 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Aim
To review the experience and to evaluate the results of stereotactic body radiation therapy (SBRT) via helical tomotherapy (HT), for the treatment of brachytherapy-unsuitable cervical cancer.
Methods
Between September 1, 2008 to January 31, 2012, nine cervical cancer patients unsuitable for brachytherapy were enrolled. All of the patients received definitive whole pelvic radiotherapy with or without chemotherapy, followed by SBRT via HT.
Results
The actuarial locoregional control rate at 3 years was 78%. The mean biological equivalent dose in 2-Gy fractions of the tumor, rectum, bladder, and intestines was 76.0 ± 7.3, 73.8 ± 13.2, 70.5 ± 10.0, and 43.1 ± 7.1, respectively. Only two had residual tumors after treatment, and the others were tumor-free. Two patients experienced grade 3 acute toxicity: one had diarrhea; and another experienced thrombocytopenia. There were no grade 3 or 4 subacute toxicities. Three patients suffered from manageable rectal bleeding in months 11, 14, and 25, respectively. One stage I VA patient experienced fistula formation in month 3.
Conclusion
SBRT via HT provides the possibility for treatment of locally advanced cervical cancer in patients who are unsuitable for brachytherapy. Long-term follow up and enrollment of more such patients to receive SBRT via the HT technique are warranted.
Background
For patients with contraindications to intracavity brachytherapy (BT), further conformal external beam radiotherapy (EBRT), in the form of a cervical boost, may provide benefits of increasing the dose to the central pelvis.Citation1 Nevertheless, a previous study has shown that EBRT with conventional techniques used throughout the treatment course for cervical cancer is associated with poor outcomes and a high incidence of side effects.Citation2
The application of intensity-modulated radiotherapy (IMRT) to gynecologic malignancies has been reported to provide excellent planning target volume (PTV) coverage and is associated with fewer sequelae (by normal tissue sparing) than conventional radiotherapy (RT). Additionally, using these characteristics of IMRT as a final boost in gynecologic malignancies with contraindications for BT has also shown encouraging results.Citation3
Helical tomotherapy (HT), an image-guided IMRT, can deliver highly conformal dose distributions and provides a more impressive critical organ–sparing ability for cervical cancer than does IMRT.Citation4 With the uniqueness of HT, the applications of stereotactic body RT (SBRT), administered via a HT system in place of BT, could be an effective and well-tolerated treatment for cervical cancer.Citation5
Here, we report on patients with locally advanced cervical cancer that were treated with HT-guided SBRT rather than BT because clinical judgment indicated contraindications to the use of that type of conventional treatment modality.
We aimed to assess the tolerance, clinical outcomes, and toxicities.
Methods
Patient characteristics
During the period between September 1, 2008 to January 31, 2012, nine patients undergoing whole pelvic radiotherapy (WPRT) for locally advanced cervical cancer contraindicated for BT were retrospectively enrolled, with the approval of the Institutional Review Board. Staging investigations included complete history and physical examination, fiber-optic endoscopic evaluation, complete blood count, liver and renal function tests, chest X-ray, and magnetic resonance imaging (MRI) scans or computed tomography (CT) scans of the pelvic region. The disease was staged according to the International Federation of Gynecology and Obstetrics (FIGO) criteria.Citation6
Radiotherapy
Radiotherapy was administered as WPRT followed by SBRT via HT. The total dose of SBRT delivered to patients was 27-16 Gy/5–9 fractions. Weekly cisplatin, beginning on the first day of radiation, was administered during external radiation. A dose of 40 mg/m2 cisplatin (maximum dose, 70 mg) was used and administered to patients via a peripheral vein, if patients received concurrent chemoradiation therapy (CCRT).
Delineation of target volumes
All patients underwent a CT planning scan (Somatom® Plus 4 CT scanner; Siemens AG, Munich, Germany), from the diaphragm to 5 cm below the ischial tuberosities. CT with 3 mm slice thickness was taken for treatment planning. Target objects and normal structures were contoured on a PinnacleCitation3 treatment planning system (Philips Medical System, Fitchburg, WI, USA). Delineation and constraints were based on Radiation Therapy Oncology Group (RTOG) 0418 protocol, the International Commission on Radiation Units and Measurements (ICRU) Report 50 and Report 62 recommendations, and our hospital guidelines.Citation4 Briefy, the gross tumor volume was defined as all known gross disease determined from CT, clinical information, and MRI. The gross tumor volume plus a 7 mm expansion was defined as the primary tumor clinical target volume (CTV), excluding the bowel, bladder, and rectum, if they were not clinically involved. The internal target volume was defined as the volume of the vagina and paravaginal soft tissues that was in both the empty and full bladder CT scans. The PTV provided a 7 mm margin around the nodal CTV and internal target volume with three-dimension (3D) expansion. The treatment plan was carried out on the full bladder scan. Identification of the nodal CTV usually began with the identification of the iliac vessels down to the level of S3. The average margin was 7 mm. Bone, iliopsoas muscle, and the intraperitoneal small bowel were excluded from the nodal CTV. Approximately 1.5 cm of tissue anterior to the S1-3 sacral segments was usually added to the CTV in order to include the presacral lymph nodes and uterosacral ligaments. The CTV of the nodes ended 7 mm from L4/L5 interspace, to account for the PTV. The PTV for nodes was stopped at the L4/L5 interspace. The lateral margin of the vaginal PTV extended to the obturator muscle, and at least 3 cm of the vagina needed to be treated. The 90% isodose surface covered between 95% and 98% of the PTV 50.4; volumes of overdose exceeding 115% < 5% of the PTV 50.4 volume could be considered acceptable.
Normal structures were contoured using the full-bladder CT scan. Dose-volume constraints for normal tissues were as follows: small bowel (2 cm above the most superior vessel contour) ,30% to receive ≥ 40 Gy; rectum < 60% to receive ≥ 30 Gy; bladder < 35% to receive ≥ 45 Gy; femoral head ≤ 15% to receive ≥ 30 Gy; pelvic bone marrow, V10 < 95% and V20 < 76%.
SBRT via HT substitute for BT
The CTVboost was defined as the area of residual tumor and gross disease determined from primary CT or MRI which will be boosted by SBRT. The PTVboost provided a 5 mm margin around the CTVboost with 3D expansion. The treatment plans were carried out with a full bladder scan and with, or without, rectal balloon (30–40 cc) insertion. All patients received megavoltage CT (MVCT) scanning every time before SBRT treatment. Patients were treated every day or every other day after WPRT was completed.
Toxicity
Interruptions in RT were at times necessitated by uncontrolled diarrhea or other acute complications. If RT was held, then chemotherapy was also held. Chemotherapy stopped at the completion of RT. Radiation was only stopped in cases of grade 4 hematologic or nonhematologic toxicity and until toxicity was resolved to at least grade 3; however, cisplatin was withheld in any case involving grade 3 toxicity until the toxicity regressed to any grade of <3, and in patients with grade 3 toxicity that persisted > 2 weeks, chemotherapy was no longer administered.
Determination of organs at risk (OARs) dose and complications
The mean and maximum doses for the bladder, intestine, and rectum were recorded and summed with the previous plan for evaluation. The resulting dose was calculated into the biological equivalent dose (BED) in 2-Gy fractions (EQD2) using a linear-quadratic model, assuming á/â ratio = 10 for tumor and á/â = 3 for OARs. Doses were normalized using this formula and were denoted by Gy3 (critical normal organs) or Gy10 (tumor). The BED for the bladder, intestine, and rectum was determined by adding the components of EBRT and SBRT The equation used in the calculation for the total mean and max BED is as follows:
(1) where Nd is the tumor or OARs dose of EBRT in Gray, d is the fraction dose of EBRT in Gray Sb is the tumor or OARs dose of SBRT in Gray, and b is the fraction dose of SBRT in GrayCitation7
Follow up
Upon treatment completion, patients were evaluated every 3 months for the first year, every 4 months during the second year, every 6 months during the third year, and annually thereafter. At each visit, a physical and pelvic examination, blood count clinical chemistry, and chest x-ray were performed. A Papanicolaou (PAP) test, CT or MRI scan, ultrasound and other imaging studies were conducted when appropriate. Suspected cases of persistent or recurrent disease were confirmed by biopsy, whenever possible. Acute and late (occurring >90 days after beginning RT) toxicities were defined and graded according to the Common Terminology Criteria for Adverse Events (CTCAE) v 3.0.Citation8
Statistical methods
Descriptive statistics (mean, median, proportions) were calculated to characterize the patient, disease, and treatment features as well as toxicities after treatment. The overall survival (OS), disease-free survival, locoregional control, and metastases-free survival rates were estimated using the Kaplan–Meier product-limit method. All analyses were performed using the Statistical Package for the Social Sciences ([SPSS] v 12.0; IBM Corporation, Armonk, NY, USA).
Results
Patient characteristics
Nine women were included. They had a median age of 68 years (range, 46–93 years) and belonged to FIGO Stage IIB to IVA (). The medium tumor volume was 41.6 (6.6–200.7) cmCitation3. During WPRT, six received CCRT and three had RT alone. The medium length of cycles of chemotherapy was 6 weeks. All of the patients were treated with WPRT, with or without chemotherapy, followed by image-guided SBRT. A total dose of 70.4–78 Gy was given to seven patients (78%). Two patients received 65.4–66.4 Gy. The mean EQD2 of the tumor, rectum, bladder, and intestines were 76.0 ± 7.3, 73.8 ± 13.2, 70.5 ± 10.0, and 43.1 ± 7.1, respectively (). The most frequent reason for being unable to perform intracavitary treatment was inability to cannulate the cervical os (44%) and the second most common was medical unsuitability or contraindication (33%). One patient was unfit for intracavitary treatment because of the risk of anaphylactic shock in anesthesia. The other patient showed unusual anatomic configurations for the uterus due to myoma, and frequent contact bleeding.
Table 1 Patients’ characteristics and received techniques
Table 2 The equivalent dose in 2-Gy fractions (EQD2), using a linear-quadratic model and assuming á/â ratio = 10 for tumor and á/â = 3 for organs at risk (OARs)
Treatment outcome
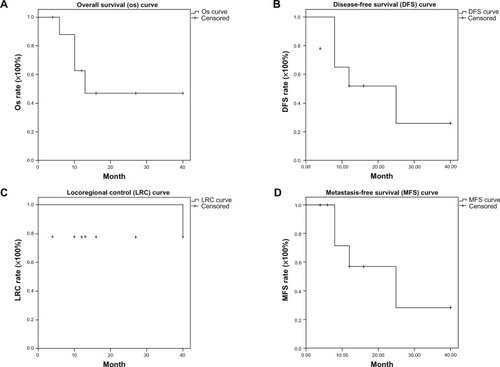
The median survival was 13 months (range, 4–40 months). The actuarial 3-year OS, disease-free survival, locoregional control, and metastases-free survival rates were 46.9%, 25.9%, 77.8%, and 28.6%, respectively (). Two stage IVA patients without concurrent chemotherapy had residual tumors after radiotherapy was completed. The others (7/9) had no locoregional recurrence upon confirmation of follow-up images and PAP. Four of nine (44%) patients experienced distant metastasis: Patient 1 had bone metastasis; Patient 5 had lung metastasis; Patient 7 had bone and lung metastasis; and Patient 9 experienced liver metastasis. Fifty-six percent of patients (5/9) were surviving at the time of this report.
Figure 1 Locally advanced cervical cancer patients received whole pelvic radiotherapy concurrent with or without chemotherapy, followed by stereotactic body radiation therapy via helical tomotherapy. (A) Overall survival curve; (B) disease-free survival curve; (C) locoregional control curve; and (D) metastases-free survival curve.
Acute and subacute toxicity
Acute toxicity and late toxicity are detailed in . One patient presented with grade 3 diarrhea and another had grade 3 thrombocytopenia during treatment. There were no grade 3 toxicities for anemia, leucopenia, nausea, vomiting, genitourinary effects, or body weight loss. There were no grade 3 or 4 subacute toxicities for hematologic, genitourinary, or gastrointestinal effects. However, 3 patients suffered from manageable rectal bleeding in months 11, 14, and 25, respectively. One stage IVA patient experienced fistula formation in month 3.
Table 3 Acute and late toxicity for patients with locally advanced cervical cancer who received whole pelvic radiotherapy concurrent with or without chemotherapy, followed by stereotactic body radiation therapy via helical tomotherapy
Discussion
There are a variety of situations in which BT cannot be carried out, such as difficult cannulation of the cervical os, anesthetic comorbidity,Citation1 or unusual anatomic configuration.Citation5 In these situations, EBRT could be an alternative to BT in cervical cancer patients.Citation1,Citation3,Citation5
Where EBRT has been used to replace BT, local recurrence has been documented in 6.6%–65% of cases, with most occurring within 3 years. Ferreira et alCitation9 reported that the locoregional failure rates for use of EBRT as replacement of BT was 65%, which was inferior to EBRT followed by BT (49%). In a 1991 report,Citation10 EBRT plus BT had a lower local failure rate (41%) than EBRT alone (67%), for stage IIIB cancer of the uterine cervix. However, other evidence showed that results of EBRT alone were comparable to the results of the combination of BT and external irradiation. In the report by Akine et al,Citation11 the local control rate for stage IIIB patients treated with EBRT was 19%. Montana et alCitation12 obtained better relapse-free survival for the combination therapy groups at 2 years (61% vs 36%), but this difference was not sustained beyond 5 years. Mollà et alCitation3 also confirmed the concept of using IMRT to deliver a final boost, and this approach might well be considered an acceptable alternative to BT. In our previous case report of pathology findings,Citation5 replacement of BT with image-guided SBRT via HT also resulted in a disease-free state, without local failure. Barraclough et alCitation1 reported that the 3-year, cancer-specific OS rate was 49% for patients who were treated with EBRT and that the local recurrence rate was 41%. Ulmer et alCitation13 used external irradiation alone in 119 patients with stage III tumors and obtained a 5-year survival rate of 30.3%. In the current study, the actuarial 3-year OS and local recurrence rates were 47%, and 22%, respectively, and with the exception of the two patients with stage IV who had residual tumors after treatment, seven of the nine (78%) patients whose treatment was delivered by SBRT had no local failure. The current results suggest the possibility of replacing BT with image-guided SBRT via HT for those patients who are unsuitable for BT treatment.
Favorable outcomes, with pelvic control rates of 69%–76%, were presentedCitation14–Citation16 with the BED 65–100.9 Gy10 (EQD2 = 54.2–84.1 Gy) at point A, with a combination of EBRT and high-dose rate BT for an advanced stage tumor of the uterine cervix. Ito et alCitation14 suggested that a 76% pelvic control rate could be achieved with the BED 65–90.4 Gy10 (EQD2 = 54.2–75.3 Gy) at point A. The University of Wisconsin also shared their experiences with the BED 85–100.9 Gy10 (EQD2 = 70.8–84.1 Gy) at point A, showing that 71% of 3-year pelvic control rates could be achieved.Citation15 Similarly, Toita et alCitation16 also confirmed that BED 70–80 Gy10 (EQD2 = 58.3–66.7 Gy) at point A could provide an impressive 3-year pelvic control rate (76%) for advanced stage diseases. In the current study, the range of BED and the mean doses of BED for tumors were 77.5–99.2 Gy10 (EQD2 = 64.6–82.7 Gy) and 91.2 ± 8.8 Gy10 (EQD2 = 76.0 ± 7.3 Gy), respectively. The 3-year locoregional control was 78%, which suggested the BED at 77.5–99.2 Gy10 (EQD2 = 64.6–82.7 Gy) might be adequate to control advanced cervical cancer in patients who cannot receive BT and in whom BT is replaced with image-guided SBRT. However, this should be evaluated with caution, since the number of cases is still limited and not all the patients in the current study were receiving CCRT.
Several investigators have analyzed probability of late complications as a function of total BED at the ICRU 38 reference points. Ranging from 97–169 Gy3 (EQD2 = 80.8–140.8 Gy), with median or mean doses for the BED of the rectum, the late complication rate was 11%–52%.Citation7,Citation16–Citation18 Ogino et alCitation17 demonstrated that the incidence of rectal complication was correlated with BED and suggested that rectal complications could decrease to less than 10% when BED does not exceed 146 Gy3 (EQD2 = 121.7 Gy). The data from Clark et alCitation18 showed that the rectum BED, without the development of complications, for the CCRT and RT alone groups was 162 Gy3 (EQD2 = 135 Gy) and 125 Gy3 (EQD2 = 104.2 Gy), respectively. Similarly, Toita et alCitation16 also suggested that the cumulative BED at the rectal reference point should be kept below 100–120 Gy3 (EQD2 = 83.3–100 Gy) to prevent rectal complication. Cheng et alCitation7 found that patients with a total maximal proximal rectal BED more than 110 Gy (EQD2 = 91.7 Gy) presented with a significantly increased frequency of Grade 2 or greater rectal complications.
Except for BED of the rectum, the total doses to the rectum have also contributed to complications. Perez et alCitation19 and Pourquier et alCitation20 reported that with doses below 75–80 Gy delivered in limited volumes, the incidence of grade 2 and 3 complications was less than 5%; however, with higher doses, the incidence of complications increased to 10%–15%. Cheng et alCitation7 recommended a proximal rectal dose < 62 Gy of a direct dose sum from WPRT and BT, to avoid an increased frequency of grade 2 or greater rectal complications. In the current study, the minimal values of mean and maximal doses of rectum for these rectal bleeding patients were 80.5 Gy3 and 137 Gy3 for BED (EQD2 = 55.3 and 97.7 Gy, respectively) and 44.3 Gy and 81.3 Gy for sum doses, respectively (). Three of the nine patients experienced manageable rectal bleeding. Toxicities are of concern and may pose a limitation for this technique. Obviously, an analysis based only on mean and maximal BED or sum doses at the rectum might be insufficient to draw conclusions; nevertheless, this should be considered and stimulate further improvement in the techniques of SBRT to decrease rectal complications, so that this can be an alternative to BT.
Calculated total BED at the bladder reference point had no significant correlation with the incidence of bladder complications.Citation16 In high-dose radiation following a course of conventionally fractionated radiation (50.4 Gy) without toxicity, the maximal limitation for bladder and intestines recommended by RTOG 9708 were 25 and 10 Gy, respectively.Citation21 In the current study, neither complication of the bladder, nor of the intestines, was noted. The maximal mean dose of the bladder and intestines for sum dose and BED Gy3 were 63.6 and 34.4 Gy, 108 and 61.7 Gy3 (EQD2 = 90.3 and 51.4), respectively. The maximal mean dose of the bladder and intestines after WPRT were 16.6 and 13.0 Gy, respectively. For the bladder and intestines, an analysis of the sum dose and maximal mean dose after conventionally fractionated radiation might be a way to evaluate the treatment plan, to avoid complications when replacing BT with SBRT via HT.
There are some limitations in our current study. First, because of the number of cases and retrospective study design, no statistical conclusions can be drawn. Second, the follow-up period was short, so long-term results and close monitoring are further required. Third, it is not easy to con-firm that the sum of maximal doses for bladder, intestine, and rectum are coming from the same points, although we tried to use the concepts of BED to provide a means for judging the correlation between doses and toxicities. Fourth, not all the patients had implanted fiducial markers and so the radiotherapy margin could not be reduced effectively, even with image-guided technique.
Conclusion
The present study effectively used image-guided SBRT via HT to provide impressive results for cervical carcinoma patients with contraindications to BT. The proposed technique may be considered an acceptable alternative to BT, by further improving on techniques and its consideration of both mean and maximal BED, and sum doses at the rectum, to avoid rectal complications. Long-term follow up is needed to confirm these preliminary findings.
Acknowledgment
This study was supported by grants (FEMH-2012-C-055; FEMH-101-2314-B418-010-MY3) from the Far Eastern Memorial Hospital, Taipei, Taiwan.
Disclosure
The authors report no conflicts of interest in this work.
References
- BarracloughLHSwindellRLivseyJEHunterRDDavidsonSEExternal beam boost for cancer of the cervix uteri when intracavitary therapy cannot be performedInt J Radiat Oncol Biol Phys200871377277818207658
- LogsdonMDEifelPJFigo IIIB squamous cell carcinoma of the cervix: an analysis of prognostic factors emphasizing the balance between external beam and intracavitary radiation therapyInt J Radiat Oncol Biol Phys199943476377510098431
- MollàMEscudeLNouetPFractionated stereotactic radiotherapy boost for gynecologic tumors: an alternative to brachytherapy?Int J Radiat Oncol Biol Phys200562111812415850911
- HsiehCHWeiMCLeeHYWhole pelvic helical tomotherapy for locally advanced cervical cancer: technical implementation of IMRT with helical tomotherapyRadiat Oncol200946220003321
- HsiehCHWeiMCHsuYPShould helical tomotherapy replace brachytherapy for cervical cancer? Case reportBMC Cancer20101063721092235
- BenedetJLEditorialInt J Gynaecol Obstet200070220720810960610
- ChengJCPengLCChenYHHuangDYWuJKJianJJUnique role of proximal rectal dose in late rectal complications for patients with cervical cancer undergoing high-dose-rate intracavitary brachytherapyInt J Radiat Oncol Biol Phys20035741010101814575832
- Protocol Development [webpage on the Internet]National Cancer Institute2006[updated December 15, 2010] Available from: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htmAccessedSeptember 1, 2008
- FerreiraPRBraga-FilhoABarlettaAIlhaLARadiation therapy alone in stage III-B cancer of the uterine cervix – a 17-year old experience in southern BrazilInt J Radiat Oncol Biol Phys199945244144610487568
- LancianoRMMartzKCoiaLRHanksGETumor and treatment factors improving outcome in stage III-B cervix cancerInt J Radiat Oncol Biol Phys1991201951001993635
- AkineYHashidaIKajiuraYCarcinoma of the uterine cervix treated with external irradiation aloneInt J Radiat Oncol Biol Phys1986129161116163759588
- MontanaGSFowlerWCVariaMAWaltonLAMackYShemanskiLCarcinoma of the cervix, stage III. Results of radiation therapyCancer19865711481543940615
- UlmerHUFrischbierHJTreatment of advanced cancers of the cervix uteri with external irradiation aloneInt J Radiat Oncol Biol Phys1983968098126863055
- ItoHKutukiSNishiguchiIRadiotherapy for cervical cancer with high-dose rate brachytherapy correlation between tumor size, dose and failureRadiother Oncol19943132402478066207
- PetereitDGSarkariaJNPotterDMSchinkJCHigh-dose-rate versus low-dose-rate brachytherapy in the treatment of cervical cancer: analysis of tumor recurrence – the University of Wisconsin experienceInt J Radiat Oncol Biol Phys19994551267127410613322
- ToitaTKakinohanaYOgawaKCombination external beam radiotherapy and high-dose-rate intracavitary brachytherapy for uterine cervical cancer: analysis of dose and fractionation scheduleInt J Radiat Oncol Biol Phys20035651344135312873679
- OginoIKitamuraTOkamotoNLate rectal complication following high dose rate intracavitary brachytherapy in cancer of the cervixInt J Radiat Oncol Biol Phys19953147257347860383
- ClarkBGSouhamiLRomanTNChappellREvansMDFowlerJ FThe prediction of late rectal complications in patients treated with high dose-rate brachytherapy for carcinoma of the cervixInt J Radiat Oncol Biol Phys19973859899939276363
- PerezCABreauxSBedwinekJMRadiation therapy alone in the treatment of carcinoma of the uterine cervix. II. Analysis of complicationsCancer19845422352466722748
- PourquierHDuboisJBDelardRCancer of the uterine cervix: dosimetric guidelines for prevention of late rectal and rectosigmoid complications as a result of radiotherapeutic treatmentInt J Radiat Oncol Biol Phys1982811188718956818192
- GrevenKWinterKUnderhillKFontenesciJCooperJBurkeTFinal analysis of RTOG 9708: adjuvant postoperative irradiation combined with cisplatin/paclitaxel chemotherapy following surgery for patients with high-risk endometrial cancerGynecol Oncol2006103115515916545437