Abstract
Purpose
To review the clinical data and treatment efficacy of bevacizumab in Chinese patients with metastatic colorectal cancer (mCRC).
Patients and methods
A total of 96 patients with mCRC treated by chemotherapy plus bevacizumab in the PLA General Hospital between December 2005 and August 2012 were analyzed retrospectively by overall response rate, disease-control rate, progression-free survival (PFS), and overall survival (OS). The tumor responses were assessed by the Response Evaluation Criteria in Solid Tumors guidelines.
Results
A total of 96 patients with mCRC were identified. Median age was 53.6 years. Eastern Cooperative Oncology Group performance status was 0–2. By the end of follow-up (August 20, 2012), 54 patients exhibited progression (56.3%), and 39 (40.6%) patients had died. A total of 27 (28.1%) achieved partial response, and 48 patients (50.0%) had stable disease, exhibiting an overall response rate of 28.1% and a disease-control rate of 78.1%. The response rates of the first-line, second-line, and third-line (or later) therapy were 41.7%, 21.9%, and 15.8%, respectively. The median durations of the PFS and OS were 8.13 months and 14.80 months, respectively. The median durations of the PFS were 12.70 months, 8.30 months, and 6.40 months for first-line, second-line, and third-line (or later) therapy, respectively, and the median durations of the OS were 24.03 months, 14.90 months, and 11.03 months for first-line, second-line, and third-line (or later) therapy, respectively.
Conclusion
A bevacizumab-containing chemotherapy regimen was well tolerated and effective in Chinese patients with mCRC.
Introduction
Colorectal cancer is the fifth most common malignancy and the leading cause of cancer death in the People’s Republic of China. Recently, the incidence of colorectal cancer has been increasing steadily, and over 100,000 deaths occur annually in the People’s Republic of China.Citation1 Currently, the standard treatment of patients with metastatic colorectal cancer (mCRC) depends on chemotherapies with fluoropyrimidines, irinotecan, and oxaliplatin. In addition, antibodies against vascular endothelial growth factor (bevacizumab), epidermal growth-factor receptor (cetuximab and panitumumab) have been shown to prolong progression-free survival (PFS) in patients with mCRC.Citation2–Citation4
Bevacizumab is a humanized monoclonal antibody that can neutralize different types of vascular endothelial growth factor.Citation5,Citation6 Many studies have shown that treatment with bevacizumab and cytotoxic chemotherapy benefits patients with mCRC,Citation7–Citation10 and bevacizumab has been recommended as the first and second line of reagent for patients with mCRC.Citation11
While the benefits of treatment with bevacizumab are well documented in Caucasian patients with mCRC, the effect and safety of treatment with bevacizumab in Chinese patients with mCRC has not been clarified. This retrospective study aimed at investigating the effect and safety of treatment with bevacizumab in Chinese patients with mCRC.
Materials and methods
Patients
This retrospective study included 96 patients with mCRC who had been treated with chemotherapy plus bevacizumab between December 2005 and August 20, 2012 in the PLA General Hospital. The inclusion criteria were: histologically confirmed mCRC; age ≥ 20 years, and a life expectancy > 3 months; Eastern Cooperative Oncology Group (ECOG) performance status of 0–2; adequate hematologic (an absolute neutrophil count > 1500/μL, hemoglobin > 9.0 g/dL, and a platelet count > 75,000/μL), hepatic (bilirubin < 2.0 mg/dL and transaminase levels < 3 times the upper normal limit), and renal functions (creatinine < 1.5 mg/dL and urinary excretion ≤ 500 mg of protein per day). The exclusion criteria were the presence of clinically significant cardiovascular disease; uncontrolled hypertension; central nervous system metastasis; major surgery within 6 weeks; pregnancy or lactation; nonhealing wounds; preexisting bleeding diatheses or coagulopathies; the need for full-dose anticoagulation.
Written informed consent was obtained from individual patients and the experimental protocol was approved by the ethical committee of the PLA General Hospital.
Treatment
Among the 96 patients included, 48 patients received bevacizumab combined with oxaliplatin-containing chemotherapy, 39 patients received bevacizumab combined with irinotecan-containing chemotherapy, and nine patients received bevacizumab combined with fluorouracil plus Leucovorin (LV). Oxaliplatin-containing chemotherapy included oxaliplatin + capecitabine (XELOX) and fluoropyrimidine + oxaliplatin (FOLFOX). Irinotecan-containing chemotherapy consisted of fluoropyrimidine + irinotecan and irinotecan alone. All of the patients were treated intravenously with 5 mg/kg bevacizumab (Avastin; Genentech, San Francisco, CA, USA) over 30–minutes every 2 weeks or 7.5 mg/kg every 3 weeks, prior to the chemotherapy ().
Table 1 Details of chemotherapy regimens
Efficacy and safety evaluation
The objective of the study was to evaluate the overall response rate (ORR), disease-control rate (DCR), overall survival (OS), PFS, and toxicity of bevacizumab in patients with mCRC treated by bevacizumab plus chemotherapy. OS was defined as the duration from the initiation of the therapy to the date of death of any cause or at the end of this experiment. PFS was defined as the duration from the initiation of the therapy to the confirmation date of progressive disease, or death of any cause.
All of the patients were included in the PFS and OS analyses if they had been treated with bevacizumab at least three times. Individual patients who had severe adverse effects but without clinical or radiographic evidence of progressive disease were taken off therapy. Patients without complete clinical data were excluded.
Tumor responses were evaluated according to the Response Evaluation Criteria in Solid Tumors guidelines.Citation12 Progression was defined as a 20% increase in the sum of diameters of the target lesions. Toxicities were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (version 3.1).Citation13
Lesions of individual patients were assessed at baseline (within 4 weeks before starting chemotherapy) and every 6–8 weeks after treatment by radiology, which consisted of a bone scan, ultrasound of lymph nodes, chest computed tomography scan, and abdominopelvic computed tomography scan.
Statistical analysis
Data are expressed as real case number and percentage and were analyzed using SPSS 17.0 (IBM, Armonk, NY, USA). Survival curves were estimated using the Kaplan–Meier method and analyzed by log-rank test. A P-value of <0.05 was considered statistically significant.
Results
A total of 96 patients with mCRC received more than three cycles of bevacizumab-plus-chemotherapy treatment. The median age of the patients in the study was 53.6 years, with 72 males and 24 females. Combined chemotherapy regimens included oxaliplatin-containing chemotherapy (50.0%), irinotecan-containing chemotherapy (40.6%), and other chemotherapy (9.4%). Primary tumors of 60 patients (62.5%) were located in the colon, the rest (36, 37.5%) in the rectum. The most common metastatic sites were liver and/or lung (38.6%). Thirty-nine (40.6%) patients had received adjuvant chemotherapy after operation. Thirty-six (37.5%) patients were treated with bevacizumab combined chemotherapy as a first-line treatment, 41 (42.7%) as a second-line treatment, and 19 (19.8%) as a third-line (or later) treatment. A median of five (range 3–19) cycles of bevacizumab were administered. Major patient demographics are summarized in .
Table 2 Baseline demographic characteristics
Efficacy
At the final cutoff date (August 20, 2012), 54 patients exhibited progression (56.3%) and 39 (40.6%) patients died. Twenty-seven patients (28.1%) achieved partial response and 48 patients (50.0%) achieved stable disease (SD), exhibiting an ORR of 28.1% (complete response and partial response) and a DCR (complete response and stable disease) of 78.1%. The response rates of for the first-line, second-line, and third-line (or later) treatments were 41.7%, 21.9%, and 15.8%, respectively (). The median follow-up for all the patients is 14.70 months. The median durations of the PFS and OS were 8.13 months and 14.80 months, respectively ( and ). The median PFSs for the first-line, second-line, and third-line (or later) treatments were 12.70, 8.30, and 6.40 months, respectively. The median OSs for the first-line, second-line, and third-line (or later) treatments were 24.03 months, 14.90 months, and 11.03 months, respectively ().
Figure 1 (A) Curve for progression-free survival in patients with metastatic colorectal cancer treated with bevacizumab combined chemotherapy; (B) curve for overall survival in patients with metastatic colorectal cancer treated with bevacizumab combined chemotherapy.
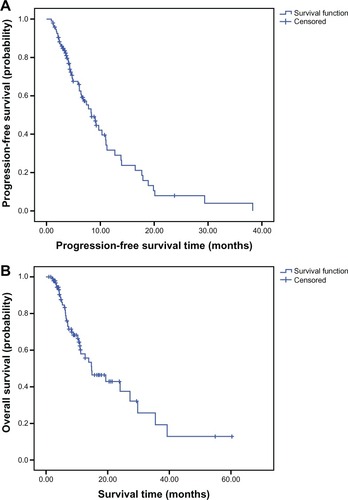
Table 3 Response to treatment
Table 4 Analysis of survival
Toxicity
There was no toxic death. Severe adverse events, such as bowel perforation, thromboembolism event, severe bleeding, or reversible posterior leukoencephalopathy syndrome, were not observed. Since we tended to withdraw the drug immediately after hypertension or hemorrhage occurred to ensure safety, three patients stopped using the drug because of the adverse events. Overall, the addition of bevacizumab to chemotherapy was well tolerated. Main adverse effects included hypertension, hemorrhage, hematochezia, poor wound healing, and thrombotic events. A total of 99 patients who received at least one dose of bevacizumab accepted safety assessment. It is worth mentioning that one patient stopped using because of uncontrolled hypertension and proteinuria, but after a 3-months interval he started again and the adverse effect became tolerable. Details of the incidence of selected adverse events are presented in .
Table 5 Selected adverse effects (total number = 99)
Discussion
Bevacizumab is a standard therapy approved for first- and second-line treatment in patients with mCRC by the FDA. Multiple clinical trials have proven the use of bevacizumab results in an improvement in PFS and OS. Approval for first-line mCRC treatment was mainly based on the supportive results of two studies: a randomized, double-blind, placebo-controlled phase III trial with 813 patients that evaluated the effect of additional bevacizumab combined with irinotecan, bolus fluorouracil, and leucovorin (IFL),Citation7 and a phase II, randomized, placebo-controlled trial that evaluated the effect of additional bevacizumab combined with fluorouracil and leucovorin,Citation8 both of which demonstrated the addition of bevacizumab to chemotherapy significantly improved PFS and OS.Citation7,Citation8
Based on these data, more clinical trials were conducted. The NO16966 study reported that PFS and OS increased in patients with mCRC treated with bevacizumab combined with XELOX or FOLFOX, which resulted in mPFS of 9.3 versus 7.4 months and mOS of 21.6 versus 19.0 months in the XELOX group, and PFS of 9.4 versus 8.6 months and mOS of 21.0 versus 18.9 months in the FOLFOX group.Citation14 Another phase III trial (CAIRO2) also demonstrated mPFS of 10.7 months and mOS of 20.3 months in the XELOX-plus-bevacizumab arm.Citation15 Although large numbers of subjects have been enrolled in clinical trials and positive results have been proved, most of the clinical trials have been performed in Western countries. Clinical trials performed in the Asian region have mostly contained small samples.Citation16 A prospective, multicenter, randomized, open-label, phase III trial conducted in the People’s Republic of China with 214 patients enrolled compared the efficacy of mIFL plus bevacizumab with mIFL alone as a first-line regimen. The results demonstrated a significant improvement in ORR (35% vs 17%), mPFS (8.3 vs 4.2 months), and mOS (18.7 vs 13.4 months).Citation17 A summary of the clinical trials investigating the efficacy of bevacizumab combined with chemotherapy in patients with mCRC is shown in .
Table 6 Summary of the clinical trials investigating the efficacy of bevacizumab combined with chemotherapy in patients with mCRC
In this present retrospective study, ORR was 28.1% for all of the patients, and mPFS and mOS were 8.13 and 14.80 months, respectively. The response rates for first-line, second-line and third-line (or later) treatments were 41.7%, 21.9%, and 15.8%, respectively.
Fifty-five of the 96 patients accepted KRAS status exam, this resulted to 28 KRAS mutations and 27 KRAS wildtype; KRAS status of the other 41 patients was unknown. Unlike cetuximab, the use of which is restricted to patients with KRAS wild-type tumors because patients with a tumor harboring a KRAS mutation are resistant to anti-epidermal growth-factor receptor therapy, the efficacy of bevacizumab is not influenced by KRAS status.Citation18,Citation19 In this study, we compared efficacy between different KRAS statuses and found no significant difference among them, which proved the status of KRAS had no prognostic value in patients using bevacizumab.
There were 21 patients also using cetuximab at some point in the continuum of care. Research has been conducted and proved that combination therapy with more than one biologic agent is not associated with improved outcomes and can cause increased toxicity. The PACCE trial demonstrated that regardless of KRAS status, the combination of bevacizumab and panitumumab with chemotherapy significantly resulted in significantly shorter PFS and inferior quality of life.Citation20 In the CAIRO2 trial, the addition of cetuximab to capecitabine, oxaliplatin, and bevacizumab showed a similar result.Citation15 A retrospective analysis provided support for a sequential use of bevacizumab, cetuximab, and three cytotoxic drugs – fluoropyrimidines, irinotecan, and oxaliplatin – was associated with increased survival, which was not found to be associated with the order in which these drugs were received.Citation21 In the present study, we compared the survival difference between patients taking both bevacizumab and cetuximab and patients taking only bevacizumab with the log-rank test. Although there was a difference in survival between these two groups, the benefit was not significant (10.30 vs 5.87 months, P = 1.03). That may have been because of the small sample size.
Conclusion
The efficacy and safety of bevacizumab plus chemotherapy in this study was basically consistent with that reported in Western patients. It could be concluded from this retrospective study that bevacizumab plus chemotherapy was effective for Chinese patients with mCRC, and the adverse events were tolerant and manageable.
Acknowledgment
This study was sponsored by Wu Jieping Medical Foundation, no 32065711201.
Disclosure
The authors report no conflicts of interest in this work.
References
- Globocan 2008Estimated cancer incidence, mortality, prevalence and disability-adjusted life years (DALYs) worldwide in 2008 Available from: http://globocan.iarc.frAccessed January 13, 2013
- KimGPSargentDJMahoneyMRPhase III noninferiority trial comparing irinotecan with oxaliplatin, fluorouracil, and leucovorin in patients with advanced colorectal carcinoma previously treated with fluorouracil: N9841J Clin Oncol2009272848285419380443
- KoopmanMAntoniniNFDoumaJSequential versus combination chemotherapy with capecitabine, irinotecan, and oxaliplatin in advanced colorectal cancer (CAIRO): a phase III randomised controlled trialLancet200737013514217630036
- TolJKoopmanMCatsARodenburgCJChemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancerN Engl J Med200936056357219196673
- WangTFLockhartACAflibercept in the treatment of metastatic colorectal cancerClin Med Insights Oncol20126193022253552
- TakahashiSVascular endothelial growth factor (VEGF), VEGF receptors and their inhibitors for antiangiogenic tumor therapyBiol Pharm Bull2011341785178822130231
- HurwitzHFehrenbacherLNovotnyWBevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancerN Engl J Med20043502335234215175435
- KabbinavarFFHambletonJMassRDHurwitzHIBergslandESarkarSCombined analysis of efficacy: the addition of bevacizumab to fluorouracil/leucovorin improves survival for patients with metastatic colorectal cancerJ Clin Oncol2005233706371215867200
- SaltzLBClarkeSDíaz-RubioEBevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III studyJ Clin Oncol2008262013201918421054
- GiantonioBJCatalanoPJMeropolNJBevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200J Clin Oncol2007251539154417442997
- CohenMHGootenbergJKeeganPPazdurRFDA drug approval summary: bevacizumab plus FOLFOX4 as second-line treatment of colorectal cancerOncologist20071235636117405901
- EisenhauerEATherassePBogaertsJNew response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1)Eur J Cancer20094522824719097774
- Cancer Therapy Evaluation ProgramCommon terminology criteria for adverse events v 3.0 (CTCAE)2006 Available from: http://www.eortc.be/services/doc/ctc/ctcaev3.pdfAccessed February 28, 2013
- CassidyJClarkeSDíaz-RubioEXELOX vs FOLFOX-4 as first-line therapy for metastatic colorectal cancer: NO16966 updated resultsBr J Cancer2011105586421673685
- TolJKoopmanMRodenburgCJA randomised phase III study on capecitabine, oxaliplatin and bevacizumab with or without cetuximab in first-line advanced colorectal cancer, the CAIRO2 study of the Dutch Colorectal Cancer Group (DCCG). An interim analysis of toxicityAnn Oncol20081973473818272912
- ToshihikoDoiNarikazuBokuKenKatoPhase I/II study of Capecitabine plus Oxaliplatin (XELOX) plus Bevacizumab as first-line therapy in Japanese patients with metastatic colorectal cancerJpn J Clin Oncol2010401091392020462981
- GuanZZXuJMLuoRCEfficacy and safety of bevacizumab plus chemotherapy in Chinese patients with metastatic colorectal cancer: a randomized phase III ARTIST trialChin J Cancer20113068268921959045
- KarapetisCSKhambata-FordSJonkerDJK-ras mutations and benefit from cetuximab in advanced colorectal cancerN Engl J Med20083591757176518946061
- LièvreABachetJBBoigeVKRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximabJ Clin Oncol20082637437918202412
- HechtJRMitchellEChidiacTA randomized phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancerJ Clin Oncol20092767228019114685
- GrotheyASargentDGoldbergRMSchmollHJSurvival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatmentJ Clin Oncol2004221209121415051767
- ParkLCLeeHSShinSHBevacizumab as a second- or later-line of treatment for metastatic colorectal cancerWorld J Gastroenterol2012181104110922416186
- LièvreASamalinEMitryEBevacizumab plus FOLFIRI or FOLFOX in chemotherapy-refractory patients with metastatic colorectal cancer: a retrospective studyBMC Cancer2009934719785749