 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
It is unclear whether there are any differences in the induction of cytotoxic T lymphocytes (CTL) and CD4+CD25high regulatory T-cells (Tregs) among dendritic cells (DCs) fused with different pancreatic carcinomas. The aim of this study was to compare the ability to induce cytotoxicity by human DCs fused with different human pancreatic carcinoma cell lines and to elucidate the causes of variable cytotoxicity among cell lines.
Methods
Monocyte-derived DCs, which were generated from peripheral blood mononuclear cells (PBMCs), were fused with carcinoma cells such as Panc-1, KP-1NL, QGP-1, and KP-3L. The induction of CTL and Tregs, and cytokine profile of PBMCs stimulated by fused DCs were evaluated.
Results
The cytotoxicity against tumor targets induced by PBMCs cocultured with DCs fused with QGP-1 (DC/QGP-1) was very low, even though PBMCs cocultured with DCs fused with other cell lines induced significant cytotoxicity against the respective tumor target. The factors causing this low cytotoxicity were subsequently investigated. DC/QGP-1 induced a significant expansion of Tregs in cocultured PBMCs compared with DC/KP-3L. The level of interleukin-10 secreted in the supernatants of PBMCs cocultured with DC/QGP-1 was increased significantly compared with that in DC/KP-3L. Downregulation of major histocompatibility complex class I expression and increased secretion of vascular endothelial growth factor were observed with QGP-1, as well as in the other cell lines.
Conclusion
The present study demonstrated that the cytotoxicity induced by DCs fused with pancreatic cancer cell lines was different between each cell line, and that the reduced cytotoxicity of DC/QGP-1 might be related to the increased secretion of interleukin-10 and the extensive induction of Tregs.
Introduction
Pancreatic and biliary cancers are relatively resistant to chemotherapy and radiation and may therefore provide an opportunity for testing the potential of immunotherapy. Clinical trials of dendritic cell (DC) vaccination for advanced pancreatic cancer patients have revealed that this therapy can stimulate an antitumoral T cell response and prolong the survival of refractory pancreatic cancer patients, indicating a promising treatment modality.Citation1–Citation5 A critical issue in optimizing DC vaccines is the choice of tumor-associated antigen for DC loading. Some tumor antigens such as mucin 1 and α-galactosylceramide have been identified as potential targets for the immunotherapy of pancreatic carcinoma.Citation6–Citation10 Some reports indicated the efficacy of combined therapy with DC vaccination and chemotherapeutic agents of gemcitabine.Citation11–Citation13 However, immunity against a single antigen may be ineffective in tumors with heterogeneous cell populations and may carry a risk of inducing tumor antigen escape variants. Although a recent study has demonstrated that the generation of cytotoxic T lymphocytes (CTL) against three or more tumor antigens correlated with clinical response,Citation14 for many tumors, no or only few antigenic epitopes have been identified. Several recent studies have demonstrated the efficacy of the induction of antitumor immunity through the generation of fused DCs with tumor cells.Citation15–Citation17 In this strategy, different tumor-associated antigens, including those that are as yet unidentified, are processed endogenously and presented by major histocompatibility complex (MHC) class I pathway in the context of costimulatory signals.
The mechanisms that mediate immune tolerance to cancer are not well understood, but recent findings have revealed them to be multifactorial, including downregulation of MHC class I molecules, loss of tumor antigens, defective death receptor signaling, and generation of immunosuppressive cells such as regulatory T cells (Tregs).Citation18–Citation22 Major populations of these cells are CD4+CD25high Tregs that specifically express the forkhead transcription factor, forkhead/winged helix transcription factor gene (FOXP3) and CD4+ type 1 Tregs that secrete high levels of interleukin-10 (IL-10). Tregs secrete the immunoregulatory cytokines, IL-10 and transforming growth factor (TGF)-β1, and inhibit T-cell proliferation. Tregs are significantly increased in a population of peripheral blood lymphocytes and tumor-infiltrating lymphocytes in some epithelial cancers.Citation19–Citation22 Prostaglandin E2 (PGE2) induces FOXP3 gene expression and Treg function in CD4+ T cells.Citation23
Very few reports have evaluated the efficacy of DC vaccination using pancreatic cancer cell lines, being almost limited to Panc02.Citation24–Citation27 However, antitumor immunity based on DCs has not yet been compared among various pancreatic carcinoma cell lines, and it remains unclear whether there are any differences in induction of CTL and Tregs among DCs fused with different pancreatic carcinoma cells. We selected four representative human pancreatic cancer cell lines, Panc-1 as undifferentiated carcinoma,Citation28 KP-1NL as highly metastatic adenocarcinoma,Citation29 QGP-1 as carcinoma of islet cell,Citation30 and KP-3L as adenosquamous carcinoma.Citation31
The aim of this study was to compare the ability to induce cytotoxicity by human DCs fused with different human pancreatic carcinoma cell lines and to elucidate the causes of variable cytotoxicity among pancreatic carcinoma cell lines.
Materials and methods
Reagents
Recombinant human granulocyte macrophage colony-stimulating factor (GM-CSF) and IL-4 were purchased from Primmune KK (Osaka, Japan). IL-2, lipopolysaccharide (LPS) from Escherichia coli, and fluorochrome-labeled PKH-26 and PKH-67 were purchased from Sigma-Aldrich (St Louis, MO, USA).
Cell culture of pancreatic carcinoma cell lines
A human pancreatic carcinoma cell line, Panc-1 (undifferentiated carcinoma), was obtained from the Cell Resource Center for Biomedical Research, Tohoku University (Miyagi, Japan). The human pancreatic carcinoma cell lines, KP-1NL (adenocarcinoma), QGP-1 (carcinoma of islet cell), and KP-3L (adenosquamous carcinoma) were purchased from Health Science Research Resources Bank (Osaka, Japan). Cells were maintained in 75 cmCitation2 cell culture flask with RPMI 1640 (Life Technologies, Carlsbad, CA, USA) supplemented with 10% fetal calf serum ([FCS] Life Technologies), 100 units/mL penicillin, 100 μg/mL streptomycin, and 50 μg/mL gentamycin, and were grown at 37°C with 5% CO2 in a humidified atmosphere.
Preparation of DCs from peripheral blood
Monocyte-derived DCs were generated as described previously.Citation32 Briefly, peripheral blood mononuclear cells (PBMCs) were isolated from peripheral blood from four healthy volunteers by Ficoll-Hypaque gradient centrifugation and were subsequently suspended in 6-well culture plates for 1 hour at 37°C. The nonadherent cells were removed. The adherent cells were harvested and cultured in RPMI 1640 supplemented with 10% FCS, 50 μM 2-mercaptoethanol, 1 μM pyruvate, 50 μg/mL gentamycin, 25 μg/mL GM-CSF, and 10 μg/mL IL-4. On day 6, DCs were harvested from the nonadherent and loosely adherent cells.
Preparation of DCs fused with carcinoma cells
Fused DCs were generated as described previously.Citation33 Briefly, DCs were harvested at day 6. DCs were mixed with carcinoma cells treated with 10 mg/mL mitomycin C (Sigma-Aldrich) for 20 minutes at a 3:1 ratio, and were incubated in serum-free RPMI 1640 containing 50% polyethylene glycol (Sigma-Aldrich) for 7 minutes. After slowly diluting with serum-free RPMI 1640, the cells were washed and resuspended in RPMI 1640 supplemented with 10% FCS, 25 μg/mL GM-CSF, and 10 μg/mL IL-4. DCs cocultured with carcinoma cells at the same ratio in RPMI 1640 supplemented with FCS, GM-CSF, and IL-4 were used as “nonfused DCs.” The next day, fused DCs and nonfused DCs were induced to mature with 1 μg/mL LPS for 24 hours. To determine fusion efficiency, DCs were labeled with PKH26 red fluorescent dye, and tumor cells were labeled with PKH67 green fluorescent dye prior to cell fusion. Individual cells or fused DCs were analyzed by flow cytometry and fluorescence microscopy. As a control, DCs generated from PBMCs and stimulated with LPS were examined which were used as “DCs alone.”
Flow cytometry analysis
Cells were washed with 0.01 M phosphate-buffered saline, pH 7.4 and incubated with antibodies directed against MHC class I (W6/32; mouse IgG2aK; Dako, Glostrup, Denmark), MHC class II (HLA-DR) (mouse IgG1; Nichirei, Tokyo, Japan), CD40 (LOB-11; mouse IgG1; Santa Cruz Biotechnology, Santa Cruz, CA, USA), CD80 (MAB104; mouse IgG1; Immunotech, Marseille, France), and CD86 (BU63; mouse IgG1, Ylem, Roma, Italy) for 30 minutes on ice. After washing with phosphate-buffered saline, the cells were incubated with fluorescent isothiocyanate (FITC)-conjugated goat anti-mouse IgG (Beckman Coulter, Marseille, France) for 30 minutes on ice. In the case of FITC-conjugated antibody against ligand (Beckman Coulter), cells were incubated for 30 minutes on ice. The appropriate respective mouse isotype controls (Dako) were employed. Samples were washed fixed in 4% paraformaldehyde, and analyzed by FACS-Calibur and CellQuest software (BD, Franklin Lakes, NJ, USA).
Cytotoxicity assay
Fused DCs, nonfused DCs, and DCs alone were cocultured with autologous nonadherent PBMCs in a 1: 20 ratio, in the presence of 25 units/mL IL-2. Fifty percent of the medium was replaced on days 3 and 5, and the cultures were restimulated by freshly isolated DCs with culture medium containing 25 units/mL IL-2 on day 7. On day 14, the stimulated PBMCs were harvested and washed three times in serum-free medium and applied as effectors at various effector/target ratios. Pancreatic carcinoma cells as target cells (1 × 10Citation4) were cocultured with effector cells for 4 hours at 37°C. To measure lactate dehydrogenase (LDH) release from targets, the supernatants were harvested and cytotoxicity was examined using the LDH Cytotoxicity Detection Kit (Takara Bio, Tokyo, Japan) as described previously.Citation34 Briefly, 100 μL of LDH substrate was added to the 96-well flat-bottomed plate and incubated for 30 minutes at room temperature. The optical absorbance of red formazan was determined spectrophotometrically at a wavelength of 490 nm. Spontaneous release of LDH by effectors or targets was assessed by incubation of the effectors or targets alone, respectively. Maximum release of LDH was determined by incubation of the targets in 1% Triton X-100. The percentage of specific cytotoxicity was calculated according to the following formula:
To obtain DCs and PBMCs from peripheral blood this study was carried out in accordance with the Declaration of Helsinki and the Ethics Code for Human Experimentation, Yamagata University School of Medicine, Yamagata, Japan. Institutional review board approval was obtained and all donors signed informed consent.
Quantification of cytokine secretion
The supernatants of the cocultures were harvested 48 hours after the second stimulation with fused DCs, and the concentrations of human cytokines, interferon-γ, IL-10 (Endogen, Rockford IL, USA), TGF-β1, and PGE2 (R&D Systems, Minneapolis, MN, USA) were quantifed by enzyme-linked immunosorbent assay (ELISA). In other experiments, the supernatants of the media were harvested from 48-hour-cultured respective pancreatic carcinoma cells, DCs, and fused DCs at a density of 2 × 105/mL in 6-well plate, and the concentrations of human cytokines, vascular endothelial growth factor (VEGF) (Endogen), IL-10, TGF-β1, and PGE2 were quantifed by ELISA.
Reverse transcription-polymerase chain reaction (RT-PCR) for IL-21
RNA was extracted from pancreatic carcinoma cells and PBMCs using RNeasy Mini Kit (Qiagen, Hilden, Germany) and was reverse-transcribed by QIAGEN OneStep RT-PCR Kit (Qiagen) according to the manufacturer’s instructions. Amplification was performed in a total volume of 25 μL for 35 cycles of 30 seconds at 94°C, 30 seconds at 54°C, and 30 seconds at 72°C. PCR products were resolved by electrophoresis on 2% agarose gels (Funakoshi, Tokyo, Japan), the bands were visualized by ethidium bromide (Bio-Rad Laboratories, Hercules, CA, USA) staining, and photographs of the gels were taken. The positive control for the expression of IL-21 mRNA was RNA from normal PBMCs treated in anti-CD3-coated plates for 24 hours.
Primers used to detect IL-21 mRNA (GenBank accession number: BC066258) were 5′-ACAGACTA ACATGCCCTTCA-3′ for the forward primer, and 5′-TCTTCACTTCCGTGTGTTCT-3′ for the reverse primer, which produced PCR products of 134 bps. IL-21 primers were synthesized by Nihon Gene Research Laboratories (Miyagi, Japan). Primers used to detect β-actin mRNA were 5′-GATCAGCAAGCAGGAGTATG-3′ for the forward primer, and 5′-GGCCATTCTCCTTAGAGAGA-3′ for the reverse primer, which produced PCR products of 390 bps.
Analysis of CD4+CD25high T cells in cocultured PBMCs
PBMCs cocultured with fused DCs or DCs alone for 14 days were washed and incubated with a FITC-conjugated antibody against CD4 (13B8.2; mouse IgG1; Beckman Coulter) and a phycoerythrin-conjugated antibody against CD25 (B1.49.4; mouse IgG2aK; Immunotech) for 30 minutes on ice. Mouse isotype controls were employed. Cells were washed again and analyzed by flow cytometry using a live gate set around viable lymphocytes based on their forward scatter/side scatter characteristics. As a control, CD4+CD25high T cells in PBMCs before coculture were analyzed.
Expression of FOXP3 and IL-10 in cocultured PBMCs
Cocultured PBMCs were immunostained for FOXP3 to recognize CD4+CD25high Tregs. Briefly, PBMCs cocultured with fused DCs or DCs alone for 14 days were washed and cytospins from PBMCs were air-dried and fixed for 10 minutes in acetone. Endogenous peroxidase activity was blocked with methanol containing 0.3% hydrogen peroxide. Monoclonal antibody anti-FOXP3 (ab20034; mouse IgG1; Abcam, Cambridge, England) was incubated at 4°C overnight on the cytospin slides. The labeled streptavidin-biotin-peroxidase method (Ultratech HRP Streptavidin-biotin Universal Detection System; Dako) was used and a positive reaction was detected as a brown color with 3,3′-diaminobenzidine (Dojin Chemicals, Kumamoto, Japan). The slides were counterstained with 1% methyl green (Muto Pure Chemicals, Tokyo, Japan). Positive cells were counted in at least 50 fields under a light microscope (x200) by two observers independently. As a control, FOXP3+ T cells in PBMCs before coculture were analyzed respectively. Next, phycoerythrin-conjugated antibody against IL-10 (JES3-19F1; rat IgG2a; PharMingen, San Diego, CA, USA) was incubated at 4°C overnight on the cytospin slides. The number of positive cells was counted in at least 50 fields under a fluorescence microscopy (×200).
Statistical analysis
Data were expressed as the mean ± standard deviation. Statistical significance was determined by the Student’s t-test for paired data using StatView-software (Abacus Concepts, Berkeley, CA, USA). Differences were considered statistically significant for P < 0.05.
Results
Characterization of DCs fused with pancreatic carcinoma cells
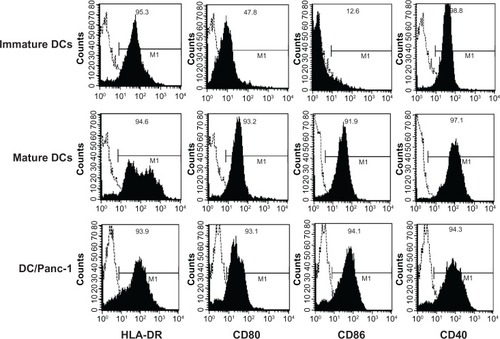
Fusion efficiency of DCs labeled with PKH26 and carcinoma cells labeled with PKH67 was confirmed by fluorescence microscopy. The population of fused DCs was 43.9% ± 4.85% of total cells by flow cytometry. The expression of MHC class II and costimulatory molecules on DCs was then analyzed by flow cytometry. Unstimulated (immature) nonfused DCs strongly expressed MHC class II (HLA-DR) and CD40, and low levels of CD80 and CD86 (). Nonfused DCs stimulated by LPS (mature DCs) strongly expressed MHC class II and costimulatory molecules such as CD80, CD86, and CD40. The immunophenotype of fused DCs (Panc-1 [], KP-1NL, KP-3L, and QGP-1 [data not shown]) was similar to that of mature DCs.
Figure 1 Flow cytometric characterization of dendritic cells (DCs) fused with pancreatic carcinoma cells.
Abbreviation: MHC, major histocompatibility complex.
Induction of cytotoxicity against pancreatic carcinoma cell lines
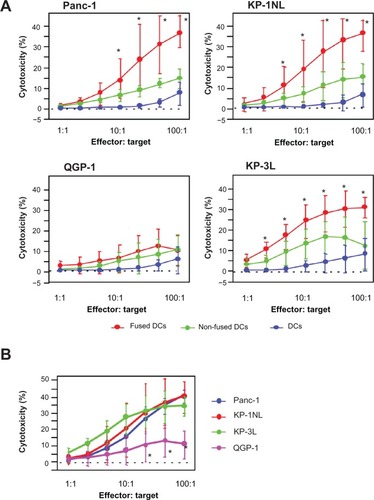
To assess the induction of antitumor immune response by fused DCs against the pancreatic carcinoma cell lines, fused DCs were cocultured with autologous PBMCs. As a control, PBMCs were also cocultured with nonfused DCs or DCs alone. PBMCs cocultured with DCs fused with Panc-1 (DC/Panc-1), KP-1NL (DC/KP-1NL), or KP-3L (DC/KP-3L) induced significant cytotoxicity against tumor targets compared with those cocultured with DCs alone (P < 0.05; ). By contrast, PBMCs cocultured with DCs fused with QGP-1 (DC/QGP-1) induced only a low level of cytotoxicity and there was no significant difference between fused DCs and controls (nonfused and DCs alone). Furthermore, when the cytotoxicity of PBMCs cocultured with fused DCs was compared among pancreatic carcinoma cell lines, the level of cytotoxicity in DC/QGP-1 was significantly lower compared with that of other carcinoma cell lines (P < 0.05; ).
Figure 2 Cytotoxicity against the respective tumor target of peripheral blood mononuclear cells (PBMCs) cocultured with dendritic cells (DCs) fused with pancreatic carcinoma cells, such as Panc-1, KP-1NL, QGP-1, and KP-3L. PBMCs were harvested from the cocultures with fused DCs, nonfused DCs, and DCs alone. Cytotoxicity induced by PBMCs was analyzed to use with lactate dehydrogenase (LDh) cytotoxicity detection kit. (A) Cytotoxicity of PBMCs cocultured with fused DCs, nonfused DCs, and DCs alone. (B) Cytotoxicity of PBMCs cocultured with fused DCs compared among pancreatic carcinoma cell lines. (B) Is an amalgam of separate components of (A).
Secretion of immunoregulatory cytokines from pancreatic carcinoma cell lines
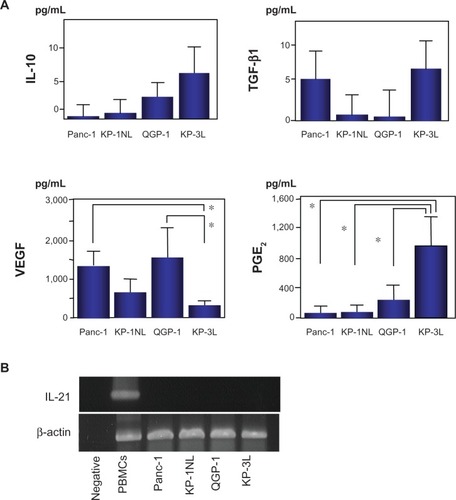
It was investigated whether or not the four carcinoma cell lines produced immunoregulatory cytokines, such as IL-10, TGF-β1, VEGF, and PGE2 by ELISA. IL-10 and TGF-β1 were secreted at very low levels from the four carcinoma cell lines (). A greater amount of VEGF, which suppresses the differentiation and maturation of DCs, was secreted from Panc-1 and QGP-1 compared with KP-3L (P < 0.05). PGE2 was secreted from KP-3L at a higher level than from the other cell lines. IL-21 mRNA, that likely suppressed the maturation of DCs and failed to induce antigen-specific T cell proliferation, was not expressed in any tumor cells by RT-PCR (). Moreover, to confirm the maturation of fused DCs, the expression of MHC class II and costimulatory molecules, such as CD80, CD86, and CD40, on DC/QGP-1 or DC/KP-3L was investigated by flow cytometry. The expression of these molecules was increased, consistent with the immunophenotype of mature DCs, and no suppression of the maturation of fused DCs was observed.
Figure 3 (A) Immunoregulatory cytokine secretion from pancreatic carcinoma cell lines and dendritic cells (DCs). Forty-eight hours after suspending carcinoma cells and DCs at a density of 2 × 105/mL in the 6-well plate, the supernatants of the medium were harvested, and quantifed for secreted interleukin-10 (IL-10), transforming growth factor (TGF)-β 1, vascular endothelial growth factor (VEGF), and prostaglandin E2 (PGE2) by enzyme linked immunosorbent assay (ELISA). results are expressed as the mean ± standard deviation (SD) of four independent experiments, and compared by Student’s t-test. *P < 0.05. (B) IL-21 mRNA by reverse transcription-polymerase chain reaction (RT-PCR).
Secretion of immunoregulatory cytokines from PBMCs cocultured with fused DCs
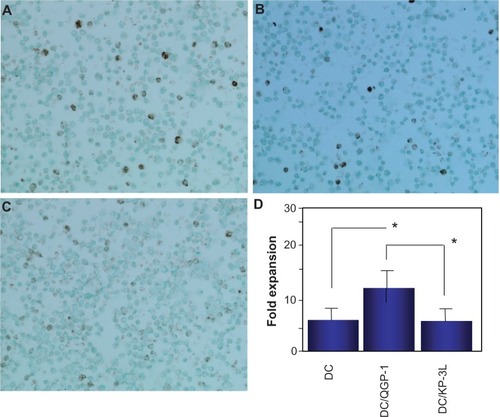
ELISA was performed to assess interferon-γ, IL-10, TGF-β1, and PGE2 secretion in the supernatants of PBMCs 48 hours after the second stimulation by DCs fused with QGP-1, as a cell line that induced a low level of cytotoxicity, or DCs fused with KP-3L, as a cell line that induced a high level of cytotoxicity. As a control, cytokines in the supernatants of PBMCs cocultured with DCs alone were assessed. Interferon-γ secretion in PBMCs cocultured with DC/QGP-1 or DC/KP-3L was significantly higher than that in DCs alone (P < 0.05; ). There was no significant difference between DC/QGP-1 and DC/KP-3L. IL-10 secretion in PBMCs cocultured with DC/QGP-1 was significantly higher than that with DC/KP-3L and DCs alone (P < 0.05). The level of TGF-β1 secretion was not significantly different among the three experimental groups. PGE2 secretion in the PBMCs cocultured with DC/QGP-1 or DC/KP-3L was increased significantly, compared with that with DCs alone. There was no significant difference between DC/QGP-1 and DC/KP-3L. To investigate whether PBMCs cocultured with DC/QGP-1 secreted IL-10, cocultured PBMCs were immunofluorescently stained with anti-IL-10 antibody. The number of IL-10+ cells in PBMCs cocultured with DC/QGP-1 was significantly increased compared with that with DC/KP-3L and DCs alone (P < 0.05; ).
Figure 4 Immunoregulatory cytokine secretion from peripheral blood mononuclear cells (PBMCs) cocultured with dendritic cells (DCs) fused with QGP-1 (DC/QGP-1), DCs fused with KP-3L (DC/KP-3L), or DCs alone.
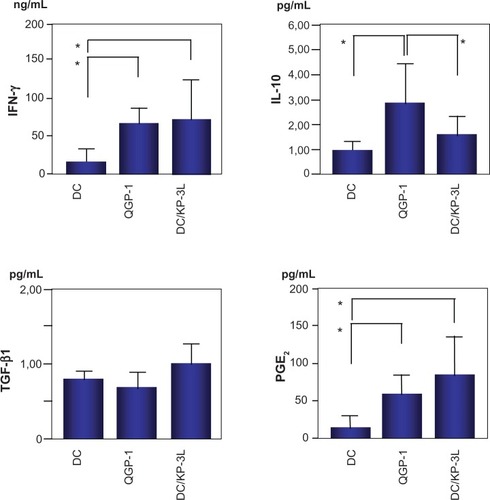
Figure 5 The induction of interleukin-10 (IL-10+) cells in the peripheral blood mononuclear cells (PBMCs) cocultured with dendritic cells (DCs). IL-10+ cells in PBMCs cocultured with DCs fused with QGP-1 (DC/QGP-1) (A), KP-3L (DC/ KP-3L) (B), or DCs alone (C) (x200). The number of positive cells was counted in at least 50 fields under a light microscopy. The number of IL-10+ cells after coculture with DC/QGP-1 was increased. results are expressed as the mean ± standard deviation (SD) of five independent experiments (D).
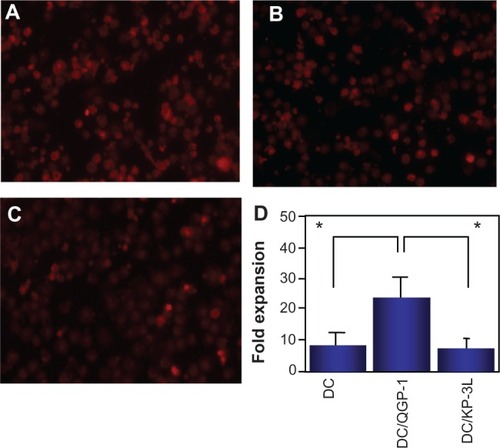
Expansion of CD4+CD25high Tregs
The relative expansion of Tregs in PBMCs cocultured with DC/QGP-1 and DC/KP-3L for 14 days was compared with that in the PBMCs before coculture. CD4+CD25high Tregs in PBMCs cocultured with DC/QGP-1 were significantly increased compared with those in PBMCs cocultured with DC/KP-3L and DCs alone by flow cytometry (CD4+CD25high) () and immunocytochemistry (FOXP3) ().
Figure 6 CD4+CD25high T cells in peripheral blood mononuclear cells cocultured with DC/QGP-1 were significantly increased compared with those in peripheral blood mononuclear cells cocultured with DC/KP-3L and DCs alone.
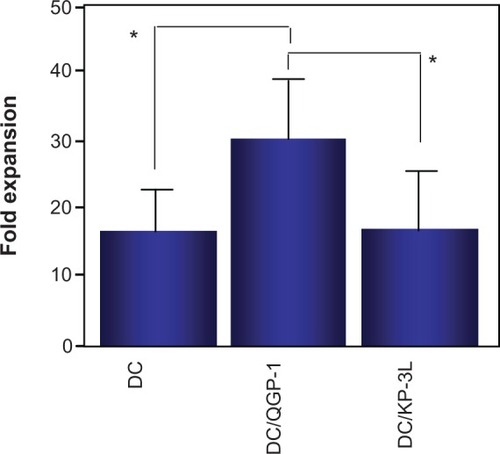
Figure 7 The induction of FOXP3+ cells in the peripheral blood mononuclear cells (PBMCs) cocultured with dendritic cells (DCs). FOXP3+ cells in PBMCs cocultured with dendritic cells (DCs) fused with QGP-1 (DC/QGP-1) (A), DCs fused with KP-3L (DC/KP-3L) (B), or DCs alone (C) (×200). The number of positive cells was counted in at least 50 fields under a light. FOXP3+ cells in PBMCs cocultured with DC/QGP-1 were significantly increased compared with those in PBMCs cocultured with DC/KP-3L and DCs alone (*P < 0.05). results are expressed as the mean ± standard deviation (SD) of five independent experiments (D).
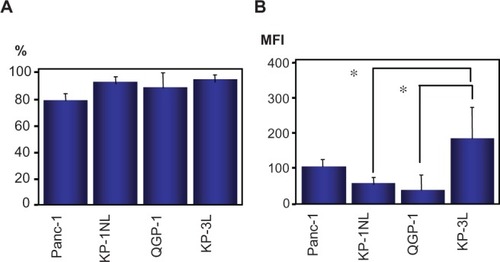
Expression of MHC class I in pancreatic carcinoma cell lines
The expression of MHC class I in carcinoma cell lines was evaluated by flow cytometry. Almost all tumor cells in the cell lines were positive for MHC class I antigen () and there was no significant difference among cell lines. By contrast, from the perspective of mean fluorescent intensity, MHC class I antigen in KP-1NL and QGP-1 was significantly lower compared with that in KP-3L (P < 0.05; ).
Figure 8 Expression of major histocompatibility complex (MHC) class I in pancreatic carcinoma cell lines by flow cytometry. Results are expressed as percentage of positive cells (A), or as mean fluorescence intensity (MFI) (B).
Expression of Fas ligand in pancreatic carcinoma cells
Fas ligand was not detectable in carcinoma cells by flow cytometry (data not shown).
Discussion
The current study firstly investigated whether DCs fused with pancreatic carcinoma cells induced cytotoxicity against the tumor targets in four human pancreatic carcinoma cell lines, and examined the contribution of immunosuppressive factors against the cytotoxicity of PBMCs cocultured with fused DCs among cell lines.
This study demonstrated that DC/Panc-1, DC/KP-1NL, and DC/KP-3L significantly induced cytotoxicity against the tumor targets compared with DCs alone, excluding DC/QGP-1. When the cytotoxicity induced by fused DCs was compared among the pancreatic carcinoma cell lines, the level of cytotoxicity in QGP-1 was significantly lower compared with that in the other cell lines.
Recent studies have demonstrated that the mechanisms of tumor escape from immune recognition/destruction are likely to be multifactorial, such the downregulation of MHC class I molecules, loss of tumor antigens, defective death receptor signaling, production of immunosuppressive cytokines, and the existence of suppressive cells.Citation18 To reveal the factors that suppressed cytotoxicity of PBMCs cocultured with fused DCs, we examined these factors among pancreatic carcinoma cell lines and demonstrated that significantly higher levels of VEGF were detected in the supernatants of Panc-1 and QGP-1 compared with KP-3L. The previous study indicated that caudal related homeobox gene 2 (CDX2), an intestine-specific tumor suppressor gene, was expressed at a high level in QGP-1 cells, and cell proliferation and cyclin D1 mRNA level were inhibited significantly after CDX2 transfection in pancreatic cancer cells, suggesting that CDX2 might play a role in inhibiting cell proliferation and repressing cyclin D1 transcriptional activity.Citation35 However, in our study there were not any significant differences in induction of cytotoxicity among DCs alone, nonfused DCs, and fused DCs (DC/QGP-1) (). Thus, the immunosuppressive potential of DC/QGP-1 may be due to only “active” in the context of professional antigen presentation from immune cells rather than an intrinsic property of QGP-1. VEGF is produced by many tumors and is important not only for tumor vascularization, but is also a key factor produced by solid tumors to inhibit immune recognition and to prevent DC differentiation and maturation.Citation36 Furthermore, to examine whether increased production of VEGF was associated with the suppression of cytotoxicity against pancreatic carcinoma cell lines, this study investigated the expression of MHC class II and costimulatory molecules in DC/QGP-1 or DC/KP-3L by flow cytometry. The expression of these molecules was similar to the expression of surface antigens in mature DCs. DC/QGP-1 and DC/KP-3L matured sufficiently to induce the immune response. Independently, expression of MHC class I antigens in KP-1NL and QGP-1 was significantly downregulated compared with KP-3L, when these results were expressed as fluorescent intensity. Downregulation of MHC class I was recognized in QGP-1 as well as KP-1NL, which induced a higher degree of cytotoxicity. MHC class I molecule down-regulation occurs frequently in many cancers, and the altered phenotypes of MHC class I antigen expression can allow tumors to avoid recognition or survive attack by cytotoxic CD8+ T-cells.Citation37 Kasuya et al have also demonstrated that the hypervascular pancreatic tumor, QGP-1 secreted a higher level of VEGF under a hypoxic environment than the hypovascular pancreatic ductal cell lines (BxPC-3 and AsPC-1).Citation38 These findings did not demonstrate that high secretion of VEGF and downregulation of MHC class I could be related to the immunosuppression of DC/QGP-1, despite a significant difference between QGP-1 and KP-3L. Furthermore, there was the elevated cytotoxicity of the nonfused DCs/tumor cell mixture (). Nonfused DCs/tumor cell mixture may recognize a low level of soluble antigens secreted from tumor cells or surface antigens expressed weakly on the cell surface of tumor cells.
Recent studies have reported that CD4+CD25high Tregs are immunoregulatory and are important in immunological tolerance to self-antigens.Citation19—Citation22 The present study demonstrated that CD4+CD25high Tregs in PBMCs cocultured with DC/QGP-1 were significantly increased compared with those in PBMCs cocultured with DC/KP-3L or DCs alone by flow cytometry (CD4+CD25high) and immunocytochemistry (FOXP3). To investigate whether Tregs induced an immunosuppressive cytokine profile, the secretion of IL-10 and TGF-β1 was subsequently analyzed in the supernatants of PBMCs cocultured with fused DCs. There was no significant difference in the level of TGF-β1 secretion among the three experimental conditions. Although a recent study demonstrated the induction of Treg function in CD4+ T cells by PGE2 secreted from carcinoma cells and the present study revealed that PGE2 was secreted to a much higher level in the supernatants of KP-3L but not QGP-1 in the supernatants of PBMCs cocultured with DC/QGP-1 or DC/KP-3L compared with that in DCs alone, there was no significant difference between DC/QGP-1 and DC/KP-3L. Cytokines (IL-10 and TGF-β), immature myeloid DCs, CpG-ODN stimulated plasmacytoid DCs, and immunomodulatory drugs (vitamin D3) can induce CD4+CD25– T-cells to differentiate into CD4+CD25+ T cells.Citation19–Citation22,Citation39 In this study, IL-10 secretion in PBMCs cocultured with DC/QGP-1 was significantly higher than that with DC/KP-3L or DCs alone, suggesting that the source of increasing IL-10 may include DCs functionally altered by fusion mechanism and IL-10 secreting Tregs such as CD4+CD25high T cells and CD4+ type 1 Tregs.Citation40 Subsequently, increasing IL-10 may be related to the expansion of Tregs and the induction of the immunosuppressive response. Leao et alCitation24 demonstrated that effective depletion of Treg cells allows the recruitment of mesothelin-specific CD8+ T cells to the antitumor immune response against a mesothelin-expressing mouse pancreatic adenocarcinoma. Yamamoto et alCitation41 also reported that Treg depletion combined with DC/tumor cell fusion hybrid vaccine enhanced the efficacy of immunotherapy in pancreatic cancer by activating CTLs. Similar effectiveness of Treg depletion in DC vaccination has been indicated in eradication of lymphoma cells in mice.Citation42
Although the overall 5-year survival for patients of pancreatic adenocarcinoma is only 1%–4%, that of malignant pancreatic endocrine carcinoma was 49%.Citation43 DC-based vaccination against pancreatic endocrine carcinoma with liver metastases revealed a decreased tumor marker level and a tumor regression of metastases.Citation44 However, information about effects of DC-based immunotherapy against pancreatic endocrine carcinoma is quite limited. Thus, it is critical to compare the difference in induction of cytotoxicity between pancreatic ductal carcinoma and endocrine carcinoma, as shown in this study.
In conclusion, the present study firstly demonstrated that there were significant differences in the induction of CTL and CD4+CD25high Tregs, and the amount of IL-10 among DCs fused with different pancreatic carcinomas. These findings suggest that the reduced cytotoxicity of DC/QGP-1 may be related to the increased secretion of IL-10 and the extensive induction of CD4+CD25high Tregs.
Acknowledgments
We thank the Yamagata Red Cross Blood Center (Yamagata, Japan) for providing the buffy coat of peripheral blood samples. We also thank Mr Suzuki H and Ms Abe K of the Department of Pathology for their technical support, and Dr Okumoto K of the Department of Internal Medicine for providing β-actin primers in Yamagata University School of Medicine.
Disclosure
The authors report no conflicts of interest in this work.
References
- StiftAFriedlJDubskyPDendritic cell-based vaccination in solid cancerJ Clin Oncol200321113514212506182
- NakamuraMWadaJSuzukiHTanakaMKatanoMMorisakiTLong-term outcome of immunotherapy for patients with refractory pancreatic cancerAnticancer Res200929383183619414316
- SusoEMDuelandSRasmussenAMhTERT mRNA dendritic cell vaccination: complete response in a pancreatic cancer patient associated with response against several hTERT epitopesCancer Immunol Immunother201160680981821365467
- BauerCDauerMSarajSDendritic cell-based vaccination of patients with advanced pancreatic carcinoma: results of a pilot studyCancer Immunol Immunother20116081097110721547597
- NakamuraIKanazawaMSatoYClinical evaluation of dendritic cells vaccination for advanced cancer patients at Fukushima Medical UniversityFukushima J Med Sci2012581404822790891
- PecherGHäringAKaiserLThielEMucin gene (MUC1) transfected dendritic cells as vaccine: results of a phase I/II clinical trialCancer Immunol Immunother20025111–1266967312439613
- NagarajSZiskeCStrehlJMessmerDSauerbruchTSchmidt-WolfIGDendritic cells pulsed with alpha-galactosylceramide induce anti-tumor immunity against pancreatic cancer in vivoInt Immunol20061881279128316772371
- NagarajSNeumannJWinzenBPancreas carcinoma antigen fused to invariant chain elicits T-cell response and tumor growth inhibitionPancreas200837332132718815556
- LepistoAJMoserAJZehHA phase I/II study of a MUC1 peptide pulsed autologous dendritic cell vaccine as adjuvant therapy in patients with resected pancreatic and biliary tumorsCancer Ther20086B95596419129927
- BéraudECollignonAFranceschiCOliveDLombardoDMasEInvestigation of a new tumor-associated glycosylated antigen as target for dendritic cell vaccination in pancreatic cancerOncoimmunology201211566122720212
- HirookaYItohAKawashimaHA combination therapy of gemcitabine with immunotherapy for patients with inoperable locally advanced pancreatic cancerPancreas2009383e69e7419276867
- BauerCBauernfeindFSterzikADendritic cell-based vaccination combined with gemcitabine increases survival in a murine pancreatic carcinoma modelGut20075691275128217395611
- DauerMHertenJBauerCChemosensitization of pancreatic carcinoma cells to enhance T cell-mediated cytotoxicity induced by tumor lysate-pulsed dendritic cellsJ Immunother200528433234216000951
- BanchereauJPaluckaAKDhodapkarMImmune and clinical responses in patients with metastatic melanoma to CD34+ progenitor-derived dendritic cell vaccineCancer Res200161176451645811522640
- BoghossianSRobinsonSVon DelwigAManasDWhiteSImmunotherapy for treating metastatic colorectal cancerSurg Oncol2012212677721292476
- AviganDRosenblattJKufeDDendritic/tumor fusion cells as cancer vaccinesSemin Oncol201239328729522595051
- XuFYeYJLiuWKongMHeYWangSDendritic cell/tumor hybrids enhances therapeutic efficacy against colorectal cancer liver metastasis in SCID miceScand J Gastroenterol201045670771320205622
- SadunRESachsmanSMChenXImmune signatures of murine and human cancers reveal unique mechanisms of tumor escape and new targets for cancer immunotherapyClin Cancer Res200713134016402517606736
- LeDTJaffeeEMRegulatory T-cell modulation using cyclophosphamide in vaccine approaches: a current perspectiveCancer Res201272143439344422761338
- SchmidtAOberleNKrammerPHMolecular mechanisms of tregmediated T cell suppressionFront Immunol201235122566933
- RaynorJLagesCSShehataHHildemanDAChougnetCAHomeostasis and function of regulatory T cells in agingCurr Opin Immunol201224448248722560294
- FacciabeneAMotzGTCoukosGT-regulatory cells: key players in tumor immune escape and angiogenesisCancer Res20127292162217122549946
- BaratelliFLinYZhuLProstaglandin E2 induces FOXP3 gene expression and T regulatory cell function in human CD4+ T cellsJ Immunol200517531483149016034085
- LeaoICGanesanPArmstrongTDJaffeeEMEffective depletion of regulatory T cells allows the recruitment of mesothelin-specific CD8 T cells to the antitumor immune response against a mesothelin-expressing mouse pancreatic adenocarcinomaClin Transl Sci20081322823920357913
- ZiskeCEtzrodtPEEliuASIncrease of in vivo antitumoral activity by CD40L (CD154) gene transfer into pancreatic tumor celldendritic cell hybridsPancreas200938775876519546834
- NagarajSNeumannJWinzenBPancreas carcinoma antigen fused to invariant chain elicits T-cell response and tumor growth inhibitionPancreas200837332132718815556
- NagarajSZiskeCStrehlJMessmerDSauerbruchTSchmidt-WolfIGDendritic cells pulsed with alpha-galactosylceramide induce anti-tumor immunity against pancreatic cancer in vivoInt Immunol20061881279128316772371
- MaddenMESarrasMPJrMorphological and biochemical characterization of a human pancreatic ductal cell line (PANC-1)Pancreas1988355125283141917
- FunakoshiAKonoAGrowth inhibition of human pancreatic cancer cells by cholecystokinin receptor antagonist in tissue culture and in nude miceGastroenterol Jpn199227178821555749
- KakuMNishiyamaTYagawaKAbeMEstablishment of a carcinoembryonic antigen-producing cell line from human pancreatic carcinomaGann19807155966017227711
- IkedaYEzakiMHayashiIYasudaDNakayamaKKonoAEstablishment and characterization of human pancreatic cancer cell lines in tissue culture and in nude miceJpn J Cancer Res199081109879932172194
- RamosRNChinLSDos SantosAPBergami-SantosPCLaginhaFBarbutoJAMonocyte-derived dendritic cells from breast cancer patients are biased to induce CD4+CD25+Foxp3+ regulatory T cellsJ Leukoc Biol201292367368222636320
- GongJNikruiNChenDFusions of human ovarian carcinoma cells autologous or allogenic dendritic cells induce antitumor immunityJ Immunol200016531705171110903782
- IwashitaYGotoSTominagaMDendritic cell immunotherapy with poly(D,L-2,4-diaminobutyric acid)-mediated intratumoral delivery of the interleukin-12 gene suppresses tumor growth significantlyCancer Sci200595530330715904472
- TakahashiKHiranoFMatsumotoKAsoKHanedaMHomeobox gene CDX2 inhibits human pancreatic cancer cell proliferation by down-regulating cyclin D1 transcriptional activityPancreas2009381495719106744
- ShengKCWrightMDApostolopoulosVInfammatory mediators hold the key to dendritic cell suppression and tumor progressionCurr Med Chem201118365507551822172061
- BernalMRuiz-CabelloFConchaAPaschenAGarridoFImplication of the β2-microglobulin gene in the generation of tumor escape phenotypesCancer Immunol Immunother20126191359137122833104
- KasuyaKNagakawaYSuzukiMAnti-vascular endothelial growth factor antibody single therapy for pancreatic neuroendocrine carcinoma exhibits a marked tumor growth-inhibitory effectExp Ther Med2011261047105222977618
- UrryZChambersESXystrakisEThe role of 1α,25-dihydroxyvitamin D3 and cytokines in the promotion of distinct Foxp3+ and IL-10+CD4+ T cellsEur J Immunol201242102697270822903229
- LevingsMKGregoriSTresoldiECazzanigaSBoniniCRoncaroloMGDifferentiation of Tr1 cells by immature dendritic cells requires IL-10 but not CD25+CD4+ Tr cellsBlood200510531162116915479730
- YamamotoMKamigakiTYamashitaKEnhancement of antitumor immunity by high levels of Th1 and Th17 with a combination of dendritic cell fusion hybrids and regulatory T cell depletion in pancreatic cancerOncol Rep200922233734319578774
- MaksimowMMiiluniemiMMarttila-IchiharaFJalkanenSHänninenAAntigen targeting to endosomal pathway in dendritic cell vaccination activates regulatory T cells and attenuates tumor immunityBlood200610841298130516621963
- EhehaltFSaegerHDSchmidtCMGrützmannRNeuroendocrine tumors of the pancreasOncologist200914545646719411317
- SchottMFieldkampJLettmannMSimonDScherbaumWASeisslerJDendritic cell immunotherapy in a neuroendocrine pancreas carcinomaClin Endocrinol2001552271277