Abstract
New treatment strategies are required for renal cell carcinoma (RCC) due to its relative insensitivity to conventional radio- and chemotherapies. The promising strategy of tumor inhibition using human telomerase reverse transcriptase (hTERT)-controlled herpes simplex virus thymidine kinase/ganciclovir (HSV-TK/GCV) in the hTERT promoter-driven HSV-TK/GCV suicide gene system was investigated. Tumor volume, weight, relative proliferation rate, and cell-apoptosis levels were examined in mice injected with adenovirus (Ad)-hTERT-HSV-TK and GCV. Increased cell death occurred following treatment with Ads carrying hTERT-HSV-TK/GCV or cytomegalovirus promoter-controlled (CMV)-HSV-TK/GCV for human RCC 786-0 and fibroblast MRC-5 cells. In mice, Ad-hTERT-HSV-TK/GCV more specifically inhibited tumor and RCC xenograft growth than Ad-CMV-HSV-TK/GCV (P < 0.05). Furthermore, Ad-hTERT-HSV-TK/GCV did not significantly damage normal fibroblasts or organ systems (heart, lung, liver, brain, kidney, and spleen). Thus, Ad-hTERT-HSV-TK/GCV is an effective RCC inhibitor in human cells in vitro and in vivo mouse models, indicating potential usefulness in RCC-targeted gene therapy.
Introduction
Kidney cancers account for 3% of all adult malignancies and 90%–95% of all kidney neoplasms worldwide, with incidence and mortality rates increasing as much as 2%–3% each decade.Citation1 As many as ten of every 100,000 people are affected, 25%–30% of patients develop treatment-resistant metastatic disease, and 40% develop postoperative recurrence.Citation1,Citation2 Clear-cell renal cell carcinoma (CCRCC) is the most prominent form of kidney cancer,Citation3,Citation4 and it commonly exhibits minimal or no sensitivity to conventional chemo- or radiotherapies.Citation5 Thus, new targeted treatments for RCC are urgently required.
Contemporary cancer gene therapies utilize immune-related, tumor-suppressor genes and drug-susceptible therapies, such as infection with herpes simplex virus thymidine kinase/ganciclovir (HSV-TK/GCV) suicide genes.Citation6 Such suicide gene therapies have the benefit of specifically targeting and selectively killing transformed tumor cells and simultaneously removing surrounding cells (“bystander effect”) with minimal systemic toxicity.Citation7 However, designing targeted suicide genes that properly distinguish between tumor and normal host cells without adversely affecting adjacent tissues and organ systems remains challenging.
Suicide genes code for highly toxic, high-catalytic-activity enzymes that are either entirely absent or expressed in very low concentrations in normal host cells.Citation8 Suicide gene therapy is commonly referred to as gene directed enzyme/prodrug therapy, including both cell-based therapies, tumor-based therapies, and viral vector therapies.Citation8,Citation9 The primary difference between these therapies is which agent (adjacent cells, the tumor itself, or a viral vector) carries the suicide gene. Due to the latency of the herpes viruses, viral TKs have been described as the ultimate target in suicide gene therapy, and numerous research studies have been conducted using the human cytomegalovirus (CMV), whose UL97 gene codes for a protein kinase that, like viral TKs, is activated by phosphorylation in various cancers.Citation10
The HSV-TK gene was first proposed for use in tumor treatment by Moolten et al,Citation11 and over 40 clinical trials have since reported positive results in non-small-cell lung cancer,Citation7 brain and spinal cord malignant gliomas,Citation12 hepatocellular carcinoma,Citation13 and ovarian tumors.Citation14 GCV is a structural analogue of the second messenger cyclic guanosine monophosphate.Citation10 Notably, the HSV-TK enzyme has 1000 × higher affinity for substrate GCV than the host cell TK, and HSV-TK has high affinity for various other nucleoside analogues of GCV, including acyclovir and penciclovir.Citation15 Thus, tumor cell death is caused by the conversion of GCV into a toxic metabolite at high concentrations in tumor cells but not in normal host cells, widely used in targeted cancer therapy.Citation8
Tumor cells are characterized by their unlimited proliferative capacity, widely believed to be associated with preservation of telomeres by activated telomerase, which is composed of an enzyme protein and RNA responsible for telomerase activation.Citation16 Telomerase is inactive in normal somatic cells and benign tumors; however, upregulation of expression of human telomerase reverse transcriptase (hTERT) has been associated with telomerase activation in numerous human cancers.Citation17 Notably, Kim et alCitation18 reported telomerase activity in 100 human tumor cell lines from 18 cancer types compared to 22 normal human somatic cell lines using telomere repeat amplification protocol, revealing a 98% positive rate.
Modern therapeutic strategies for inhibition of telomerase activity primarily target telomerase RNA and hTERT, though competitive inhibition of reverse transcription has also been explored.Citation19 Recently, the hTERT promoter has been employed to control therapeutic gene expression, thus facilitating specific targeting and destruction of tumor cells by altering telomerase activation.Citation20,Citation21 The hTERT promoter has also been used to regulate therapeutic genes, such as B-cell lymphoma 2-associated X protein, caspase 8, and suicide genes to achieve specific expression levels in telomerase-positive tumor.Citation22 Clinically, the hTERT-driven HSV-TK/GCV system has inhibited tumor growth successfully in human cell lines and animal models of breast,Citation22 pulmonary,Citation7 and ovarian cancers.Citation23 Thus, selective regulation of exogenous suicide gene expression in telomerase-positive tumor cells can dramatically improve targeted therapies.
The hTERT promoter was employed to control the adenovirus (Ad)-HSV-TK/GCV suicide gene system, exploiting the proliferative character of malignant RCC cells to achieve a recombinant Ad-hTERT-HSV-TK/GCV with high specificity and efficacy for RCC cells both in vivo and in vitro. Consistent with previous successes in other cancer types,Citation7,Citation22,Citation23 this method provides a potential tool for improving RCC patient prognosis.
Materials and methods
Cell lines and cultures
Human (hCCRCC) 786-0 and human fibroblast (hFB) MRC-5 cells were cultured at 37°C in a 5% CO2 atmosphere and 0.25% trypsin (Sigma-Aldrich, St Louis, MO, USA) for cell digestion before passaging. hCCRCC 786-0 cells were cultured in 1640 medium containing 10% fetal bovine serum (Life Technologies, Carlsbad, CA, USA) and 100 μg/mL ampicillin (Sigma). hFB MRC-5 cells were cultured in Eagle’s minimal essential medium containing 10% fetal bovine serum (Life Technologies).
Adenovirus construction
Replication-deficient recombinant adenoviral vectors pCA13 and pBGHE3 were used in Ad construction (Microbix Biosystems, Mississauga, ON, Canada). Briefly, pCA13-CMV-HSV/TK and pCA13-hTERT-HSV/TK; pCA13-CMV-HSV/TK and pCA13-hTERT-HSV/TK; pCA13-CMV-EGFP and pCA13-hTERT-EGFP; and pCA13-CMV-EGFP and pCA13-hTERT-EGFP were constructed by digesting plasmids pUC19-HSV/TK (Second Military Medical University collection); pCA13; plasmid pUC19-EGFP; and pSG-CMV-EGFP and pSG-hTERT-EGFP, respectively, with EcoRI and/or XbaI. These produced the 1143 bp HSV/TK fragment, which was ligated with digested pSG-CMV and pSG-TP (pSG-CMV-HSV/TK and pSG-hTERT-HSV/TK), fragments ligated with the digested pCA13 vector (pCA13-CMV-HSV/TK and pCA13-hTERT-HSV/TK), fragments ligated with digested pSG-CMV and pSG-TP (pSG-CMV-EGFP and pSG-hTERT-EGFP), and CMV-EGFP and hTERT-EGFP fragments ligated with digested pCA13 vector (pCA13-CMV-EGFP and pCA13-hTERT-EGFP).
Recombination and expansion of Ad-Her
Plasmids pCA13-CMV-HSV/TK and pCA13-hTERT-HSV/TK, pCA13-CMV-EGFP, and pCA13-hTERT-EGFP were cotransfected with pBGHE3 (containing the entire 5-Ad except 188–1339 bp) using Lipofectamine 2000 in 293 cells to obtain the recombinant virus. The viral plaque appeared after 9 days, and the recombinant adenovirus was obtained by purification (3×). DNA of the recombinant virus was extracted by the QIAamp DNA Kit (Qiagen, Venlo, Netherlands) and characterized by polymerase chain reaction, producing clones Ad-CMV-HSV/TK, Ad-hTERT-HSV/TK, Ad-CMV-EGFP, and Ad-hTERT-EGFP The recombinant virus and the wild-type adenovirus were characterized by polymerase chain reaction.
Virus infection
hCCRCC 786-0 cells and hFB MRC-5 cells were seeded in six-well plates at 1 × 105/well and 1 × 106/well (Thermo Fisher Scientific, Waltham, MA, USA), respectively, and incubated at 37°C in a 5% CO2 atmosphere for 24 hours. Cells were then placed in serum-free medium containing the nonproliferative recombinant Ad-CMV-EGFP and Ad-hTERT-EGFP with GFP genes at multiplicities of infection (MOIs) of 0, 1, 5, 10, 50, 100, and 200 for 2 hours followed by culture medium containing 5% serum. GFP expression was observed by fluorescence microscopy (TE300; Nikon, Tokyo, Japan) 2 and 7 days after infection.
Recombinant Ad effect on cells was determined by cell-viability assay using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), as previously described.Citation24
Mouse RCC model
Forty BALB/C nude mice aged 4 and 6 weeks were purchased from the Animal Center of the Chinese Academy of Medical Sciences and housed under sterile conditions at the Third Hospital of Hebei Medical University and used in nude mice model xenograft models of RCC. Briefly, 5 × 107/mL (0.1 mL) of 786-0 cell suspension was subcutaneously injected into the left, front dorsa of each mouse, and all mice were killed when tumor growth reached a diameter of ~6 mm.
Mice were divided randomly into four groups: A, no treatment (control group); B, intraperitoneal injection of GCV (50 mg/kg) on days 2–18; C, Ad-hTERT-HSV-TK 6.0 × 107/kg tail vein injection on days 1, 7, and 13, plus intraperitoneal injection of phosphate-buffered saline solution on days 2–18; and D, similar intravenous injection of Ad-hTERT-HSV-TK and similar intraperitoneal injection with GCV (treatment group).
Tumor diameters were measured with a vernier caliper every 3 days for 30 days. Mice were then killed and tumor weight, pathological condition, and visceral organ condition were determined. All tissues were further subjectively assessed using visual analysis by an experienced researcher, and distinctive qualitative observations were reported.
Immunohistochemical and pathological examinations
Excised tumors were dissected fixed in 10% formalin, embedded in paraffin, cut into thin slices, dewaxed, stained with hematoxylin and eosin, and sealed with resin. Expression of proliferating cell nuclear antigen (PCNA) was determined by immunohistochemical staining with mouse anti-human PCNA antibody (1:100; Abcam, Cambridge, UK) or phosphate-buffered saline in negative controls. Nuclei of PCNA-positive cells (dark brown) were observed and the proportion of PCNA-positive cells per field in ten fields was recorded (mean = PCNA proliferation index).
TUNEL assay
Paraffin sections were dewaxed hydrated dried digested with 20 μg/mL proteinase K at room temperature, fixed for 5 minutes with 4% paraformaldehyde, and incubated with terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) working solution (8.3 UTdT, 0.83 nmol Dig-dUTP, 50 μL TdT buffer) at 37°C for 60 minutes. The reaction was terminated by 2 × saline-sodium citrate. Sections were incubated with alkaline phosphatase-labeled anti-Dig Fab fragment (1:1000; Abcam) at 25°C, detected by 5-bromo-4-chloro-3-indolyl-phosphate/nitro blue tetrazolium, dyed with neutral red and sealed. Results were determined by examining positive cells (granular/diffuse brown staining) in ten randomly selected and consecutive high power (×400) fields (mean = apoptotic index).
Statistical analysis
All statistical analyses were performed using SPSS version 11.0 (IBM, Armonk, NY, USA). All measured data were expressed as means ± standard deviation. Differences between groups were compared by t-tests or variance analyses, and unpaired t-tests were used to compare data between cell lines. Tumor weight, apoptosis, and proliferation were compared with controls using χ2 and least significant difference t-tests. Rates were compared with χ2 and rank-sum tests. P-values less than 0.05 were considered statistically significant (P < 0.05).
Results
Characteristics of viral infection
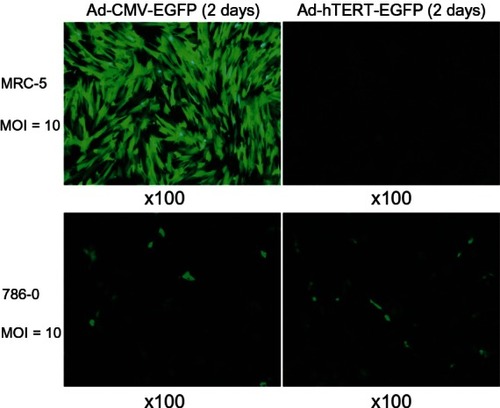
Efficient infection in MRC-5 and 786-0 cells was achieved using the recombinant Ad-CMV-EGFP at an MOI of 10. Expression of GFP was highest after postinfection day 1, demonstrating expressions in excess of 65% of MRC-5 and 786-0 cells. MRC-5 cells were better infected. For Ad-hTERT-EGFP at an MOI of 10, only 786-0 was appreciably infected. Highest GFP expression was observed on postinfection day 2, demonstrating expressions in excess of 55% of 786-0 cells. No infection was observed in MRC-5 cells using Ad-hTERT-EGFP at MOI values of 1, 5, 10, 20, 30, 50, and 100 ().
Figure 1 Expression of adenovirus cytomegalovirus-enhanced green fluorescent protein (Ad-CMV-EGFP) and Ad-human telomerase reverse transcriptase (hTERT)-EGFP in MRC-5 and 786-0 cells.
Ad-hTERT-HSV-TK/GCV-mediated specific cytotoxicity to RCC cells in vitro
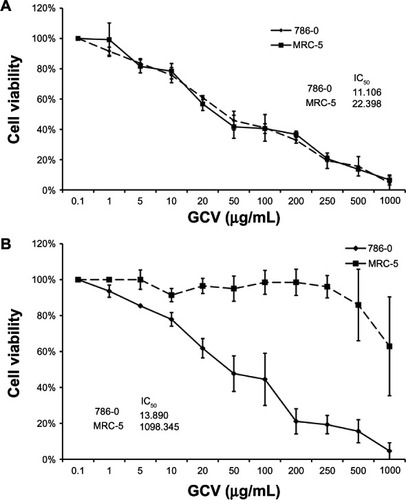
At similar MOIs of Ad-CMV-HSV-TK, half-maximal inhibitory concentration (IC50) values of GCV in in vitro cell lines 786-0 and MRC-5 were not significantly different (11.106 μg/mL and 22.398 μg/mL, respectively; P > 0.05) (). At similar MOIs of Ad-hTERT-HSV-TK, however, IC50 values of GCV for 786-0 and MRC-5 cells were significantly different (13.890 μg/mL and 1098.345 μg/mL, respectively; P < 0.05) (), indicating specific cytotoxicity of Ad-hTERT-HSV-TK/GCV for RCC cancer cells.
Figure 2 (A and B) Cell viability comparison of MRC-5 and 786-0 cell lines infected by either adenovirus human telomerase reverse-transcriptase herpes simplex virus thymidine kinase/ganciclovir (Ad-hTERT-HSV-TK/GCV) or Ad-cytomegalovirus (CMV)-HSV-TK/GCV. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay was used to measure the cell viability after 7 days. Half-maximal inhibitory concentration (IC50) was compared using unpaired t-tests. P-values less than 0.05 were considered statistically significant. (A) Cell-viability comparison of MRC-5 and 786-0 cell lines infected by Ad-CMV-HSV-TK/GCV. (B) Cell-viability comparison of MRC-5 and 786-0 cell lines infected by Ad-hTERT-HSV-TK/GCV.
Ad-hTERT-HSV-TK/GCV inhibited RCC tumor growth and prolonged survival in vivo
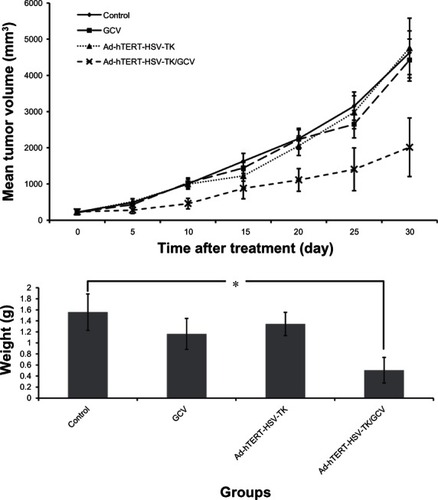
In in vivo mouse models, injection with recombinant Ad-hTERT-HSV-TK/GCV produced significantly smaller mean tumor volume on postinjection days 13, 17, 21, 25, and 30 in the treatment group than in the other 3 groups as controls (P < 0.05) (). At postinjection day 30, the tumor inhibition rate reached 76.98% in Ad-hTERT-HSV-TK/GCV-treated mice (<26.49% in all other groups) (). Tumor weights in Ad-hTERT-HSV-TK/GCV-treated mice were significantly lower than those in all other groups (). By postinjection day 14, surviving mice numbered six (control group), four (GCV group), and five (Ad-hTERT-HSV-TK group). Conversely, all Ad-hTERT-HSV-TK/GCV-treated mice survived (P < 0.05).
Figure 3 (A and B) Comparison of tumor weight and volume in a nude mice model of renal cell carcinoma (RCC). (A) growth curve of nude mice RCC. (B) Tumor weight of nude mice RCC.
Abbreviation: Ad-hTERT-HSV-TK/GCV, adenovirus human telomerase reverse-transcriptase herpes simplex virus thymidine kinase/ganciclovir.
Pathological characteristics
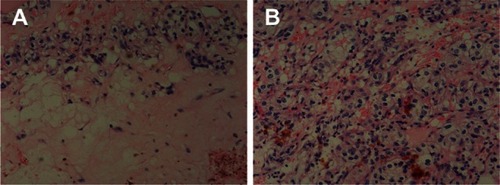
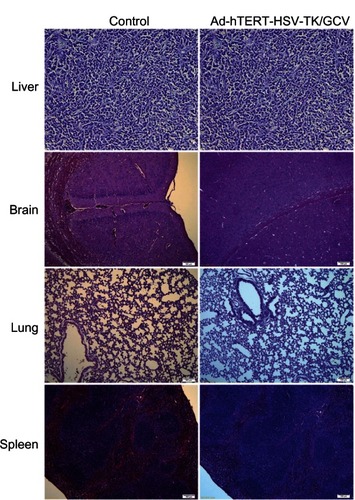
Treatment-group specimens exhibited dregs and coke-like necrosis upon pathological assessment, while no obvious necrosis and more neovascularization was observed in control-group specimens. Furthermore, pathological examination revealed that tumor cells treated with Ad-hTERT-HSV-TK/GCV displayed a unique pattern of scattered, sparse, and flaky necrosis, and tumor vessels evidenced significant reduction, disorder was observed in tumor structure, and replacement of neoplastic parenchyma with fibrous tissue was apparent. In some tumor cells, cytoplasmic vacuolar degeneration, reduced nuclei numbers, loosened chromatin, lightly stained cytoplasm, and nuclear pyknosis were observed (). Notably, the heart, lung, liver, brain, kidney, and spleen tissues evidenced no significant morphological differences following Ad-hTERT-HSV-TK/GCV treatment ().
Ad-hTERT-HSV-TK/GCV inhibits proliferation and induces apoptosis in RCC cells
Ad-hTERT-HSV-TK/GCV-treated mice exhibited large numbers of apoptotic cells and cells with vacuolar degeneration, while few or no apoptotic cells were observed in tumors from other groups (). The apoptotic index of the Ad-hTERT-HSV-TK/GCV treatment, control, and GCV and Ad-hTERT-HSV-TK groups was 3.2% ± 0.3%, 0.1% ± 0.1%, 0.2% ± 0.1%, and 0.2% ± 0.1%, respectively (P < 0.05) ().
Figure 6 (A and B) Histology of apoptosis and proliferation in nude mice renal cell carcinoma xenografts from adenovirus human telomerase reverse-transcriptase herpes simplex virus thymidine kinase/ganciclovir (Ad-hTERT-HSV-TK-GCV)-infected or control mice. (A) Histology of apoptosis cells from Ad-hTERT-HSV-TK-GCV-infected (left panel) or control mice by terminal deoxynucleotidyl transferase dUTP nick-end labeling (right panel). (B) Histology of proliferative cells from Ad-hTERT-HSV-TK-GCV-infected (left panel) or control mice by nuclear antibody staining (right panel).
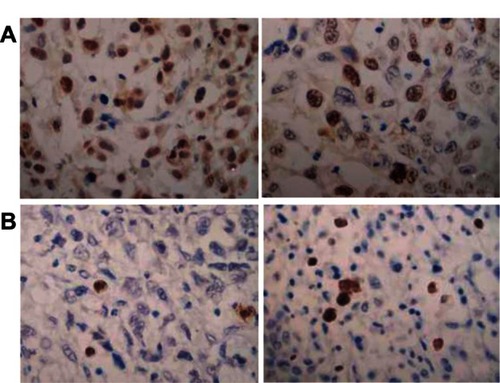
Table 1 Index of apoptosis and proliferation in renal cell carcinoma xenograft
The PCNA proliferation index of the Ad-hTERT-HSV-TK/GCV treatment, control, and GCV and Ad-hTERT-HSV-TK groups were 27.57% ± 9.36%, 71.2% ± 13.1%, 69.8% ± 11.6%, and 72.4% ± 12.3%, respectively. Ad-hTERT-HSV-TK/GCV significantly inhibited the proliferation of RCC cells compared to effects observed in the other 3 groups as controls (P < 0.01) ( and ).
Discussion
Ad-hTERT-HSV-TK/GCV can not only inhibit the growth of RCC xenografts in mice but it can also prolong the survival time of tumor-bearing mice. Furthermore, Ad-hTERT-HSV-TK/GCV therapy was shown to produce no significant tissue damage in nude mice in adjacent cells, indicating a limited bystander effect. Ad-hTERT-HSV-TK/GCV caused selective tumor cell death in vitro and also reduced the volume and weight of mice RCC xenografts by increasing apoptosis and reducing proliferation of the tumor cells. This novel suicide gene therapy for RCC may thus provide an effective future clinical method for improving patient outcomes.
Currently, HSV-TK/GCV suicide therapy has been successfully applied in experimental research and phase I clinical trials,Citation13 but no studies of RCC have been previously reported. Leveraging the unique proliferative property of malignant tumors, the current study employed an Ad carrying the HSV-TK gene under the regulation of the hTERT promoter to limit the expression of HSV-TK in telomerase-positive tumors. Mehle et alCitation25 detected telomerase activity in 71 cases of kidney cancer, reporting a positive rate of 78.9% in RCC tumor tissues and 0% in adjacent tissues. These results identify hTERT expression as a specific characteristic of RCC cells, a finding consistent with the current results. In both RCC 786-0 cells and normal human fibroblast MRC-5 cells of the present study, efficient Ad-CMV-EGFP infection was achieved at an MOI of 10 and 30, respectively. These findings indicate CMV vector-mediated, high-level, nonselective expression of the exogenous gene. Ad-hTERT-EGFP infected 786-0 cells effectively at the same MOI, but did not infect MRC-5 cells at any MOI, suggesting that the hTERT-mediated gene expression is highly tumor-specific. These findings are consistent with the results published by previous reports.Citation26,Citation27
Both Ad-CMV-HSV-TK/GCV and Ad-hTERT-HSV-TK/GCV demonstrated the ability to limit the proliferation of RCC tumor cells in the current study. The variant IC50 values for GCV observed in tumor cells versus normal cells suggests that introduction of the hTERT promoter reduced nonspecific cytotoxicity to normal cells and improved the efficacy/toxicity ratio of the recombinant adenovirus. Furthermore, Ad-hTERT-HSV-TK/GCV inhibited the growth of tumor cells in vivo in mice, without excessive damage to adjacent tissues and organs through the bystander effect. Notably, this effect has been associated with gap-junctional intercellular communication, which allows toxic metabolites of GCV to surrounding cells despite nonexpression in these cells both in vitro and in vivo.Citation28 The reason for minimization of this effect is currently undetermined, though the notably low level of adjacent tissue damage merits further investigation and may offer insights into possible molecular strategies for enhancing the extent of tumor cell death without producing excessive bystander effects. Although Ad-CMV-HSV-TK/GCV mediated higher expression levels of HSV-TK, thereby producing stronger inhibition of tumor cell proliferation, its main deficiency was a lack of specific targeting. Thus, further study of appropriate targeting mechanisms must be explored before clinical implementation is considered.
The unique degenerative characteristics of tumor cells treated with Ad-hTERT-HSV-TK/GCV, including visible necrosis upon pathological examination and in vivo reductions in tumor growth and cell proliferation, indicate that this treatment may act in both direct and indirect mechanisms, as suggested by previous studies of other cancer cell lines.Citation28–Citation30 Notably, these mechanisms exhibited no harmful effects on major organ systems, such as the heart, lung, liver, kidney, and spleen, in the current study. These findings further indicate the safety of Ad-hTERT-HSV-TK/GCV treatment. While previous studies have shown that intratumoral injection of Ad-CMV-HSV-TK may be effective, these studies have still reported severe toxicity associated with treatment.Citation31 The current route of administration, however, produced limited systemic toxicity.
In the current study, tumors treated with Ad-hTERT-HSV-TK or GCV showed no statistically significant reduction in size compared to controls, further confirming that isolated Ad-hTERT-HSV-TK or GCV treatments exhibit no antitumor effects. Zhang et alCitation7 found that the exogenous gene expression peaked 3 days after the intratumoral injection of the adenovirus Ad-hTERT-E1A-TK, suggesting a time-sensitive parameter associated with GCV administration. Further study, however, will be required to assess the effects of GCV injection timing on outcomes. The relationship between Ad distribution in tumors and exogenous gene-expression levels should also be further clarified prior to clinical implementation. Furthermore, future work should carefully examine the high immunogenicity induced by anti-Ad antibodies immediately after intravenous administration, which may compound the findings of the current study.
The therapeutic antitumor effects of Ad-hTERT-HSV-TK/GCV for the treatment of RCC were apparent in the current study, the first reported application of HSV-TK/GCV for RCC treatment. Tumor-specific regulation of the hTERT promoter was able to ensure cancer-specific expression of HSV-TK in malignant RCC cells, with minimal harm to surrounding normal cells and organ systems. Because systemic toxicity is limited in this treatment strategy, future clinical strategies may be designed to limit damage to organ systems through the bystander effect and improve overall patient quality of life. The current study provides valuable preliminary data for the clinical application of Ad-hTERT-HSV-TK/GCV suicide gene therapy for RCC treatment.
Acknowledgments
We would like to express our appreciation to Professor Han Ruifa from the Department of Urology, Second Hospital of Tianjin Medical University, Tianjin (People’s Republic of China) for providing us with human renal clear-cell carcinoma cell line 786-0 and human fibroblast cell line MRC-5.
Disclosure
The authors report no conflicts of interest in this work.
References
- GuptaKMillerJDLiJZRussellMWCharbonneauCEpidemiologic and socioeconomic burden of metastatic renal cell carcinoma (mRCC): a literature reviewCancer Treat Rev200834319320518313224
- XuKDingQFangZSilencing of HIF-1alpha suppresses tumorigenicity of renal cell carcinoma through induction of apoptosisCancer Gene Ther201017321222219816521
- PatardJJLerayERioux-LeclercqNPrognostic value of histologic subtypes in renal cell carcinoma: a multicenter experienceJ Clin Oncol200523122763277115837991
- SchachterLRCooksonMSChangSSSecond prize: frequency of benign renal cortical tumors and histologic subtypes based on size in a contemporary series: what to tell our patientsJ Endourol200721881982317867935
- MorikawaTSugiyamaAKumeHIdentification of Toll-like receptor 3 as a potential therapeutic target in clear cell renal cell carcinomaClin Cancer Res200713195703570917908959
- MaattaAMTenhunenAPasanenTNon-small cell lung cancer as a target disease for herpes simplex type 1 thymidine kinase-ganciclovir gene therapyInt J Oncol200424494394915010834
- ZhangJFWeiFWangHPPotent anti-tumor activity of telomerase-dependent and HSV-TK armed oncolytic adenovirus for non-small cell lung cancer in vitro and in vivoJ Exp Clin Cancer Res2010295220487549
- SaukkonenKHemminkiATissue-specific promoters for cancer gene therapyExpert Opin Biol Ther20044568369615155160
- YakkundiAMcErlaneVMurrayMTumor-selective drug activation: a GDEPT approach utilizing cytochrome P450 1A1 and AQ4NCancer Gene Ther200613659860516410820
- ShugarDViral and host-cell protein kinases: enticing antiviral targets and relevance of nucleoside, and viral thymidine, kinasesPharmacol Ther1999822–331533510454209
- MooltenFLWellsJMCurability of tumors bearing herpes thymidine kinase genes transferred by retroviral vectorsJ Natl Cancer Inst19908242973002299679
- MaattaAMSamaranayakeHPikkarainenJWirthTYla-HerttualaSAdenovirus mediated herpes simplex virus-thymidine kinase/ganciclovir gene therapy for resectable malignant gliomaCurr Gene Ther20099535636719860650
- SangroBMazzoliniGRuizMA phase I clinical trial of thymidine kinase-based gene therapy in advanced hepatocellular carcinomaCancer Gene Ther2010171283784320689572
- KimballKJRiveraAAZinnKRNovel infectivity-enhanced oncolytic adenovirus with a capsid-incorporated dual-imaging moiety for monitoring virotherapy in ovarian cancerMol Imaging20098526427719796604
- DeyDEvansGRDSuicide gene therapy by herpes simplex virus-1 thymidine kinase (HSV-TK)YouPYTargets in Gene TherapyRijeka, CroatiaInTech2011
- EbaraSShimuraSNasuYGene therapy for prostate cancer: toxicological profile of four HSV-tk transducing adenoviral vectors regulated by different promotersProstate Cancer Prostatic Dis20025431632512627218
- StrazisarMMlakarVGlavacDThe expression of COX-2, hTERT, MDM2, LATS2 and S100A2 in different types of non-small cell lung cancer (NSCLC)Cell Mol Biol Lett200914344245619238334
- KimNWPiatyszekMAProwseKRSpecific association of human telomerase activity with immortal cells and cancerScience19942665193201120157605428
- AgrawalADangSGabraniRRecent patents on anti-telomerase cancer therapyRecent Pat Anticancer Drug Discov20127110211721854360
- FujiwaraTShirakawaYKagawaSTelomerase-specific oncolytic virotherapy for human gastrointestinal cancerExpert Rev Anticancer Ther201111452553221504319
- KyoSTakakuraMFujiwaraTInoueMUnderstanding and exploiting hTERT promoter regulation for diagnosis and treatment of human cancersCancer Sci20089981528153818754863
- ChhikaraMHuangHVlachakiMTEnhanced therapeutic effect of HSV-tk+GCV gene therapy and ionizing radiation for prostate cancerMol Ther20013453654211319915
- YuBZhangYZhanYCo-expression of herpes simplex virus thymidine kinase and Escherichia coli nitroreductase by an hTERT-driven adenovirus vector in breast cancer cells results in additive anti-tumor effectsOncol Rep201126125526421573500
- IsobeIYanagisawaKMichikawaM3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) causes Akt phosphorylation and morphological changes in intracellular organellae in cultured rat astrocytesJ Neurochem200177127428011279283
- MehleCPiatyszekMALjungbergBShayJWRoosGTelomerase activity in human renal cell carcinomaOncogene19961311611668700542
- LinWHYehSHYangWJTelomerase-specific oncolytic adenoviral therapy for orthotopic hepatocellular carcinoma in HBx transgenic miceInt J Cancer201313261451146222886913
- LiJTBianKZhangALTargeting different types of human meningioma and glioma cells using a novel adenoviral vector expressing GFP-TRAIL fusion protein from hTERT promoterCancer Cell Int20111113522035360
- MesnilMYamasakiHBystander effect in herpes simplex virus-thymidine kinase/ganciclovir cancer gene therapy: role of gap-junctional intercellular communicationCancer Res200060153989399910945596
- BauzonMJinFKretschmerPHermistonTIn vitro analysis of cidofovir and genetically engineered TK expression as potential approaches for the intervention of ColoAd1-based treatment of cancerGene Ther20091691169117419458647
- AhnMLeeSJLiXEnhanced combined tumor-specific oncolysis and suicide gene therapy for prostate cancer using M6 promoterCancer Gene Ther2009161738218772902
- TsuchiyamaTNakamotoYSakaiYMukaidaNKanekoSOptimal amount of monocyte chemoattractant protein-1 enhances antitumor effects of suicide gene therapy against hepatocellular carcinoma by M1 macrophage activationCancer Sci200899102075208219016769