glycolysis (eg, phosphofructokinase), pentose phosphate pathway (PPP; eg, glucose-6-phosophate dehydrogenase [G6PD], phosphogluconate dehydrogenase, transketolase, transaldolase [TALDO]-1) and lipogenesis (eg, malic enzyme [ME]-1, isocitrate dehydrogenase 1) have been shown to be upregulated by Nrf2. Accordingly, the amplified activity of Nrf2 has been associated with oncogenic metabolism with, eg, high levels of lactate or inosine monophosphate and low levels of fructose-6-phosphate, sedoheptulose-7 phosphate, or pyruvate. Some (eg, TALDO1, ME1) of these genes are direct ARE-regulated targets of Nrf2. Moreover, the cellular repertoire of glycolytic, PPP, or tricarboxylic acid cycle enzymes, as well as of electron transport-chain components, can change and result in a shift toward anabolic/catabolic pathways by epigenetic effects through Nrf2-inducible miRNAs, eg, miR-29a/b, miR-320a, and miR-143. Similarly, through its inducing effect on HDAC4, Nrf2 leads to downregulation of miR1 and miR206, thereby increasing the expression of PPP-associated genes (eg, G6PD, TALDO1) and favoring a tumor-proliferative phenotype.Citation253
Nrf2 and drug/radiotherapy resistance of cancer
A marked property of tumor cells exhibiting elevated Nrf2 activity is a profound protection from anticancer drugs or radiotherapy-induced cell death. Therefore, Nrf2 is a potent determinant of chemo- and/or radioresistance of cancer. This includes resistance of NSCLC, cervical cancer, endometrial serous carcinoma, or cholangiocarcinoma cells to treatment with cisplatin,Citation177,Citation254,Citation255 pancreatic cancer cells to treatment with gemcitabine,Citation256 colon and gastric cancer cells to treatment with 5-fluorouracil (5-FU),Citation257,Citation258 hepatocellular carcinoma cells to treatment with doxorubicin,Citation259 or prostate cancer cells to treatment with mitoxantrone or topotecan.Citation260
Additionally, anticancer drugs themselves can elicit Nrf2-dependent cytoprotection. For example, oxaliplatinCitation261 and PIs like bortezomib (BTZ) are strong inducers of Nrf2, and thereby can lead to chemoresistance. BTZ has been shown to induce drug resistance in neuroblastoma cells through Nrf2-dependent HO1 expression. Likewise, proteasome inhibition results in upregulation of proteasomal gene expression due to increased Nrf2 activation, and obviously also Nrf1 activation, which confers not only resistance to PIs but also further growth advantages to cancer cells.
A detailed secretome analysis identified a cluster of drug-metabolizing and prosurvival Nrf2 target genes in cancer stem cells, underscoring the pivotal role of Nrf2 in intrinsic chemoresistance.Citation262 For example, ABC transporters like MRP3, MRP4, or MRP5 are upregulated by Nrf2, facilitating the efflux of anticancer drugs like cisplatin, 5-FU, or gemcitabine out of cancer cells.Citation256,Citation263 In addition, Nrf2-inducible metabolizing enzymes, such as AKR1B10,Citation264 affecting drugs like daunorubicin or idarubicin,Citation259 NQO1, or HO1 affecting drugs like gemcitabine, cisplatin, doxorubicin, or daunorubicin contribute to the detoxification of anticancer drugs and thereby to chemoresistance (collectively discussed by Na and SurhCitation265). Furthermore, Nrf2-induced expression of survival genes like BCL2 contributes to reduced apoptotic responses to anticancer drugs, and the Nrf2-dependent expression of proteasomal genes promotes growth and survival-related pathways depending on the ubiquitin/proteasome pathway.
Likewise, the sensitivity of cells to irradiation-induced cell damage and thereby cell death is under the control of Nrf2. A number of Nrf2 target genes, such as HO1 and PRX1, have been shown to directly suppress cytotoxic effects by irradiation,Citation265 which manifest through ROS formation and ROS-dependent DNA damage. Accordingly, radioresistance due to Nrf2 amplificationCitation266 has been reported for, eg, NSCLC, head and neck, pancreatic or prostate cancer, glioma, and esophageal squamous carcinoma.
Nrf2 inhibition in cancer therapy
As outlined earlier and demonstrated by numerous experimental and preclinical studies within the last decade, Nrf2 inhibitors represent rational tools to prevent malignant progression in cancer, whereas activators of Nrf2 are obviously more suited for cancer prevention. As long as the action of Nrf2 fits into still-homeostatic control of cellular and tissue integrity, its pharmacological activation by bioactive compounds seems to be a reasonable measure for prevention of diseases, like cancer. However, upon exposure to persistent oxidative stress (eg, during chronic inflammation) and/or when adapting a premalignant phenotype, cells may take advantage of Nrf2 activation to undergo malignant transformation. Therefore, the molecular mechanisms defining these two conditions need to be unraveled in order to assess the use of Nrf2 either as an activation target in prevention strategies or as an inhibition target in anticancer therapy. As outlined earlier, genetic and epigenetic alterations or long-lasting and/or insufficiently compensated environmental stress (toward ROS and xenobiotics) are conditions making Nrf2 protumorigenic. This property of Nrf2 then manifests even more when facing additional oncogenic pathways ().
Regardless of the still-limited knowledge of the exact modalities by which Nrf2 switches from being anti- to protumorigenic, numerous experimental attempts have been made to block tumor growth and progression or to improve anticancer therapy through inhibiting Nrf2. There have been many Nrf2 short hairpin RNA (shRNA)- or small interfering RNA-based studiesCitation197,Citation226,Citation240,Citation254,Citation255,Citation267 in cell-culture settings or using mouse models that demonstrated sensitization of tumor cells to apoptosis, suppression of tumor-cell proliferation or lower invasiveness, and reduced tumor vascularization. Furthermore, reexpression of miR-507, -634, -450a, and -129-5p has been shown to suppress esophageal squamous cell carcinoma through dampening Nrf2 signaling.Citation159
However, while all these studies indicated substantial benefits from genetic silencing of Nrf2 to reduce proliferation, viability, and chemo/radioresistance of cancer cells, its immediate application is expected to be limited by insufficient delivery of such nucleic acids as miRNAs or shRNAs into cells. In this respect, small molecules are preferable for clinical applications. More emphasis has now been given to Nrf2 inhibition by using pharmacological compounds affecting Nrf2 activation.
Strategies for pharmacological Nrf2 inhibition
A number of studies have already reported Nrf2-inhibitory effects of established compounds, but validation of these effects has either proven unsuccessful or a transfer into clinical application that way has seemed unlikely. For example, all-trans-retinoic acid and other RARα ligands are able to inhibit Nrf2,Citation92 but the significance of the negative interference between RARα and Nrf2 is not clear, because a positive effect of retinoic acids on Nrf2 has been reported as well.Citation268 Nevertheless, it would be interesting to see whether inhibiting Nrf2 in retinoid-based chemotherapy might act synergistically with other anticancer drugs. Tarumoto et al reported that ascorbic acid, a reducing reagent, can overcome resistance to the anticancer drug imatinib by inhibiting Nrf2 activity,Citation269 but this effect was indirect. Kweon et al showed that epigallocatechin 3-gallate can inhibit Nrf2 activity and thereby HO1 expression in A549 NSCLC cells, but only at rather high concentrations,Citation270 limiting its safe use in the clinic.
When considering novel, naturally derived compounds for inhibiting Nrf2, one should keep in mind that these may have considerable toxicities. As an example, the mycotoxin ochratoxin A is an efficient inhibitor of Nrf2, but exerts nephrotoxicity and kidney carcinogenic effects.Citation271 Therefore, depending on the cellular context, biological drugs may be inappropriate or at least strongly limited in their use in clinical settings. Therefore, the number of reports on more or less specific Nrf2 inhibitors that may be biologically safe in such attempts is still quite few.
One of the first reports on drug-mediated Nrf2 inhibition described a chemosensitizing effect of brusatol, a quassinoid compound from an evergreen shrub from Southeast Asia, on A549 NSCLC cells.Citation272 Brusatol potently and reversibly inhibited Nrf2 through an increasing effect on Keap1-mediated polyubiquitination of Nrf2, thereby promoting Nrf2 degradation and suppressing Nrf2 activity. In that way, brusatol decreased Nrf2 target gene expression and increased anticancer drug-induced but not basal apoptosis. Moreover, brusatol sensitized A549 tumors for chemotherapy with cisplatin in a murine xenograft model in an Nrf2-dependent fashion, without obvious toxicity. Interestingly, even in the presence of certain mutant-Keap1 variants, brusatol exerted Nrf2 inhibitory effects, thus having the potential to compensate for an impaired Keap1-inhibitory function. However, previous studies have shown that brusatol can activate NF-κB.Citation273 Thereby, on the one hand, brusatol may lead to increased growth and survival of tumor cells through its inducing effect on the NF-κB pathway. On the other hand, through the reported negative interference of NF-κB with Nrf2 activation,Citation189,Citation190 brusatol may inhibit Nrf2 via NF-κB. Therefore, brusatol can exert differential effects on two crucial stress- and tumor-related pathways depending on its dose and presumably the tumor type as well. Another property of brusatol – the rapid reversibility of Nrf2 inhibition – may further limit its efficacy in cancer therapy. This would imply the need for an extended administration of the drug to efficiently target the amplified Nrf2. In addition, a compensatory increase of Nrf2 activity could occur as a rebound effect from brusatol treatment.
Similarly in A459 cells, the vegetable-derived flavonoid luteolin has been shown to suppress Nrf2 activation independently of Keap1 through an enhanced turnover of Nrf2 mRNA. Along with decreasing Nrf2 activity, luteolin augmented the responsiveness of A459 and also MCF7 breast cancer or Caco2 colon cancer cells to anticancer drug (oxaliplatin, bleomycin, doxorubicin)-induced apoptosis, and thereby acted as a potent chemosensitizer. However, this Nrf2-inhibitory effect was limited to a particular dose of luteolin, and may considerably depend on the cellular context, because this compound has been shown to efficiently induce Nrf2 activation in several other tumor cell lines, thereby acting cytoprotectively.Citation274,Citation275 Moreover, protection from azoxymethane/dextran sulfate sodium-induced colorectal cancer in mice by luteolin has been ascribed to its Nrf2-inducing properties.Citation276 Therefore, the use of luteolin as an Nrf2 inhibitor in chemosensitization may be hampered by its dual effects on Nrf2.
The synthetic compound IM3829 (cyclohexyl-ethoxy-aniline) was demonstrated to enhance the responsiveness of NSCLC cells to radiotherapy-induced apoptosis through inhibiting Nrf2 and expression of its target genes. As observed with luteolin, IM3829 affects the stability of Nrf2 mRNA independently of Keap1. In a mouse tumor xenograft model, IM8329 potently enhanced the efficacy of radiotherapy.Citation277 Further studies are needed to demonstrate the safety of this compound for its use as radiosensitizer.
The coffee-derived alkaloid trigonelline has been previously shown to exert a profound inhibitory effect on Nrf2.Citation278 A recent study further reported inhibition of Nrf2 activation in several pancreatic cancer cell lines by trigonelline.Citation138 It was shown that trigonelline is capable of preventing the nuclear accumulation of Nrf2, an effect insensitive to treatment with the nuclear export inhibitor leptomycin B. Thereby, trigonelline potently inhibited the expression of Nrf2 target genes, including those of proteasomal subunit proteins. This was accompanied by a decrease of proteasome activity, making pancreatic cancer cells more sensitive to anticancer drugs as well as to death ligands, eg, TRAIL. In a mouse xenograft tumor model, trigonelline treatment also exerted a profound Nrf2 inhibitory and a marked chemosensitizing effect without any measurable toxicity. A major advantage of using this substance in ongoing studies is given by the fact that trigonelline has been already tested in human clinical trials and is well tolerated.Citation279,Citation280
Another study reported suppression of Nrf2 in pancreatic cancer cells by the PI3K-p110a inhibitor PIK-75.Citation256 By reducing Nrf2 protein levels, PIK-56 decreased the expression of Nrf2 target genes, including MRP5, the expression of which is induced by anticancer drugs like gemcitabine. PIK-75 treatment augmented the apoptosis of pancreatic cancer cells and potentiated gemcitabine toxicity through blocking Nrf2-dependent MRP5 expression. Accordingly, a marked reduction of in vivo tumor growth in a mouse xenograft model was observed after PIK-75 treatment, and a synergistic effect of PIK-75 was observed when given in combination with gemcitabine.Citation256 PIK-75 is thus a promising drug, as it has a great impact on tumor-cell viability.
The same holds true for the natural flavonoids chrysin and apigenin, which have been described as effective adjuvant sensitizers to reduce anticancer-drug resistance of HCC cells by downregulating Nrf2 activation.Citation171,Citation259 Through affecting the PI3K/Akt- and Erk-dependent activation of Nrf2, these compounds have been shown to increase the responsiveness to anticancer drugs. This effect could be attributed to decreased expression of Nrf2 targets like HO-1, MRP5, and AKR1B10. Another natural flavonoid, wogonin, has been shown to decrease Nrf2 protein levels and thereby expression of HO-1 and NQO1 in MCF7 breast cancer cells, accompanied by sensitization to doxorubicin.Citation281 This action of wogonin is obviously also dependent on suppression of the PI3K/Akt/Nrf2 pathway. However, it still needs to be validated whether these PI3K/Akt interfering compounds are safe in clinical settings.
Another approach investigated the effect of treatment with 2-deoxy-D-glucose (2-DG) and 6-aminonicotinamide (6-AN) on the radiosensitivity of head and neck squamous carcinoma cells. It was shown that 2-DG/6-AN treatment reduced Nrf2 activity through upregulated Keap1 expression, thereby decreasing Nrf2-dependent resistance to radiotherapy.Citation282
Most if not all of these strategies described so far deal with substances that affect other pathways as well. The use of them may be thus restricted to tumor cells exhibiting Nrf2 amplification, whereas in other conditions in chemo/radioresistant cancer cells, eg, amplified NF-κB, these drugs should rather be excluded from cancer therapy. More data are thus needed to assess under what conditions these compounds will have beneficial effects, eg, in chemosensitization, and when they will not. The ongoing search for other drugs – in particular, those having greater selectivity for inhibiting Nrf2 – is certainly an important task. However, it is quite obvious that plant-derived substances are very often limited in their specificity for a selected target. Owing to the broad target reactivity, these substances have been evolutionarily conserved to provide greatest protection of the host from environmental threats. Synthetic derivatives with greater target selectivity are therefore needed to get more reliable Nrf2 inhibitors.
Conclusion and outlook
Regardless of the dual role of Nrf2 in oncogenesis, deregulated Nrf2 activity in tumors essentially contributes to the manifestation of cancer hallmarks and associates with poor prognosis and therapy resistance. This undeniably underscores the dark side of Nrf2. From experimental data using mouse models, it has become clear that Nrf2 affects tumorigenesis in a time- and context-dependent fashion. However, it could also be learned that a generalized prediction of the ultimate outcome of tumor development with respect to the Nrf2 status is rather difficult. This is exemplified by two studies on kRas-driven cancer in mice. On the one hand, it was reported that Nrf2 prevents the initiation of chemically induced lung cancer, but favors its malignant progression later on depending on mutated kRas.Citation163 On the other hand, it was shown that Nrf2 exerts its protumorigenic effects at an earlier stage of pancreatic carcinogenesis initiated by mutated kRas.Citation13 Given the advantages transformed cells can gain from Nrf2 activation, greater survival and growth-favoring metabolic effects may pave the way quite early for local tumor development. Later on, the multiple effects of Nrf2 on tumor vascularization, invasiveness, and cancer stem cell self-renewal may contribute to cancer progression and metastasis formation as well. It is therefore evident that the decision to make use of Nrf2 as an activation or inhibition target for a given therapy requires an accurate estimation of its different roles during each step of tumorigenesis, which can vary from tumor to tumor.
Certainly, the use of Nrf2 as an inducible target in cancer-prevention strategies is still an attractive option when considering, eg, acute/temporary exposure to environmental hazards like aflatoxin, benzo(a)pyrene, or diesel exhaust. However, long-term application seems not to be suitable as long as it is not fully known how Nrf2 is kept under homeostatic control and when/how this control gets lost. Nrf2 can be regarded instead as a useful marker for the assessment of particular cancer phenotypes, such as chemo- and radioresistance or autophagy deficiency. Under these conditions, Nrf2 gain-of-function mutations or amplified Nrf2-inducing signaling pathways directly relate to the malignant phenotype, whereas Keap1 mutations may have additional effects. Therefore, predicting the clinical outcome of alterations in the Keap1/Nrf2 pathway may be sometimes difficult, and from the plethora of data it is also clear that cross talk with other tumor-related pathways has a pivotal role in shaping the effects of Nrf2 on the cellular phenotype. This includes effects on proliferation and senescence, which are particularly relevant in oncogenic kRas background on the one hand and p53 status on the other hand. While mutated kRas drives proliferative pathways, accompanied by ROS formation giving rise to additional growth advantages through the induction of Nrf2-dependent cytoprotection, certain p53 mutants could still negatively interfere with Nrf2, but others might not. Likewise, NF-κB, albeit having the potential of directly or indirectly inducing Nrf2, can competitively suppress Nrf2 too. Therefore, during inflammation, Nrf2 can add its cytoprotective actions to the prosurvival effect of NF-κB, but Nrf2 and NF-κB can also negatively interfere with each other. As another example, nuclear hormone receptors, such as RARs, ERα, or the ARs exert complex interactions with the Keap1/Nrf2 pathway, and thereby define the role of Nrf2 to act as either a tumor suppressor or tumor promoter.
In view of the complexity of the cross talk between Nrf2 and its numerous signaling-network partners, it is clear that we still know too little about the direction in which Nrf2 may act during tumorigenesis to judge its general use as a therapeutic inhibition target. Nevertheless, its temporary use as a sensitizer in chemo/radiotherapy will be certainly advantageous if really specific and safe-in-use Nrf2 inhibitors are established. As already outlined, most existing Nrf2-inhibiting compounds do not fit these criteria so far. On the one hand, these compounds can have severe cytotoxicities when affecting vital tissue-protection mechanisms, eg, in the kidney. On the other hand, drugs affecting upstream pathways like the PI3K/Akt pathway can exert antitumor effects independently of Nrf2. In addition, possible limitations of manipulating the Keap1/Nrf2 pathway may relate to the numerous actions of Keap1 independently of controlling Nrf2. Therefore, Keap1 influences NF-κB activation through interfering with IκB kinase phosphorylation, PPARγ, and several other pathways. Therefore, much effort is still needed in the search for novel compounds and engineered small molecules that target Nrf2 more specifically.
Acknowledgments
The authors wish to thank the Deutsche Forschungsgemeinschaft and the German Cluster of Excellence “Inflammation at Interfaces” for funding.
Disclosure
The authors report no conflicts of interest in this work.
References
- KangKWLeeSJKimSGMolecular mechanism of nrf2 activation by oxidative stressAntioxid Redox Signal2005711–121664167316356128
- OsburnWOKenslerTWNrf2 signaling: an adaptive response pathway for protection against environmental toxic insultsMutat Res20086591–2313918164232
- KasparJWNitureSKJaiswalAKNrf2:INrf2 (Keap1) signaling in oxidative stressFree Radic Biol Med20094791304130919666107
- SuzukiTMotohashiHYamamotoMToward clinical application of the Keap1-Nrf2 pathwayTrends Pharmacol Sci201334634034623664668
- GiudiceAMontellaMActivation of the Nrf2-ARE signaling pathway: a promising strategy in cancer preventionBioessays200628216918116435293
- LauAVilleneuveNFSunZWongPKZhangDDDual roles of Nrf2 in cancerPharmacol Res2008585–626227018838122
- KwakMKKenslerTWTargeting NRF2 signaling for cancer chemopreventionToxicol Appl Pharmacol20102441667619732782
- HayesJDMcMahonMChowdhrySDinkova-KostovaATCancer chemoprevention mechanisms mediated through the Keap1-Nrf2 pathwayAntioxid Redox Signal201013111713174820446772
- SpornMBLibyKTNRF2 and cancer: the good, the bad and the importance of contextNat Rev Cancer201212856457122810811
- JaramilloMCZhangDDThe emerging role of the Nrf2-Keap1 signaling pathway in cancerGenes Dev201327202179219124142871
- SheltonPJaiswalAKThe transcription factor NF-E2-related factor 2 (Nrf2): a protooncogene?FASEB J201327241442323109674
- ZhangDDThe Nrf2-Keap1-ARE signaling pathway: The regulation and dual function of Nrf2 in cancerAntioxid Redox Signal201013111623162620486759
- DeNicolaGMKarrethFAHumptonTJOncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesisNature2011475735410610921734707
- CadenasEBiochemistry of oxygen toxicityAnnu Rev Biochem198958791102673022
- HalliwellBGutteridgeJMFree Radicals in Biology and Medicine3rd edOxfordOxford University Press1999
- InoueMSatoEFNishikawaMMitochondrial generation of reactive oxygen species and its role in aerobic lifeCurr Med Chem200310232495250514529465
- ConnerEMGrishamMBInflammation, free radicals, and antioxidantsNutrition19961242742778862535
- PoliGLeonarduzziGBiasiFChiarpottoEOxidative stress and cell signallingCurr Med Chem20041191163118215134513
- KnightJADiseases related to oxygen-derived free radicalsAnn Clin Lab Sci19952521111217785961
- KlaunigJEKamendulisLMThe role of oxidative stress in carcinogenesisAnnu Rev Pharmacol Toxicol20044423926714744246
- ReuterSGuptaSCChaturvediMMAggarwalBBOxidative stress, inflammation, and cancer: how are they linked?Free Radic Biol Med201049111603161620840865
- ChoHYReddySPKleebergerSRNrf2 defends the lung from oxidative stressAntioxid Redox Signal200681–2768716487040
- NguyenTNioiPPickettCBThe Nrf2-antioxidant response element signaling pathway and its activation by oxidative stressJ Biol Chem200928420132911329519182219
- ChorleyBNCampbellMRWangXIdentification of novel NRF2-regulated genes by ChIP-Seq: influence on retinoid X receptor alphaNucleic Acids Res201240157416742922581777
- MalhotraDPortales-CasamarESinghAGlobal mapping of binding sites for Nrf2 identifies novel targets in cell survival response through ChIP-Seq profiling and network analysisNucleic Acids Res201038175718573420460467
- JohnsonCHPattersonADIdleJRGonzalezFJXenobiotic metabolomics: major impact on the metabolomeAnnu Rev Pharmacol Toxicol201252375621819238
- IdleJRGonzalezFJMetabolomicsCell Metab20076534835117983580
- WilliamsGMJeffreyAMOxidative DNA damage: endogenous and chemically inducedRegul Toxicol Pharmacol200032328329211162722
- LiXSchulerMABerenbaumMRMolecular mechanisms of metabolic resistance to synthetic and natural xenobioticsAnnu Rev Entomol20075223125316925478
- LewisDFHuman P450s in the metabolism of drugs: molecular modelling of enzyme-substrate interactionsExpert Opin Drug Metab Toxicol2005115816922648
- XuCLiCYKongANInduction of phase I, II and III drug metabolism/transport by xenobioticsArch Pharm Res200528324926815832810
- HayesJDDinkova-KostovaATThe Nrf2 regulatory network provides an interface between redox and intermediary metabolismTrends Biochem Sci201439419921824647116
- HirotsuYKatsuokaFFunayamaRNrf2-MafG heterodimers contribute globally to antioxidant and metabolic networksNucleic Acids Res20124020102281023922965115
- MoiPChanKAsunisICaoAKanYWIsolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control regionProc Natl Acad Sci U S A19949121992699307937919
- ItohKWakabayashiNKatohYKeap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domainGenes Dev199913176869887101
- CullinanSBGordanJDJinJHarperJWDiehlJAThe Keap1-BTB protein is an adaptor that bridges Nrf2 to a Cul3-based E3 ligase: oxidative stress sensing by a Cul3-Keap1 ligaseMol Cell Biol200424198477848615367669
- KatohYIidaKKangMIEvolutionary conserved N-terminal domain of Nrf2 is essential for the Keap1-mediated degradation of the protein by proteasomeArch Biochem Biophys2005433234235015581590
- KobayashiAKangMIWataiYOxidative and electrophilic stresses activate Nrf2 through inhibition of ubiquitination activity of Keap1Mol Cell Biol200626122122916354693
- TongKIKobayashiAKatsuokaFYamamotoMTwo-site substrate recognition model for the Keap1-Nrf2 system: a hinge and latch mechanismBiol Chem200638710–111311132017081101
- RachakondaGXiongYSekharKRStamerSLLieblerDCFreemanMLCovalent modification at Cys151 dissociates the electrophile sensor Keap1 from the ubiquitin ligase CUL3Chem Res Toxicol200821370571018251510
- ZhangDDLoSCSunZHabibGMLiebermanMWHanninkMUbiquitination of Keap1, a BTB-Kelch substrate adaptor protein for Cul3, targets Keap1 for degradation by a proteasome-independent pathwayJ Biol Chem200528034300913009915983046
- VilleneuveNFTianWWuTUSP15 negatively regulates Nrf2 through deubiquitination of Keap1Mol Cell2013511687923727018
- NitureSKKasparJWShenJJaiswalAKNrf2 signaling and cell survivalToxicol Appl Pharmacol20102441374219538984
- HuangHCNguyenTPickettCBRegulation of the antioxidant response element by protein kinase C-mediated phosphorylation of NF-E2-related factor 2Proc Natl Acad Sci U S A20009723124751248011035812
- CullinanSBZhangDHanninkMArvisaisEKaufmanRJDiehlJANrf2 is a direct PERK substrate and effector of PERK-dependent cell survivalMol Cell Biol200323207198720914517290
- ApopaPLHeXMaQPhosphorylation of Nrf2 in the transcription activation domain by casein kinase 2 (CK2) is critical for the nuclear translocation and transcription activation function of Nrf2 in IMR-32 neuroblastoma cellsJ Biochem Mol Toxicol2008221637618273910
- SunZChinYEZhangDDAcetylation of Nrf2 by p300/CBP augments promoter-specific DNA binding of Nrf2 during the antioxidant responseMol Cell Biol200929102658267219273602
- ChenZYeXTangNThe histone acetylranseferase [sic] hMOF acetylates Nrf2 and regulates anti-drug responses in human non-small cell lung cancerBr J Pharmacol2014171133196321124571482
- KawaiYGardunoLTheodoreMYangJArinzeIJAcetylation-deacetylation of the transcription factor Nrf2 (nuclear factor erythroid 2-related factor 2) regulates its transcriptional activity and nucleocytoplasmic localizationJ Biol Chem201128697629764021196497
- KangSJRyooIGLeeYJKwakMKRole of the Nrf2-heme oxygenase-1 pathway in silver nanoparticle-mediated cytotoxicityToxicol Appl Pharmacol20122581899822036727
- YuRChenCMoYYActivation of mitogen-activated protein kinase pathways induces antioxidant response element-mediated gene expression via a Nrf2-dependent mechanismJ Biol Chem200027551399073991310986282
- YuRMandlekarSLeiWFahlWETanTHKongANp38 Mitogen-activated protein kinase negatively regulates the induction of phase II drug-metabolizing enzymes that detoxify carcinogensJ Biol Chem200027542322232710644681
- SakamotoKIwasakiKSugiyamaHTsujiYRole of the tumor suppressor PTEN in antioxidant responsive element-mediated transcription and associated histone modificationsMol Biol Cell20092061606161719158375
- JainAKJaiswalAKGSK-3β acts upstream of Fyn kinase in regulation of nuclear export and degradation of NF-E2 related factor 2J Biol Chem200728222165021651017403689
- BlankVSmall Maf proteins in mammalian gene control: mere dimerization partners or dynamic transcriptional regulators?J Mol Biol2008376491392518201722
- MotohashiHShavitJAIgarashiKYamamotoMEngelJDThe world according to MafNucleic Acids Res19972515295329599224592
- KannanMBSolovievaVBlankVThe small MAF transcription factors MAFF, MAFG and MAFK: current knowledge and perspectivesBiochim Biophys Acta20121823101841184622721719
- BenkhelifaSProvotSNabaisEEychèneACalothyGFelder-SchmittbuhlMPPhosphorylation of MafA is essential for its transcriptional and biological propertiesMol Cell Biol200121144441445211416124
- Sii-FeliceKPouponnotCGilletSMafA transcription factor is phosphorylated by p38 MAP kinaseFEBS Lett2005579173547355415963504
- RoYTJangBKShinCYParkEUKimCGYangSIAkt regulates the expression of MafK, synaptotagmin I, and syntenin-1, which play roles in neuronal functionJ Biomed Sci2010171820233453
- TanigawaSLeeCHLinCSJun dimerization protein 2 is a critical component of the Nrf2/MafK complex regulating the response to ROS homeostasisCell Death Dis20134e92124232097
- OyakeTItohKMotohashiHBach proteins belong to a novel family of BTB-basic leucine zipper transcription factors that interact with MafK and regulate transcription through the NF-E2 siteMol Cell Biol19961611608360958887638
- HoshinoHKobayashiAYoshidaMOxidative stress abolishes leptomycin B-sensitive nuclear export of transcription repressor Bach2 that counteracts activation of Maf recognition elementJ Biol Chem200027520153701537610809773
- DhakshinamoorthySJainAKBloomDAJaiswalAKBach1 competes with Nrf2 leading to negative regulation of the antioxidant response element (ARE)-mediated NAD(P)H:quinone oxidoreductase 1 gene expression and induction in response to antioxidantsJ Biol Chem200528017168911690015734732
- JyrkkänenHKKuosmanenSHeinäniemiMNovel insights into the regulation of antioxidant-response-element-mediated gene expression by electrophiles: induction of the transcriptional repressor BACH1 by Nrf2Biochem J2011440216717421812759
- MitsuishiYTaguchiKKawataniYNrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogrammingCancer Cell2012221667922789539
- NitureSKKhatriRJaiswalAKRegulation of Nrf2 – an updateFree Radic Biol Med201466364423434765
- MitsuishiYMotohashiHYamamotoMThe Keap1-Nrf2 system in cancers: stress response and anabolic metabolismFront Oncol2012220023272301
- ZhangDDHanninkMDistinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stressMol Cell Biol200323228137815114585973
- YamamotoTSuzukiTKobayashiAPhysiological significance of reactive cysteine residues of Keap1 in determining Nrf2 activityMol Cell Biol20082882758277018268004
- MageshSChenYHuLSmall molecule modulators of Keap1-Nrf2-ARE pathway as potential preventive and therapeutic agentsMed Res Rev201232468772622549716
- KumarHKimISMoreSVKimBWChoiDKNatural product-derived pharmacological modulators of Nrf2/ARE pathway for chronic diseasesNat Prod Rep201431110913924292194
- LoSCHanninkMPGAM5 tethers a ternary complex containing Keap1 and Nrf2 to mitochondriaExp Cell Res200831481789180318387606
- NitureSKJaiswalAKInhibitor of Nrf2 (INrf2 or Keap1) protein degrades Bcl-xL via phosphoglycerate mutase 5 and controls cellular apoptosisJ Biol Chem201128652445424455622072718
- KomatsuMKurokawaHWaguriSThe selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1Nat Cell Biol201012321322320173742
- InamiYWaguriSSakamotoAPersistent activation of Nrf2 through p62 in hepatocellular carcinoma cellsJ Cell Biol2011193227528421482715
- ChenWSunZWangXJDirect interaction between Nrf2 and p21 (Cip1/WAF1) upregulates the Nrf2-mediated antioxidant responseMol Cell200934666367319560419
- ChowdhrySZhangYMcMahonMSutherlandCCuadradoAHayesJDNrf2 is controlled by two distinct beta-TrCP recognition motifs in its Neh6 domain, one of which can be modulated by GSK-3 activityOncogene201332323765378122964642
- RadaPRojoAIEvrard-TodeschiNStructural and functional characterization of Nrf2 degradation by the glycogen synthase kinase 3/beta-TrCP axisMol Cell Biol201232173486349922751928
- FaraonioRVergaraPDi MarzoDp53 Suppresses the Nrf2-dependent transcription of antioxidant response genesJ Biol Chem200628152397763978417077087
- ChenWJiangTWangHDoes Nrf2 contribute to p53-mediated control of cell survival and death?Antioxid Redox Signal201217121670167522559194
- KaloEKogan-SakinISolomonHMutant p53R273H attenuates the expression of phase 2 detoxifying enzymes and promotes the survival of cells with high levels of reactive oxygen speciesJ Cell Sci2012125Pt 225578558622899716
- NitureSKJainAKSheltonPMJaiswalAKSrc subfamily kinases regulate nuclear export and degradation of transcription factor Nrf2 to switch off Nrf2-mediated antioxidant activation of cytoprotective gene expressionJ Biol Chem201128633288212883221690096
- SunZZhangSChanJYZhangDDKeap1 controls postinduction repression of the Nrf2-mediated antioxidant response by escorting nuclear export of Nrf2Mol Cell Biol200727186334634917636022
- YuMLiHLiuQNuclear factor p65 interacts with Keap1 to repress the Nrf2-ARE pathwayCell Signal201123588389221262351
- KimWDKimYWChoIJLeeCHKimSGE-cadherin inhibits nuclear accumulation of Nrf2: implications for chemoresistance of cancer cellsJ Cell Sci2012125Pt 51284129522302998
- BakinAVStourmanNVSekharKRSmad3-ATF3 signaling mediates TGF-beta suppression of genes encoding phase II detoxifying proteinsFree Radic Biol Med200538337538715629866
- BrownSLSekharKRRachakondaGSasiSFreemanMLActivating transcription factor 3 is a novel repressor of the nuclear factor erythroid-derived 2-related factor 2 (Nrf2)-regulated stress pathwayCancer Res200868236436818199529
- KratschmarDVCalabreseDWalshJSuppression of the Nrf2-dependent antioxidant response by glucocorticoids and 11β-HSD1-mediated glucocorticoid activation in hepatic cellsPLoS One201275e3677422606287
- ZhouWLoSCLiuJHHanninkMLubahnDBERRβ: a potent inhibitor of Nrf2 transcriptional activityMol Cell Endocrinol20072781–2526217920186
- YaoYBrodieAMDavidsonNEKenslerTWZhouQInhibition of estrogen signaling activates the NRF2 pathway in breast cancerBreast Cancer Res Treat2010124258559120623181
- WangXJHayesJDHendersonCJWolfCRIdentification of retinoic acid as an inhibitor of transcription factor Nrf2 through activation of retinoic acid receptor alphaProc Natl Acad Sci U S A200710449195891959418048326
- WangHLiuKGengMRXRα inhibits the NRF2-ARE signaling pathway through a direct interaction with the Neh7 domain of NRF2Cancer Res201373103097310823612120
- BryanHKOlayanjuAGoldringCEParkBKThe Nrf2 cell defence pathway: Keap1-dependent and -independent mechanisms of regulationBiochem Pharmacol201385670571723219527
- Gañán-GómezIWeiYYangHBoyano-AdánezMCGarcía-ManeroGOncogenic functions of the transcription factor Nrf2Free Radic Biol Med20136575076423820265
- SlocumSLKenslerTWNrf2: control of sensitivity to carcinogensArch Toxicol201185427328421369766
- ChanKLuRChangJCKanYWNRF2, a member of the NFE2 family of transcription factors, is not essential for murine erythropoiesis, growth, and developmentProc Natl Acad Sci U S A1996932413943139488943040
- ChoHYKleebergerSRNrf2 protects against airway disordersToxicol Appl Pharmacol20102441435619646463
- KudohKUchinamiHYoshiokaMSekiEYamamotoYNrf2 activation protects the liver from ischemia/reperfusion injury in miceAnn Surg2014260111812724368646
- LiuMReddyNMHigbeeEMThe Nrf2 triterpenoid activator, CDDO-imidazolide, protects kidneys from ischemia-reperfusion injury in miceKidney Int201485113414124088953
- ShirasakiKTaguchiKUnnoMMotohashiHYamamotoMNF-E2-related factor 2 promotes compensatory liver hypertrophy after portal vein branch ligation in miceHepatology20145962371238224443206
- ZhaoZChenYWangJAge-related retinopathy in NRF2-deficient micePLoS One201164e1945621559389
- OsburnWOKarimBDolanPMIncreased colonic inflammatory injury and formation of aberrant crypt foci in Nrf2-deficient mice upon dextran sulfate treatmentInt J Cancer200712191883189117631644
- KhorTOHuangMTKwonKHChanJYReddyBSKongANNrf2-deficient mice have an increased susceptibility to dextran sulfate sodium-induced colitisCancer Res20066624115801158417178849
- KhorTOHuangMTPrawanAIncreased susceptibility of Nrf2 knockout mice to colitis-associated colorectal cancerCancer Prev Res (Phila)20081318719119138955
- IidaKItohKKumagaiYNrf2 is essential for the chemopreventive efficacy of oltipraz against urinary bladder carcinogenesisCancer Res200464186424643115374950
- LibyKTRoyceDBRisingsongRSynthetic triterpenoids prolong survival in a transgenic mouse model of pancreatic cancerCancer Prev Res (Phila)20103111427143420959520
- SawCLHuangMTLiuYKhorTOConneyAHKongANImpact of Nrf2 on UVB-induced skin inflammation/photoprotection and photoprotective effect of sulforaphaneMol Carcinog201150647948621557329
- XuCHuangMTShenGInhibition of 7,12-dimethylbenz(a) anthracene-induced skin tumorigenesis in C57BL/6 mice by sulforaphane is mediated by nuclear factor E2-related factor 2Cancer Res200666168293829616912211
- YatesMSKwakMKEgnerPAPotent protection against aflatoxin-induced tumorigenesis through induction of Nrf2-regulated pathways by the triterpenoid 1-[2-cyano-3-,12-dioxooleana-1, 9(11)-dien-28-oyl]imidazoleCancer Res20066642488249416489057
- McMahonMItohKYamamotoMThe Cap’n’Collar basic leucine zipper transcription factor Nrf2 (NF-E2 p45-related factor 2) controls both constitutive and inducible expression of intestinal detoxification and glutathione biosynthetic enzymesCancer Res20016183299330711309284
- Ramos-GomezMDolanPMItohKYamamotoMKenslerTWInteractive effects of nrf2 genotype and oltipraz on benzo[a]pyrene-DNA adducts and tumor yield in miceCarcinogenesis200324346146712663505
- ShenGXuCHuRModulation of nuclear factor E2-related factor 2-mediated gene expression in mice liver and small intestine by cancer chemopreventive agent curcuminMol Cancer Ther200651395116432161
- SussanTERangasamyTBlakeDJTargeting Nrf2 with the triterpenoid CDDO-imidazolide attenuates cigarette smoke-induced emphysema and cardiac dysfunction in miceProc Natl Acad Sci U S A2009106125025519104057
- ZhengHWhitmanSAWuWTherapeutic potential of Nrf2 activators in streptozotocin-induced diabetic nephropathyDiabetes201160113055306622025779
- Ramos-GomezMKwakMKDolanPMSensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient miceProc Natl Acad Sci U S A20019863410341511248092
- CheungKLLeeJHKhorTONrf2 knockout enhances intestinal tumorigenesis in Apc(min/+) mice due to attenuation of anti-oxidative stress pathway while potentiates inflammationMol Carcinog2014531778422911891
- SatohHMoriguchiTTaguchiKNrf2-deficiency creates a responsive microenvironment for metastasis to the lungCarcinogenesis201031101833184320513672
- SchultzMAHaganSSDattaANrf1 and Nrf2 transcription factors regulate androgen receptor transactivation in prostate cancer cellsPLoS One201491e8720424466341
- WangWKwokAMChanJYThe p65 isoform of Nrf1 is a dominant negative inhibitor of ARE-mediated transcriptionJ Biol Chem200728234246702467817609210
- KunduJKSurhYJNrf2-Keap1 signaling as a potential target for chemoprevention of inflammation-associated carcinogenesisPharm Res2010276999101320354764
- LiuJGuXRobbinsDProtandim, a fundamentally new antioxidant approach in chemoprevention using mouse two-stage skin carcinogenesis as a modelPLoS One200944e528419384424
- ZhaoCRGaoZHQuXJNrf2-ARE signaling pathway and natural products for cancer chemopreventionCancer Epidemiol201034552353320638930
- AshrafianHCzibikGBellahceneMFumarate is cardioprotective via activation of the Nrf2 antioxidant pathwayCell Metab201215336137122405071
- ChartoumpekisDVZirosPGPsyrogiannisAINrf2 represses FGF21 during long-term high-fat diet-induced obesity in miceDiabetes201160102465247321852674
- JohnsonJAJohnsonDAKraftADThe Nrf2-ARE pathway: an indicator and modulator of oxidative stress in neurodegenerationAnn N Y Acad Sci20081147616919076431
- LaPash DanielsCMAustinEVRockneyDEBeneficial effects of Nrf2 overexpression in a mouse model of Alexander diseaseJ Neurosci20123231105071051522855800
- UrunoAFurusawaYYagishitaYThe Keap1-Nrf2 system prevents onset of diabetes mellitusMol Cell Biol201333152996301023716596
- KenslerTWEgnerPAAgyemanASKeap1-nrf2 signaling: a target for cancer prevention by sulforaphaneTop Curr Chem201332916317722752583
- CornblattBSYeLDinkova-KostovaATPreclinical and clinical evaluation of sulforaphane for chemoprevention in the breastCarcinogenesis20072871485149017347138
- EgnerPAChenJGWangJBBioavailability of sulforaphane from two broccoli sprout beverages: results of a short-term, cross-over clinical trial in Qidong, ChinaCancer Prev Res (Phila)20114338439521372038
- KenslerTWNgDCarmellaSGModulation of the metabolism of airborne pollutants by glucoraphanin-rich and sulforaphane-rich broccoli sprout beverages in Qidong, ChinaCarcinogenesis201233110110722045030
- ZojaCBenigniARemuzziGThe Nrf2 pathway in the progression of renal diseaseNephrol Dial Transplant201429 Suppl 1i19i2423761459
- ReddyNMPottetiHRMarianiTJBiswalSReddySPConditional deletion of Nrf2 in airway epithelium exacerbates acute lung injury and impairs the resolution of inflammationAm J Respir Cell Mol Biol20114561161116821659655
- TaguchiKMotohashiHYamamotoMMolecular mechanisms of the Keap1-Nrf2 pathway in stress response and cancer evolutionGenes Cells201116212314021251164
- ListerANedjadiTKitteringhamNRNrf2 is overexpressed in pancreatic cancer: implications for cell proliferation and therapyMol Cancer2011103721489257
- SoiniYEskelinenMJuvonenPNuclear Nrf2 expression is related to a poor survival in pancreatic adenocarcinomaPathol Res Pract20142101353924189098
- ArltASebensSKrebsSInhibition of the Nrf2 transcription factor by the alkaloid trigonelline renders pancreatic cancer cells more susceptible to apoptosis through decreased proteasomal gene expression and proteasome activityOncogene201332404825483523108405
- Garcia-ManeroGTambaroFPBekeleNBPhase II trial of vorinostat with idarubicin and cytarabine for patients with newly diagnosed acute myelogenous leukemia or myelodysplastic syndromeJ Clin Oncol201230182204221022585696
- ChenNYiXAbushahinNNrf2 expression in endometrial serous carcinomas and its precancersInt J Clin Exp Pathol201041859621228930
- BarbanoRMuscarellaLAPasculliBAberrant Keap1 methylation in breast cancer and association with clinicopathological featuresEpigenetics20138110511223249627
- Al SaatiTClercPHanounNOxidative stress induced by inactivation of TP53INP1 cooperates with KrasG12D to initiate and promote pancreatic carcinogenesis in the murine pancreasAm J Pathol201318261996200423578383
- PetrelliAPerraACoraDMicroRNA/gene profiling unveils early molecular changes and nuclear factor erythroid related factor 2 (NRF2) activation in a rat model recapitulating human hepatocellular carcinoma (HCC)Hepatology201459122824123857252
- MaQHeXMolecular basis of electrophilic and oxidative defense: promises and perils of Nrf2Pharmacol Rev20126441055108122966037
- YooNJKimHRKimYRAnCHLeeSHSomatic mutations of the KEAP1 gene in common solid cancersHistopathology201260694395222348534
- HastBECloerEWGoldfarbDCancer-derived mutations in KEAP1 impair NRF2 degradation but not ubiquitinationCancer Res201474380881724322982
- ShibataTOhtaTTongKICancer related mutations in NRF2 impair its recognition by Keap1-Cul3 E3 ligase and promote malignancyProc Natl Acad Sci U S A200810536135681357318757741
- KimYROhJEKimMSOncogenic NRF2 mutations in squamous cell carcinomas of oesophagus and skinJ Pathol2010220444645119967722
- SolisLMBehrensCDongWNrf2 and Keap1 abnormalities in non-small cell lung carcinoma and association with clinicopathologic featuresClin Cancer Res201016143743375320534738
- WangRAnJJiFJiaoHSunHZhouDHypermethylation of the Keap1 gene in human lung cancer cell lines and lung cancer tissuesBiochem Biophys Res Commun2008373115115418555005
- HanadaNTakahataTZhouQMethylation of the KEAP1 gene promoter region in human colorectal cancerBMC Cancer2012126622325485
- ZhangPSinghAYegnasubramanianSLoss of Kelch-like ECH-associated protein 1 function in prostate cancer cells causes chemoresistance and radioresistance and promotes tumor growthMol Cancer Ther20109233634620124447
- YuSKhorTOCheungKLNrf2 expression is regulated by epigenetic mechanisms in prostate cancer of TRAMP micePLoS One201051e857920062804
- KhorTOHuangYWuTYShuLLeeJKongANPharmacodynamics of curcumin as DNA hypomethylation agent in restoring the expression of Nrf2 via promoter CpGs demethylationBiochem Pharmacol20118291073107821787756
- DuWJiangYZhengZFeedback loop between p66(Shc) and Nrf2 promotes lung cancer progressionCancer Lett20133371586523689140
- EadesGYangMYaoYZhangYZhouQmiR-200a regulates Nrf2 activation by targeting Keap1 mRNA in breast cancer cellsJ Biol Chem201128647407254073321926171
- van JaarsveldMTHellemanJBoersmaAWmiR-141 regulates KEAP1 and modulates cisplatin sensitivity in ovarian cancer cellsOncogene201332364284429323045278
- YangMYaoYEadesGZhangYZhouQMiR-28 regulates Nrf2 expression through a Keap1-independent mechanismBreast Cancer Res Treat2011129398399121638050
- YamamotoSInoueJKawanoTKozakiKOmuraKInazawaJThe impact of miRNA-based molecular diagnostics and treatment of NRF2-stabilized tumorsMol Cancer Res2014121586824307696
- AdamJHatipogluEO’FlahertyLRenal cyst formation in Fh1-deficient mice is independent of the Hif/Phd pathway: roles for fumarate in KEAP1 succination and Nrf2 signalingCancer Cell201120452453722014577
- KinchLGrishinNVBrugarolasJSuccination of Keap1 and activation of Nrf2-dependent antioxidant pathways in FH-deficient papillary renal cell carcinoma type 2Cancer Cell201120441842022014567
- KongBQiaCErkanMKleeffJMichalskiCWOverview on how oncogenic Kras promotes pancreatic carcinogenesis by inducing low intracellular ROS levelsFront Physiol2013424624062691
- SatohHMoriguchiTTakaiJEbinaMYamamotoMNrf2 prevents initiation but accelerates progression through the Kras signaling pathway during lung carcinogenesisCancer Res201373134158416823610445
- BauerAKHillT3rdAlexanderCMThe involvement of NRF2 in lung cancerOxid Med Cell Longev2013201374643223577226
- YamadoriTIshiiYHommaSMolecular mechanisms for the regulation of Nrf2-mediated cell proliferation in non-small-cell lung cancersOncogene201231454768477722249257
- BanerjeePBasuADattaDGasserMWaaga-GasserAMPalSThe heme oxygenase-1 protein is overexpressed in human renal cancer cells following activation of the Ras-Raf-ERK pathway and mediates anti-apoptotic signalJ Biol Chem201128638335803359021808062
- FunesJMHendersonSKaufmanROncogenic transformation of mesenchymal stem cells decreases Nrf2 expression favoring in vivo tumor growth and poorer survivalMol Cancer20141312024491031
- NagyPVargaAPircsKHegedűsKJuhászGMyc-driven overgrowth requires unfolded protein response-mediated induction of autophagy and antioxidant responses in Drosophila melanogasterPLoS Genet201398e100366423950728
- LevySFormanHJC-Myc is a Nrf2-interacting protein that negatively regulates phase II genes through their electrophile responsive elementsIUBMB Life201062323724620232342
- RomanoGThe role of the dysfunctional Akt-related pathway in cancer: establishment and maintenance of a malignant cell phenotype, resistance to therapy, and future strategies for drug developmentScientifica (Cairo)2013201331718624381788
- GaoAMKeZPShiFSunGCChenHChrysin enhances sensitivity of BEL-7402/ADM cells to doxorubicin by suppressing PI3K/Akt/Nrf2 and ERK/Nrf2 pathwayChem Biol Interact2013206110010823994249
- DengXRuiWZhangFDingWPM2.5 induces Nrf2-mediated defense mechanisms against oxidative stress by activating PIK3/AKT signaling pathway in human lung alveolar epithelial A549 cellsCell Biol Toxicol201329314315723525690
- StachelIGeismannCAdenKModulation of nuclear factor E2-related factor-2 (Nrf2) activation by the stress response gene immediate early response-3 (IER3) in colonic epithelial cells: a novel mechanism of cellular adaption to inflammatory stressJ Biol Chem201428941917192924311782
- TaguchiKSakataKOhashiWImaizumiTImamuraJHattoriYTonic inhibition by G protein-coupled receptor kinase 2 of Akt/endothelial nitric oxide synthase signaling in human vascular endothelial cells under conditions of hyperglycemia with high insulin levelsJ Pharmacol Exp Ther2014349219920824570070
- DeyNCrosswellHEDePThe protein phosphatase activity of PTEN regulates SRC family kinases and controls glioma migrationCancer Res20086861862187118339867
- HuangWLvXLiuCThe N-terminal phosphodegron targets TAZ/WWTR1 protein for SCFβ-TrCP-dependent degradation in response to phosphatidylinositol 3-kinase inhibitionJ Biol Chem201228731262452625322692215
- ChenQLiWWanYAmplified in breast cancer 1 enhances human cholangiocarcinoma growth and chemoresistance by simultaneous activation of Akt and Nrf2 pathwaysHepatology20125561820182922213475
- LernerCBittoAPulliamDReduced mammalian target of rapamycin activity facilitates mitochondrial retrograde signaling and increases life span in normal human fibroblastsAging Cell201312696697723795962
- ShibataTSaitoSKokubuASuzukiTYamamotoMHirohashiSGlobal downstream pathway analysis reveals a dependence of oncogenic NF-E2-related factor 2 mutation on the mTOR growth signaling pathwayCancer Res201070229095910521062981
- GaikwadNWYangLRoganEGCavalieriELEvidence for NQO2-mediated reduction of the carcinogenic estrogen ortho-quinonesFree Radic Biol Med200946225326218996184
- AnsellPJLoSCNewtonLGRepression of cancer protective genes by 17β-estradiol: ligand-dependent interaction between human Nrf2 and estrogen receptor alphaMol Cell Endocrinol20052431–2273416198475
- LoRMatthewsJThe aryl hydrocarbon receptor and estrogen receptor alpha differentially modulate nuclear factor erythroid-2-related factor 2 transactivation in MCF-7 breast cancer cellsToxicol Appl Pharmacol2013270213914823583297
- LamSSMakASYamJWCheungANNganHYWongASTargeting estrogen-related receptor alpha inhibits epithelial-to-mesenchymal transition and stem cell properties of ovarian cancer cellsMol Ther201422474375124419103
- ZhangTLiangXShiLEstrogen receptor and PI3K/Akt signaling pathway involvement in S-(-)equol-induced activation of Nrf2/ARE in endothelial cellsPLoS One2013811e7907524260155
- GorriniCGangBPBassiCEstrogen controls the survival of BRCA1-deficient cells via a PI3K-NRF2-regulated pathwayProc Natl Acad Sci U S A2014111124472447724567396
- MontanoMMDengHLiuMSunXSingalRTranscriptional regulation by the estrogen receptor of antioxidative stress enzymes and its functional implicationsOncogene200423142442245314676828
- ArltASchäferHKalthoffHThe ‘N-factors’ in pancreatic cancer: functional relevance of NF-κB, NFAT and Nrf2 in pancreatic cancerOncogenesis20121e3523552468
- WakabayashiNSlocumSLSkokoJJShinSKenslerTWWhen NRF2 talks, who’s listening?Antioxid Redox Signal201013111649166320367496
- LiuGHQuJShenXNF-κB/p65 antagonizes Nrf2-ARE pathway by depriving CBP from Nrf2 and facilitating recruitment of HDAC3 to MafKBiochim Biophys Acta20081783571372718241676
- HealyZRLeeNHGaoXDivergent responses of chondrocytes and endothelial cells to shear stress: cross-talk among COX-2, the phase 2 response, and apoptosisProc Natl Acad Sci U S A200510239140101401516172382
- ChenCYJangJHLiMHSurhYJResveratrol upregulates heme oxygenase-1 expression via activation of NF-E2-related factor 2 in PC12 cellsBiochem Biophys Res Commun20053314993100015882976
- MannaSKMukhopadhyayAAggarwalBBResveratrol suppresses TNF-induced activation of nuclear transcription factors NF-kappa B, activator protein-1, and apoptosis: potential role of reactive oxygen intermediates and lipid peroxidationJ Immunol2000164126509651910843709
- BalogunEForestiRGreenCJMotterliniRChanges in temperature modulate heme oxygenase-1 induction by curcumin in renal epithelial cellsBiochem Biophys Res Commun2003308495095512927811
- GanFFLingHAngXA novel shogaol analog suppresses cancer cell invasion and inflammation, and displays cytoprotective effects through modulation of NF-κB and Nrf2-Keap1 signaling pathwaysToxicol Appl Pharmacol2013272385286223899529
- KumarSSinghBKPrasadAKParmarVSBiswalSGhoshBEthyl 3′,4′,5′-trimethoxythionocinnamate modulates NF-κB and Nrf2 transcription factorsEur J Pharmacol20137001–3324123261968
- RushworthSAZaitsevaLMurrayMYShahNMBowlesKMMacEwanDJThe high Nrf2 expression in human acute myeloid leukemia is driven by NF-κB and underlies its chemo-resistanceBlood2012120265188519823077289
- ArltABauerISchafmayerCIncreased proteasome subunit protein expression and proteasome activity in colon cancer relate to an enhanced activation of nuclear factor E2-related factor 2 (Nrf2)Oncogene200928453983399619734940
- NitureSKJaiswalAKNrf2-induced antiapoptotic Bcl-xL protein enhances cell survival and drug resistanceFree Radic Biol Med20135711913123275004
- NitureSKJaiswalAKNrf2 protein up-regulates antiapoptotic protein Bcl-2 and prevents cellular apoptosisJ Biol Chem2012287139873988622275372
- RotblatBMelinoGKnightRANRF2 and p53: Januses in cancer?Oncotarget20123111272128323174755
- VurusanerBPoliGBasagaHTumor suppressor genes and ROS: complex networks of interactionsFree Radic Biol Med201252171822019631
- SuzukiSTanakaTPoyurovskyMVPhosphate-activated glutaminase (GLS2), a p53-inducible regulator of glutamine metabolism and reactive oxygen speciesProc Natl Acad Sci U S A2010107167461746620351271
- BensaadKTsurutaASelakMATIGAR, a p53-inducible regulator of glycolysis and apoptosisCell2006126110712016839880
- AsherGLotemJKamaRSachsLShaulYNQO1 stabilizes p53 through a distinct pathwayProc Natl Acad Sci U S A20029953099310411867746
- YouANamCWWakabayashiNYamamotoMKenslerTWKwakMKTranscription factor Nrf2 maintains the basal expression of Mdm2: an implication of the regulation of p53 signaling by Nrf2Arch Biochem Biophys2011507235636421211512
- CapaccioneKMPineSRThe Notch signaling pathway as a mediator of tumor survivalCarcinogenesis20133471420143023585460
- WakabayashiNSkokoJJChartoumpekisDVNotch-Nrf2 axis: regulation of Nrf2 gene expression and cytoprotection by notch signalingMol Cell Biol201434465366324298019
- KimJHThimmulappaRKKumarVNRF2-mediated Notch pathway activation enhances hematopoietic reconstitution following myelosuppressive radiationJ Clin Invest2014124273074124463449
- ZhuJWangHSunQNrf2 is required to maintain the self-renewal of glioma stem cellsBMC Cancer20131338023937621
- LiWLiuHZhouJSCaveolin-1 inhibits expression of antioxidant enzymes through direct interaction with nuclear erythroid 2 p45-related factor-2 (Nrf2)J Biol Chem201228725209222093022547061
- ZhengYMorrisASunkaraMLayneJToborekMHennigBEpigallocatechin-gallate stimulates NF-E2-related factor and heme oxygenase-1 via caveolin-1 displacementJ Nutr Biochem201223216316821447442
- VolonteDLiuZMusillePMInhibition of nuclear factor-erythroid 2-related factor (Nrf2) by caveolin-1 promotes stress-induced premature senescenceMol Biol Cell201324121852186223637463
- CossetECGodetJEntz-WerleNInvolvement of the TGFβ pathway in the regulation of α5 β1 integrins by caveolin-1 in human glioblastomaInt J Cancer2012131360161121901744
- GreenDRLevineBTo be or not to be? How selective autophagy and cell death govern cell fateCell20141571657524679527
- LauAWangXJZhaoFA noncanonical mechanism of Nrf2 activation by autophagy deficiency: direct interaction between Keap1 and p62Mol Cell Biol201030133275328520421418
- IchimuraYWaguriSSouYSPhosphorylation of p62 activates the Keap1-Nrf2 pathway during selective autophagyMol Cell201351561863124011591
- JainALamarkTSjøttemEp62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcriptionJ Biol Chem201028529225762259120452972
- BrayKMathewRLauAAutophagy suppresses RIP kinase-dependent necrosis enabling survival to mTOR inhibitionPLoS One201277e4183122848625
- ParkSHKimJHChiGYInduction of apoptosis and autophagy by sodium selenite in A549 human lung carcinoma cells through generation of reactive oxygen speciesToxicol Lett2012212325226122721804
- RaoVAKleinSRBonarSJThe antioxidant transcription factor Nrf2 negatively regulates autophagy and growth arrest induced by the anticancer redox agent mitoquinoneJ Biol Chem201028545344473445920805228
- ZhouYWangHDZhuLKnockdown of Nrf2 enhances autophagy induced by temozolomide in U251 human glioma cell lineOncol Rep201329139440023128449
- LiSLiJShenCtert-Butylhydroquinone (tBHQ) protects hepatocytes against lipotoxicity via inducing autophagy independently of Nrf2 activationBiochim Biophys Acta201418411223324055888
- KimTHHurEGKangSJNRF2 blockade suppresses colon tumor angiogenesis by inhibiting hypoxia-induced activation of HIF-1αCancer Res20117162260227521278237
- JiXWangHZhuJCorrelation of Nrf2 and HIF-1α in glioblastoma and their relationships to clinicopathologic features and survivalNeurol Res201335101044105024070025
- JiXWangHZhuJKnockdown of Nrf2 suppresses glioblastoma angiogenesis by inhibiting hypoxia-induced activation of HIF-1αInt J Cancer2014135357458424374745
- ShenHYangYXiaSRaoBZhangJWangJBlockage of Nrf2 suppresses the migration and invasion of esophageal squamous cell carcinoma cells in hypoxic microenvironmentDis Esophagus Epub9132013
- KwakMKKenslerTWInduction of 26S proteasome subunit PSMB5 by the bifunctional inducer 3-methylcholanthrene through the Nrf2-ARE, but not the AhR/Arnt-XRE, pathwayBiochem Biophys Res Commun200634541350135716723119
- KwakMKWakabayashiNGreenlawJLYamamotoMKenslerTWAntioxidants enhance mammalian proteasome expression through the Keap1-Nrf2 signaling pathwayMol Cell Biol200323238786879414612418
- SchaedlerSKrauseJHimmelsbachKHepatitis B virus induces expression of antioxidant response element-regulated genes by activation of Nrf2J Biol Chem201028552410744108620956535
- PickeringAMLinderRAZhangHFormanHJDaviesKJNrf2-dependent induction of proteasome and Pa28αβ regulator are required for adaptation to oxidative stressJ Biol Chem201228713100211003122308036
- HöhnTJGruneTThe proteasome and the degradation of oxidized proteins: Part III – Redox regulation of the proteasomal systemRedox Biol2014238839424563857
- ChenJReganRFIncreasing expression of heme oxygenase-1 by proteasome inhibition protects astrocytes from heme-mediated oxidative injuryCurr Neurovasc Res20052318919616181113
- RenSSmithMJLouroIDThe p44S10 locus, encoding a subunit of the proteasome regulatory particle, is amplified during progression of cutaneous malignant melanomaOncogene200019111419142710723133
- RhoJHQinSWangJYRoehrlMHProteomic expression analysis of surgical human colorectal cancer tissues: up-regulation of PSB7, PRDX1, and SRP9 and hypoxic adaptation in cancerJ Proteome Res2008772959297218549262
- HuZPanXFWuFQSynergy between proteasome inhibitors and imatinib mesylate in chronic myeloid leukemiaPLoS One200947e625719606213
- SebensSBauerIGeismannCInflammatory macrophages induce Nrf2 transcription factor-dependent proteasome activity in colonic NCM460 cells and thereby confer anti-apoptotic protectionJ Biol Chem201128647409114092121990354
- DuZXYanYZhangHYProteasome inhibition induces a p38 MAPK pathway-dependent antiapoptotic program via Nrf2 in thyroid cancer cellsJ Clin Endocrinol Metab2011965E763E77121346076
- EbertBKisielaMMalatkovaPEl-HawariYMaserERegulation of human carbonyl reductase 3 (CBR3; SDR21C2) expression by Nrf2 in cultured cancer cellsBiochemistry201049398499851120806931
- KraftDCDeocarisCCWadhwaRRattanSIPreincubation with the proteasome inhibitor MG-132 enhances proteasome activity via the Nrf2 transcription factor in aging human skin fibroblastsAnn N Y Acad Sci2006106742042416804021
- RushworthSABowlesKMMacEwanDJHigh basal nuclear levels of Nrf2 in acute myeloid leukemia reduces sensitivity to proteasome inhibitorsCancer Res20117151999200921212410
- KochASteffenJKrügerETCF11 at the crossroads of oxidative stress and the ubiquitin proteasome systemCell Cycle20111081200120721412055
- GorowiecMRBorthwickLAParkerSMKirbyJASaretzkiGCFisherAJFree radical generation induces epithelial-to-mesenchymal transition in lung epithelium via a TGF-β1-dependent mechanismFree Radic Biol Med20125261024103222240154
- OkitaYKamoshidaASuzukiHTransforming growth factor-beta induces transcription factors MafK and Bach1 to suppress expression of the heme oxygenase-1 geneJ Biol Chem201328828206582066723737527
- OhCJKimJYChoiYKDimethylfumarate attenuates renal fibrosis via NF-E2-related factor 2-mediated inhibition of transforming growth factor-beta/Smad signalingPLoS One2012710e4587023056222
- ChoiHKPokharelYRLimSCInhibition of liver fibrosis by solubilized coenzyme Q10: role of Nrf2 activation in inhibiting transforming growth factor-β1 expressionToxicol Appl Pharmacol2009240337738419647758
- RachakondaGSekharKRJowharDIncreased cell migration and plasticity in Nrf2-deficient cancer cell linesOncogene201029253703371420440267
- PanHWangHZhuLMaoLQiaoLSuXThe role of Nrf2 in migration and invasion of human glioma cell U251World Neurosurg2013803–436337022120303
- ZhangLWangNZhouSYeWJingGZhangMPropofol induces proliferation and invasion of gallbladder cancer cells through activation of Nrf2J Exp Clin Cancer Res2012316622901367
- JiXJChenSHZhuLKnockdown of NF-E2-related factor 2 inhibits the proliferation and growth of U251MG human glioma cells in a mouse xenograft modelOncol Rep201330115716423673813
- HanahanDWeinbergRAHallmarks of cancer: the next generationCell2011144564667421376230
- HayesJDAshfordMLNrf2 orchestrates fuel partitioning for cell proliferationCell Metab201216213914122883227
- ShimizuTTolcherAWPapadopoulosKPThe clinical effect of the dual-targeting strategy involving PI3K/AKT/mTOR and RAS/MEK/ERK pathways in patients with advanced cancerClin Cancer Res20121882316232522261800
- SinghAHappelCMannaSKTranscription factor NRF2 regulates miR-1 and miR-206 to drive tumorigenesisJ Clin Invest201312372921293423921124
- MaXZhangJLiuSHuangYChenBWangDNrf2 knockdown by shRNA inhibits tumor growth and increases efficacy of chemotherapy in cervical cancerCancer Chemother Pharmacol201269248549421842204
- JiangTChenNZhaoFHigh levels of Nrf2 determine chemoresistance in type II endometrial cancerCancer Res201070135486549620530669
- DuongHQYiYWKangHJInhibition of NRF2 by PIK-75 augments sensitivity of pancreatic cancer cells to gemcitabineInt J Oncol201444395996924366069
- AkhdarHLoyerPRauchCCorluAGuillouzoAMorelFInvolvement of Nrf2 activation in resistance to 5-fluorouracil in human colon cancer HT-29 cellsEur J Cancer200945122219222719524433
- HuXFYaoJGaoSGNrf2 overexpression predicts prognosis and 5-FU resistance in gastric cancerAsian Pac J Cancer Prev20131495231523524175806
- GaoAMKeZPWangJNYangJYChenSYChenHApigenin sensitizes doxorubicin-resistant hepatocellular carcinoma BEL-7402/ADM cells to doxorubicin via inhibiting PI3K/Akt/Nrf2 pathwayCarcinogenesis20133481806181423563091
- SinghAWuHZhangPHappelCMaJBiswalSExpression of ABCG2 (BCRP) is regulated by Nrf2 in cancer cells that confers side population and chemoresistance phenotypeMol Cancer Ther2010982365237620682644
- WangXJLiYLuoLOxaliplatin activates the Keap1/Nrf2 antioxidant system conferring protection against the cytotoxicity of anticancer drugsFree Radic Biol Med201470687724556415
- EmminkBLVerheemAVan HoudtWJThe secretome of colon cancer stem cells contains drug-metabolizing enzymesJ Proteomics201391849623835434
- MahaffeyCMZhangHRinnaAHollandWMackPCFormanHJMultidrug-resistant protein-3 gene regulation by the transcription factor Nrf2 in human bronchial epithelial and non-small-cell lung carcinomaFree Radic Biol Med200946121650165719345732
- NishinakaTMiuraTOkumuraMNakaoFNakamuraHTeradaTRegulation of aldo-keto reductase AKR1B10 gene expression: involvement of transcription factor Nrf2Chem Biol Interact20111911–318519121277289
- NaHKSurhYJOncogenic potential of Nrf2 and its principal target protein heme oxygenase-1Free Radic Biol Med20146735336524200599
- ZhouSYeWShaoQZhangMLiangJNrf2 is a potential therapeutic target in radioresistance in human cancerCrit Rev Oncol Hematol201388370671524126138
- ManandharSLeeSKwakMKEffect of stable inhibition of NRF2 on doxorubicin sensitivity in human ovarian carcinoma OV90 cellsArch Pharm Res201033571772620512470
- TanKPKosugeKYangMItoSNRF2 as a determinant of cellular resistance in retinoic acid cytotoxicityFree Radic Biol Med200845121663167318845239
- TarumotoTNagaiTOhmineKAscorbic acid restores sensitivity to imatinib via suppression of Nrf2-dependent gene expression in the imatinib-resistant cell lineExp Hematol200432437538115050748
- KweonMHAdhamiVMLeeJSMukhtarHConstitutive overexpression of Nrf2-dependent heme oxygenase-1 in A549 cells contributes to resistance to apoptosis induced by epigallocatechin 3-gallateJ Biol Chem200628144337613377216950787
- LimoncielAJenningsPA review of the evidence that ochratoxin A is an Nrf2 inhibitor: implications for nephrotoxicity and renal carcinogenicityToxins (Basel)20146137137924448208
- RenDVilleneuveNFJiangTBrusatol enhances the efficacy of chemotherapy by inhibiting the Nrf2-mediated defense mechanismProc Natl Acad Sci U S A201110841433143821205897
- CuendetMGillsJJPezzutoJMBrusatol-induced HL-60 cell differentiation involves NF-κB activationCancer Lett20042061435015019158
- WruckCJClaussenMFuhrmannGLuteolin protects rat PC12 and C6 cells against MPP+ induced toxicity via an ERK dependent Keap1-Nrf2-ARE pathwayJ Neural Transm Suppl200772576717982879
- LinCWWuMJLiuIYSuJDYenJHNeurotrophic and cytoprotective action of luteolin in PC12 cells through ERK-dependent induction of Nrf2-driven HO-1 expressionJ Agric Food Chem20105874477448620302373
- PanduranganAKAnanda SadagopanSKDharmalingamPGanapasamSLuteolin, a bioflavonoid inhibits azoxymethane-induced colorectal cancer through activation of Nrf2 signalingToxicol Mech Methods2014241132024024667
- LeeSLimMJKimMHAn effective strategy for increasing the radiosensitivity of human lung cancer cells by blocking Nrf2-dependent antioxidant responsesFree Radic Biol Med201253480781622684019
- BoettlerUSommerfeldKVolzNCoffee constituents as modulators of Nrf2 nuclear translocation and ARE (EpRE)-dependent gene expressionJ Nutr Biochem201122542644020655719
- van DijkAEOlthofMRMeeuseJCSeebusEHeineRJvan DamRMAcute effects of decaffeinated coffee and the major coffee components chlorogenic acid and trigonelline on glucose toleranceDiabetes Care20093261023102519324944
- OlthofMRvan DijkAEDeaconCFHeineRJvan DamRMAcute effects of decaffeinated coffee and the major coffee components chlorogenic acid and trigonelline on incretin hormonesNutr Metab (Lond)201181021299855
- ZhongYZhangFSunZDrug resistance associates with activation of Nrf2 in MCF-7/DOX cells, and wogonin reverses it by down-regulating Nrf2-mediated cellular defense responseMol Carcinog2013521082483422593043
- SharmaPKVarshneyR2-Deoxy-D-glucose and 6-aminonicotinamide-mediated Nrf2 down regulation leads to radiosensitization of malignant cells via abrogation of GSH-mediated defenseFree Radic Res201246121446145722946929
