Abstract
Background
In order to minimize surgical stress and preserve organs, endoscopic or robotic surgery is often performed when conducting head and neck surgery. However, it is impossible to physically touch tumors or to observe diffusely invaded deep organs through the procedure of endoscopic or robotic surgery. In order to visualize and safely resect tumors even in these cases, we propose using an indocyanine green (ICG) fluorescence method for navigation surgery in head and neck cancer.
Objective
To determine the optimum surgical time for tumor resection after the administration of ICG based on the investigation of dynamic ICG fluorescence imaging.
Methods
Nine patients underwent dynamic ICG fluorescence imaging for 360 minutes, assessing tumor visibility at 10, 30, 60, 120, 180, and 360 minutes. All cases were scored according to near-infrared (NIR) fluorescence imaging visibility scored from 0 to 5.
Results
Dynamic NIR fluorescence imaging under the HyperEye Medical System indicated that the greatest contrast in fluorescent images between tumor and normal tissue could be observed from 30 minutes to 1 hour after the administration of ICG. The optimum surgical time was determined to be between 30 minutes to 2 hours after ICG injection. These findings are particularly useful for detection and safe resection of tumors invading the parapharyngeal space.
Conclusion
ICG fluorescence imaging is effective for the detection of head and neck cancer. Preliminary findings suggest that the optimum timing for surgery is from 30 minutes to 2 hours after the ICG injection.
Introduction
Complete resection is essential for obtaining an improved prognosis in the treatment of head and neck cancer. However, it is difficult to successfully resect advanced cases while simultaneously preserving organs. This is particularly complicated when the cancer has diffusely invaded deep organs. Tumors that cannot be physically touched or observed are often dealt with through endoscopic or robotic surgery. These procedures are highly beneficial during surgery in visualizing tumors that have invaded deep behind critical organs.
Optical imaging using near-infrared (NIR) fluorescence is a new technique that can be utilized to visually observe the operation field in real time during surgery.Citation1 NIR fluorophores are probes that have gained immense interest in various fields of biomedicine. This is due to factors such as minimal interference of absorption and fluorescence found in biological samples, minimal scattering, enhanced tissue penetration depth, and low autofluorescence, which provides high-quality contrast.Citation2,Citation3
In this study, we used indocyanine green (ICG) with infrared camera imaging (the HyperEye Medical System [HEMS]; Mizuho Medical Co, Ltd, Tokyo, Japan) in order to investigate dynamic imaging as observed between normal and diseased tissue. To our knowledge, this is the first study to identify dynamic imaging for image-guided surgery with ICG NIR fluorescence imaging in head and neck cancer in vivo.
Our objective was to determine the optimum surgical time for tumor resection after administration of ICG based on the investigation of dynamic ICG fluorescence imaging as observed between normal and diseased tissue.
Materials and methods
Nine patients were included in this study. The numbers of oral, pharyngeal, and other cancers were two, five, and two, respectively. The number of cases of primary cancers and lymph node metastasis were five and four, respectively. Untreated cases and recurrent cases were six and three, respectively ().
Table 1 Patient characteristics and the visibility score of dynamic NIR fluorescence imaging
Real-time NIR fluorescent imaging
ICG 0.5 mg/kg (Diagnogreen; Daiichi Sankyo, Tokyo, Japan) was injected via the cephalic vein. Observation of the dynamic fluorescent image was conducted with an infrared camera (HEMS). Fluorescent images were recorded at periods of 10 minutes, 30 minutes, 1 hour, 2 hours, 3 hours, and 6 hours after the initial injection. All cases were scored according to NIR fluorescence imaging visibilityCitation4 scored from 0 to 5. A score of 0 means a lesion is not visible; a score of 1 refers to the presence of a lesion that is hardly visible; a score of 2 indicates that the contrast is weak – that is, the lesion is detectable only when its exact location is known; a score of 3 indicates that the contrast of the lesion is clearly detectable yet inferior when compared with the contrast observed in the other surrounding structures; a score of 4 indicates that the contrast of the lesion is similar to that of the other structures; and a score of 5 indicates that the contrast of the lesion dominated the image.Citation5
Two cases were administered with ICG on two occasions. The first time was to evaluate real-time imaging, while the second was at the time of resection. This protocol was approved by the institutional review board at Juntendo University, Tokyo, Japan and all patients gave documented informed consent.
Results
Real-time NIR fluorescence imaging
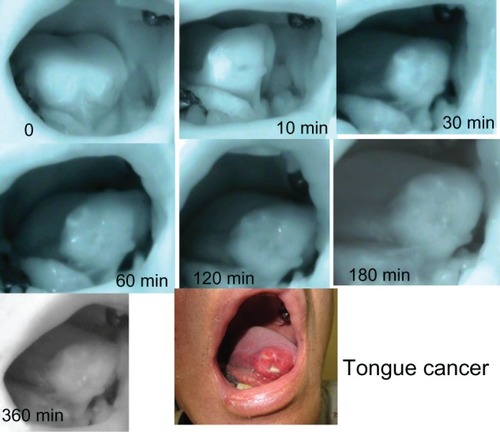
After the intravenous administration of ICG under HEMS imaging, fluorescence emissions were detected throughout the primary region and the neck immediately. There was no contrast observed between the tumor and the healthy organs. Gradually, the fluorescence emissions lost brightness in normal structures. In our study, the greatest contrast between lesions and normal structures was observed approximately 30–60 minutes after the administration of ICG (). All nine cases were examined according to the subjective NIR fluorescence imaging visibility score.Citation4 Scores were highest at the period of 30 minutes following ICG administration. After 6 hours, fluorescence emissions also decreased in the tumors. Twelve hours after, there was no contrast observable between the tumor and the normal structures. The results of our data are summarized in .
Figure 1 Changes in ICG fluorescence images in tongue cancer.
Abbreviations: HEMS, HyperEye Medical System; ICG, indocyanine green.
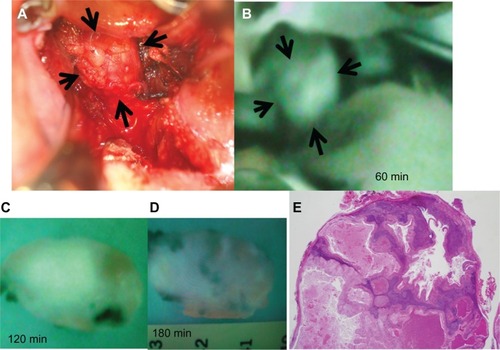
Two cases were resected under HEMS imaging 30–60 minutes after the administration of ICG. In one of the two cases, we observed recurrent retropharyngeal node from hypopharyngeal carcinoma (). First, we marked the position of the tumor on pharyngeal mucosa before incision. ( and ). We then incised the pharyngeal mucosa transorally. Even with the submucosal tumor obscured by fasciae, we were able to observe the tumor clearly under HEMS imaging ( and ). All tumors displayed bright fluorescence emissions that clearly contrasted with the normal structures. The tumor was completely resected pathologically (). As a result, we could remove the tumor safely and noninvasively to preserve pharyngeal functions.
Figure 2 MRIs of a recurrent retropharyngeal node from hypopharyngeal carcinoma. (A) T2-weighted MRI shows a left recurrent retropharyngeal node (arrow). (B) MRI with fat suppression enhances only marginal zone of the tumor (arrow).
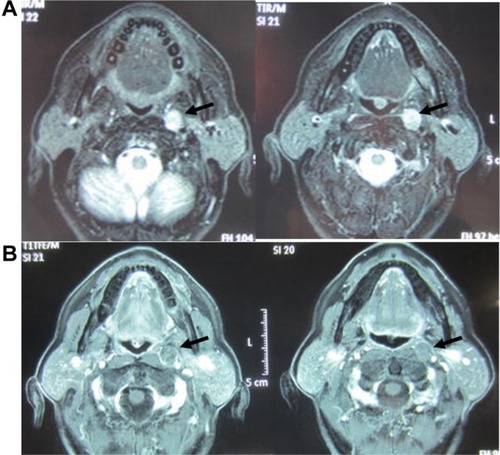
Figure 3 Examination with ICG fluorescence image for recurrent retropharyngeal node from hypopharyngeal carcinoma intraoperative findings between 0 minutes and 30 minutes after ICG injection. (A) Intrapharyngeal finding. Retropharyngeal tumor was not palpable and not visible. Tumor location marked based on ICG image (arrows). (B) ICG fluorescence image soon after ICG injection. ICG fluorescence visualizes diffusely recurrent retropharyngeal node on posterolateral oropharynx soon after ICG injection (dotted circle). (C) Pharyngeal mucosa incision. Left soft palate is incised. The tumor, covered with fasciae, cannot be observed on the white LED light 30 minutes after ICG injection. (D) ICG image at 30 minutes after ICG injection.
Abbreviation: ICG, indocyanine green.
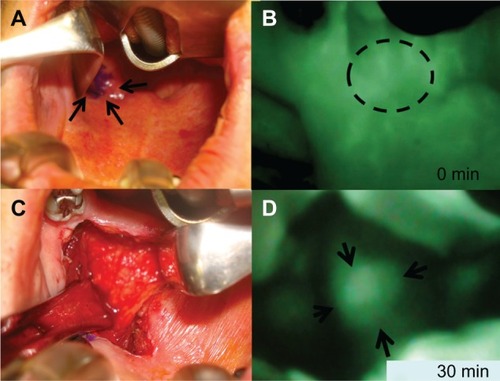
Figure 4 Examination with ICG fluorescence image for recurrent retropharyngeal node from hypopharyngeal carcinoma between 60 minutes and 180 minutes in excised specimens. (A) Intraoperative findings after 60 minutes. The tumor is observed clearly on the white LED light. (B) ICG image 60 minutes after ICG injection. ICG image visualized the tumor clearly under HEMS (Mizuho Medical Co, Ltd, Tokyo, Japan) imaging. (C) 120 minutes after ICG injection. Under HEMS imaging, the excised tumor is moderately fluorescent. (D) 180 minutes after ICG injection. Under HEMS imaging, the excised tumor is slightly fluorescent. (E) Pathological findings.
Discussion
Recently, ICG fluorescence imaging has been utilized for a variety of purposes in oncologic surgery. ICG fluorescence imaging can potentially be used for (1) sentinel lymph node detection;Citation6,Citation7 (2) evaluation of lymphedema;Citation8 (3) microvascular circulation of free flaps in reconstructive surgery;Citation9 and (4) endoscopic marking of colorectal tumors.Citation7
ICG is a water-soluble, anionic, amphiphilic tricarbocyanin probe with a hydrodynamic diameter of 1.2 nm and excitation and emission wavelengths in serum at 778 and 830, respectively.Citation10,Citation11 ICG is a widely used diagnostic reagent approved for use in the examination of the hepatic function and cardiac output.Citation12,Citation13 After the intravenous administration of ICG, the vascular hyperpermeability of cancer towards macromolecules results in a higher fluorescence intensity in cancers observed during the extravascular phase than observed around normal tissue. In addition, ICG is cleared from the vascular compartment by the liver.Citation14 After intravenous injection of ICG, the dye quickly binds to globulins preferentially to a1-lipoproteins within 1 to 2 seconds.Citation15 ICG is not metabolized in the body, and hepatic elimination is the main source of clearance.
In recent years, sentinel node biopsy with ICG NIR imaging has been used extensively in head and neck cancer treatment.Citation16,Citation17 Several studies have reported that sentinel node biopsy with ICG NIR imaging produces results that are as good as those of a radioactive tracer; however, there have been no reports concerning image-guided surgery for the purpose of minimum invasive surgery using endoscopic and robotic surgery for the treatment of head and neck cancer.Citation15,Citation16 To our knowledge, this study is the first study to describe clinical application of ICG NIR dynamic imaging for examining its usefulness in head and neck cancer surgery.
Our evaluation of the dynamic imaging of ICG fluorescence emissions suggests that the contrast between ICG fluorescence tumors and normal tissue is maximally distinguished 30–60 minutes after the administration of ICG. We concluded that the optimum time for carrying out surgical procedures is between 30 minutes and 2 hours after ICG administration in head and neck cancer.
The vascular hyperpermeability of cancers toward macromolecules resulted in the saturation of fluorescence dyes in neoplastic lesions when compared to normal structures.Citation12 During experimentation under laser scanning confocal microscopic imaging, we clearly observed the accumulation of ICG in tumor masses and no fluorescent emission in normal tissues.Citation18 In our experimental study, we determined that the optimum surgical time was between 3 and 12 hours after the ICG injection.Citation18 The optimum surgical time in the experimental study was significantly later than that of our study. The reason for this time lag was that not all ICG was administered via the fine tail vein,Citation18 and some of the leaked ICG was gradually reabsorbed by veins. However, in these clinical cases, all ICG was administered via the cephalic vein over a short period of time and the optimum surgical time ranged from 30 minutes to 120 minutes. Different species’ metabolism of ICG may also influence differences regarding the optimal surgical time using ICG fluorescence. However, it is important for us to pay attention to the fact that the accumulation of ICG may not be limited to cancerous tissues. ICG would also be expected to accumulate in inflammatory tissues and areas of surgical trauma.Citation19
A report has indicated that ICG NIR fluorescence imaging-guided surgery is efficient in liver cancer surgery at detecting not only large cancers, but also microcancers.Citation20 In this study, we suggest the usefulness of ICG NIR fluorescence imaging not only in liver cancer surgery, but also in head and neck cancer surgery.
In this study we used a new HEMS handheld device. The HEMS can detect tumors by ICG-enhanced images with vivid color and can be freely operated intraoperatively by surgeons. This is a high-sensitivity charge-coupled device camera and custom-made optical filter with non-Bayer color filter arrays. It can detect visible and NIR rays from 380–1200 nm without bias in color balance at 30 frames per second.Citation21 We use the HEMS for sentinel node biopsy and fluorescent angiography in daily surgical practice. It has high sensitivity due to the color CCD camera used in ICG fluorescence imaging.Citation22
Because of its high sensitivity, NIR imaging under HEMS can help to resect tumors that have invaded parapharyngeal space behind the internal carotid artery, internal jugular vein, and lower cranial nerves. We suggest that complete tumor resection, even for advanced cancer, can be carried out under ICG NIR fluorescent imaging without major complications, eg, causing dysfunction to important cranial nerves. Macroscopic imaging with HEMS can secure adequate tumor-free margins while minimizing surgical morbidity and preservation of function. In this study we have demonstrated a successful method of distinguishing cancer from normal tissue and optimum surgical time with HEMS in animal models.Citation18 These results demonstrate that this method could be applied to head and neck cancer surgery. Application of endoscopic and robotic surgery for oral, pharynx, and skull base lesions enables minimally invasive surgery with superior results. We need to be able to detect tumors in deeper and invisible areas when palpation is not possible. This is required in order to resect tumors safely and can be achieved through tumor detection carried out with ICG NIR fluorescent imaging. Further investigations may lead to the development of a new, minimally invasive surgical therapy achieving both better prognosis and function preservation in head and neck cancer.
Conclusion
ICG fluorescence imaging is effective for the detection of head and neck cancer. Preliminary findings suggest that the optimum timing for surgery is from 30 minutes to 2 hours after the ICG injection. Combined with endoscopic and robotic surgery for oral, pharynx, and skull base cancers, ICG fluorescence image-guided surgery enables minimally invasive surgery with good results in head and neck cancer.
Author contributions
TA and SO conceived of the study and participated in its design and coordination. MK and RY drafted the manuscript. JY and KI were involved in revising the manuscript. All authors read and approved the final manuscript.
Acknowledgment
This study was supported in part by Grants-in Aid for Scientific Research from the Ministry of Education, Culture, Sports, and Technology (22591920) of Japan.
Disclosure
The authors report no conflicts of interest in this work.
References
- OgawaMKosakaNChoykePLKobayashiHIn vivo molecular imaging of cancer with a quenching near-infrared fluorescent probe using conjugates of monoclonal antibodies and indocyanine greenCancer Res20096941268127219176373
- EscobedoJORusinOLimSStronginRMNIR dyes for bioimaging applicationsCurr Opin Chem Biol2010141647019926332
- KirchherrAKBrielAMäderKStabilization of indocyanine green by encapsulation within micellar systemsMol Pharm20096248049119228053
- PoellingerABurockSGrosenickDBreast cancer: early – and late-fluorescence near-infrared imaging with indocyanine green – a preliminary studyRadiology2011258240941621177396
- GrosenickDMoestaKTMöllerMTime-domain scanning optical mammography: I. Recording and assessment of mammograms of 154 patientsPhys Med Biol200550112429244915901947
- MurawaDHircheCDreselSHünerbeinMSentinel lymph node biopsy in breast cancer guided by indocyanine green fluorescenceBr J Surg200996111289129419847873
- NouraSOhueMSekiYEvaluation of the lateral sentinel lymph node by indocyanine green for rectal cancer based on micrometastasis determined by reverse transcriptase polymerase chain reactionOncol Rep200820474575018813813
- MausEATanICRasmussenJCNear-infrared fluorescence imaging of lymphatics in head and neck lymphedemaHead Neck201234344845322311465
- BetzCSZhorzelSSchachenmayrHEndoscopic measurements of free-flap perfusion in the head and neck region using red-excited indocyanine green: preliminary resultsJ Plast Reconstr Aesthet Surg200962121602160819036663
- OhnishiSLomnesSJLaurenceRGGogbashianAMarianiGFrangioniJVOrganic alternatives to quantum dots for intraoperative near-infrared fluorescent sentinel lymph node mappingMol Imaging20054317218116194449
- GurfinkelMThompsonABRalstonWPharmacokinetics of ICG and HPPH-car for the detection of normal and tumor tissue using fluorescence, near-infrared reflectance imaging: a case studyPhotochem Photobiol20007219410210911733
- TanakaEChenFYFlaumenhaftRGrahamGJLaurenceRGFrangioniJVReal-time assessment of cardiac perfusion, coronary angiography, and acute intravascular thrombi using dual-channel near-infrared fluorescence imagingJ Thorac Cardiovasc Surg2009138113314019577070
- DejaMAhlersOMacguillMChanges in hepatic blood flow during whole body hyperthermiaInt J Hyperthermia20102629510020146563
- HagenAGrosenickDMacdonaldRLate-fluorescence mammography assesses tumor capillary permeability and differentiates malignant from benign lesionsOpt Express20091719170161703319770920
- BrouwerORKlopWMBuckleTFeasibility of sentinel node biopsy in head and neck melanoma using a hybrid radioactive and fluorescent tracerAnn Surg Oncol20121961988199422207047
- BredellMGSentinel lymph node mapping by indocyanin green fluorescence imaging in oropharyngeal cancer – preliminary experienceHead Neck Oncol201023121034503
- BakerKJBinding of sulfobromophthalein (BSP) sodium and indocyanine green (ICG) by plasma alpha-1 lipoproteinsProc Soc Exp Biol Med196612249579635918158
- FujimakiMYokoyamaJOhbaSDynamic imaging in determining the optimum surgical time for NIR fluorescent image-guided surgery – a preliminary studyHead Neck Oncol2012425023104532
- MartirosyanNLCavalcantiDDEschbacherJMUse of in vivo near-infrared laser confocal endomicroscopy with indocyanine green to detect the boundary of infiltrative tumorJ Neurosurg201111561131113821923240
- IshizawaTFukushimaNShibaharaJReal-time identification of liver cancers by using indocyanine green fluorescent imagingCancer2009115112491250419326450
- HandaTKatareRGNishimoriHNew device for intraoperative graft assessment: HyperEye charge-coupled device camera systemGen Thorac Cardiovasc Surg2010582687720155342
- YokoyamaJItoSOhbaSFujimakiMIkedaKA novel approach to translymphatic chemotherapy targeting sentinel lymph nodes of patients with oral cancer using intra-arterial chemotherapy – preliminary studyHead Neck Oncol201134221929818