Abstract
Accumulating evidence shows that enhancer of zeste homolog 2 (E2H2) is upregulated in a broad range of cancer types, such as breast cancer, prostate cancer, ovarian cancer, and colon cancer. Therefore, inhibiting EZH2 expression may be a promising strategy for anticancer therapy. This review focuses on the current understanding of the mechanisms underlying EZH2 regulation that are involved in cancer progression. Also, it introduces two EZH2 inhibitors that target EZH2 and could be potentially applied in the treatment of cancer in the future.
Introduction
Enhancer of zeste homolog 2 (EZH2) encodes a histone methyltransferase, which is the catalytic core protein of the polycomb repressor complex 2 (PRC2).Citation1,Citation2 PRC2 is well known for initiating target gene silencing by promoting H3K27 trimethylation, which is catalyzed by EZH2. Several articles implicated that the EZH2 is involved in the cell proliferation, invasion, apoptosis, angiogenesis, and metastasis of cancer progression.
Underlying mechanism of EZH2 in cancer progression
Polycomb group proteins maintain the gene expression pattern of different cells that is set during early development by modifying chromatin structure.Citation3 In mammals, there are two main polycomb group complexes, PRC1 and PRC2. The PRC2 complex mainly consists of four core components: EZH2, suppressor of zeste 12 homolog (SUZ12), embryonic ectoderm development protein (EED), and retinoblastoma-associated protein 46/48. EZH2 via the SET domain catalyzes H3K27me3, and is associated with the silencing of tumor suppressor genes such as DAB2IP ().Citation4
Figure 1 Schematic representation of transcriptional gene repression by enhancer of zeste homolog 2 (EZH2). Proposed mechanism leads to aberrantly high levels of trimethylation on histone H3K27 in cancer.
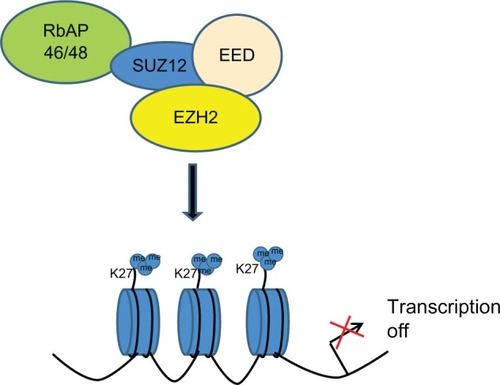
Cancer stem cells are a subgroup of cancer cells with several features: unlimited self-renewal potential, tumorigenicity, and chemoresistance.Citation5 Several papers showed that signal transducer and activator of transcription 3 (STAT3) and EZH2 are involved in the self-renewal, pluripotency, and proliferation of cancer stem cells.Citation6 Akt-dependent Ser21 phosphorylation of EZH2 has been reported in breast cancer cells treated with insulin-like growth factor 1 or estrogen.Citation7 Furthermore, Chen et al confirmed the c-Jun N-terminal kinase (JNK) regulation of STAT3 and link the JNK–STAT3–Akt signaling axis to the phosphorylation of EZH2.Citation8 Suva et al provided evidence that direct downregulated transcriptional regulation of c-Myc by EZH2 may constitute a novel mechanism underlying glioblastoma cancer stem cell maintenance.Citation9
Roles of EZH2 in cancer
The accumulated finding is that EZH2 levels are abnormally elevated in cancer tissues compared with corresponding normal tissues. Furthermore, higher EZH2 levels are correlated with advanced stages of disease and poor prognosis.
EZH2 in prostate cancer
Analyses of patient samples significantly correlate abnormally elevated EZH2 levels with increased proliferation rates, invasiveness, and metastasis of prostate cancer. Van Leenders et al showed that expression of EZH2 was significantly enhanced in tumors with a Gleason score of more than eight, extraprostatic extension, positive surgical margins, and prostate-specific antigen recurrence.Citation10 Bryant et al found that knockdown of endogenous EZH2 reduced proliferation and invasion in prostate cancer cells.Citation11 Furthermore, Ren et al indicated that EZH2 promoted prostate cancer cell invasion and metastasis via the repression of RKIP, a metastasis suppressor gene.Citation12 It is known that metastasis is associated with the balance between matrix metalloproteinases and their inhibitors, ie, tissue inhibitor of metalloproteinases. Shin et al discovered that EZH2 plays an active role in this process by repressing the expression of metallopeptidase inhibitor 2 and metallopeptidase inhibitor 3 in prostate cancer cells.Citation13 Although, there are eleven genetic variations in EZH2 in prostate cancer, genetic variations of the EZH2 gene are not responsible for the linkage of 7q to aggressive prostate cancer.Citation14 However, there is another mechanism for EZH2 in prostate cancer. Xu et al discovered that in the castration-resistant prostate cancer cells, EZH2 could be a transcriptional coactivator of androgen receptor instead of a transcriptional repressor of PCR2.Citation15 Furthermore, their study demonstrates that the phosphatidylinositol 3-kinase–Akt pathway could mediate phosphorylation of EZH2 at Ser21 and this phosphorylation could be involved in the transcriptional coactivator.
EZH2 in breast cancer
Overexpressed EZH2 has been reported as a biomarker of aggressive breast cancer and associated with invasion and cancer progression.Citation2,Citation16 EZH2 expression in 280 breast cancer patients was tested by high-density tissue microarray. EZH2 levels were elevated in patients with invasive breast carcinoma compared with normal or atypical hyperplasia.Citation16 Furthermore, it has been shown that high levels of EZH2 are associated with poor outcome to tamoxifen therapy in advanced breast cancer.Citation17,Citation18 Recently, EZH2-mediated epigenetic repression of DNA damage repair in breast tumor initiating cells was identified as a mechanism that could promote expansion of breast tumor initiating cells, and may contribute to cancer progression.Citation18 To analyze the role of EZH2 in the molecular subtypes of breast tumors (basal-like, luminal A, luminal B, human epidermal growth factor 2 (HER2)-enriched, and normal-like), Holm et al tested the EHZ2 and H3K27me3 expression in more than 400 tumors using immunohistochemistry. They found significantly high abundance of EZH2 in basal-like, triple negative, and HER2-enriched tumors, and high H3K27me3 in luminal A, HER2-enriched, and normal-like tumors.Citation19 EZH2 overexpression inhibits breast cancer type 1 susceptibility protein (BRCA1) phosphorylation (Ser1423) and thereby promotes an increase of Cdc25C, an essential player for G2/M checkpoint control.Citation20 Puppe et al observed increased EZH2 protein levels in human BRCA1-deficient tumor sections compared with other breast tumors.Citation21 Furthermore, they used the EZH2 inhibitor 3-deazaneplanocin (DZNep) to treat the BRCA1-deficient cancer cell and BRCA1-positive cancer cells. They discovered that DZNep showed remarkable selectivity in inhibiting BRCA1-def-cient tumor cells compared with BRCA1-proficient tumor cells. Their research provided another promising approach for the treatment of BRCA1-mutated breast cancers.
Besides the transcript repressor, EZH2 activation could switch to an activator via two different ways. In luminal-like estrogen receptor-positive cells, EZH2 overexpression can lead to an interaction with the Wnt signaling pathway, leading to the activation of c-Myc and cyclin D1.Citation22 In basal-like, estrogen receptor-negative cells, EZH2 activates nuclear factor-κB target genes by formation of a ternary complex with the nuclear factor-κB components RelA and RelB.Citation23
EZH2 in ovarian cancer
Several authors have indicated that EZH2 is involved with invasion and metastasis in ovarian carcinoma. Rao et al found that high expression of EZH2 was found in none of the normal ovaries, in 3% of the cystadenomas, in 23% of the borderline tumors, and in 50% of the ovarian carcinomas.Citation24 Drug resistance is a major clinical obstacle for ovarian cancer therapy. Rizzo et al found that EZH2 played a key role in the maintenance of a drug-resistant, tumor-sustaining subpopulation of cells in ovarian cancers undergoing chemotherapy.Citation25 Furthermore, ALDH1A1 – a putative marker for epithelial ovarian cancer stem cells – is found as a novel EZH2 target gene in epithelial ovarian cancer cells.Citation26 Lu et al identified EZH2 as a key regulator of tumor angiogenesis in ovarian cancer.Citation27 In endothelial cells, vascular endothelial growth factor stimulation could lead to increased expression of E2F transcription factors, which directly mediate EZH2 levels. Then, EZH2 causes the silence of VASH1 – an anti-angiogenic gene – and subsequently increases angiogenesis.
EZH2 in non-small-cell lung cancers
Kikuchi et al analyzed the immunohistochemical assessment of 157 surgically resected non-small-cell lung cancers.Citation28 They found that high EZH2 expression significantly correlated with non-adenocarcinoma histology, moderate and poor differentiation, advanced pathologic tumor classification, and high Ki-67 and cyclin E. Furthermore, Huqun et al found that positive EZH2 expression was associated significantly with larger tumor size in non-small-cell lung cancer.Citation29 Kaplan– Meier survival analyses and logrank tests demonstrated that patients whose samples were classified into the positive EZH2 expression group had a significantly shorter overall survival. The mechanisms of EZH2 in the progression of non-small-cell lung cancer are not clear. A recent published paper shows that mir-138 – a novel tumor suppressor micro-ribonucleic acid – could bind to 3′-untranslated region of EZH2 and suppress the expression of EZH2 at both messenger ribonucleic acid and protein levels.Citation30
Potential cancer therapy function
It is known that EZH2 plays an important role in cancer development. EZH2 expression can be reduced with S-adenosylhomocysteine hydrolase inhibitor DZNep, which inhibits methyltransferases and induces degradation of EZH2, SUZ12, and EED as well as the associated H3K27me3. DZNep treatment was shown to result in reactivation of EZH2 repressed target genes, inhibited cell growth, and reduced tumor formation in various cancers.Citation31 For example, Fiskus et al found that treatment with DZNep induced p16, p21, p27, and F-box protein 32 while depleting cyclin E and homeobox A9 levels in the cultured human acute myeloid leukemia cells and in primary acute myeloid leukemia cells.Citation32 Furthermore, combined with gemcitabine, DZNep synergistically enhanced the antiproliferative activity of gemcitabine, reduced the percentage of cells in the G2/M phase, and significantly increased apoptosis.Citation33 Recently, McCabe et al discovered a potent, highly selective, S-adenosylmethionine-competitive, small molecule inhibitor called GSK126. GSK126 can effectively inhibit the proliferation of EZH2 mutant diffuse large B-cell lymphoma cell lines and significantly inhibit the growth of EZH2 mutant diffuse large B-cell lymphoma xenografts in mice.Citation34
Conclusion
This review highlights that overexpression of EZH2 is correlated with cell proliferation, invasion, adhesion, and metastasis in several cancer types. Inhibitors of EZH2, including DZNep and GSK126, seem very promising anticancer agents to help reach the ultimate goal of cancer prevention and remission.
Disclosure
The authors report no conflicts of interest in this work.
References
- CaoRWangLWangHRole of histone H3 lysine 27 methylation in polycomb-group silencingScience200229855951039104312351676
- KunjuLPCookinghamCToyKAChenWSabelMSKleerCGEZH2 and ALDH-1 mark breast epithelium at risk for breast cancer developmentMod Pathol201124678679321399615
- MargueronRReinbergDThe polycomb complex PRC2 and its mark in lifeNature2011469733034334921248841
- MinJZaslavskyAFedeleGAn oncogene-tumor suppressor cascade drives metastatic prostate cancer by coordinately activating Ras and nuclear factor-κBNat Med201016328629420154697
- CreaFMathewsLAFarrarWLHurtEMTargeting prostate cancer stem cellsAnticancer Agents Med Chem20099101105111319925394
- ChangCJHungMCThe role of EZH2 in tumour progressionBr J Cancer2012106224324722187039
- ChaTLZhouBPXiaWAkt-mediated phosphorylation of EZH2 suppresses methylation of lysine 27 in histone H3Science2005310574630631016224021
- ChenBLiuJChangQBeezholdKLuYChenFJNK and STAT3 signaling pathways converge on Akt-mediated phosphorylation of EZH2 in bronchial epithelial cells induced by arsenicCell Cycle201312111212123255093
- SuvaMLRiggiNJaniszewskaMEZH2 is essential for glioblastoma cancer stem cell maintenanceCancer Res200969249211921819934320
- van LeendersGJDukersDHesselsDPolycomb-group oncogenes EZH2, BMI1, and RING1 are overexpressed in prostate cancer with adverse pathologic and clinical featuresEur Urol200752245546317134822
- BryantRJCrossNAEatonCLHamdyFCCunliffeVTEZH2 promotes proliferation and invasiveness of prostate cancer cellsProstate200767554755617252556
- RenGBaritakiSMaratheHPolycomb protein EZH2 regulates tumor invasion via the transcriptional repression of the metastasis suppressor RKIP in breast and prostate cancerCancer Res201272123091310422505648
- ShinYJKimJHThe role of EZH2 in the regulation of the activity of matrix metalloproteinases in prostate cancer cellsPLoS One201271e3039322272343
- BachmannNHoegelJHaeuslerJMutation screen and association study of EZH2 as a susceptibility gene for aggressive prostate cancerProstate200565325225916015586
- XuKWuZJGronerACEZH2 oncogenic activity in castration-resistant prostate cancer cells is polycomb-independentScience201233861131465146923239736
- KleerCGCaoQVaramballySEZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cellsProc Natl Acad Sci USA200310020116061161114500907
- ReijmEAJansenMPRuigrok-RitstierKDecreased expression of EZH2 is associated with upregulation of ER and favorable outcome to tamoxifen in advanced breast cancerBreast Cancer Res Treat2011125238739420306127
- StefanssonOAEstellerMEZH2-mediated epigenetic repression of DNA repair in promoting breast tumor initiating cellsBreast Cancer Res201113330921672285
- HolmKGrabauDLovgrenKGlobal H3K27 trimethylation and EZH2 abundance in breast tumor subtypesMol Oncol20126549450622766277
- GonzalezMELiXToyKDownregulation of EZH2 decreases growth of estrogen receptor-negative invasive breast carcinoma and requires BRCA1Oncogene200928684385319079346
- PuppeJDrostRLiuXBRCA1-deficient mammary tumor cells are dependent on EZH2 expression and sensitive to polycomb repressive complex 2-inhibitor 3-deazaneplanocin ABreast Cancer Res2009114R6319709408
- ShiBLiangJYangXIntegration of estrogen and Wnt signaling circuits by the polycomb group protein EZH2 in breast cancer cellsMol Cell Biol200727145105511917502350
- LeeSTLiZWuZContext-specific regulation of NF-κB target gene expression by EZH2 in breast cancersMol Cell201143579881021884980
- RaoZYCaiMYYangGFEZH2 supports ovarian carcinoma cell invasion and/or metastasis via regulation of TGF-β1 and is a predictor of outcome in ovarian carcinoma patientsCarcinogenesis20103191576158320668008
- RizzoSHerseyJMMellorPOvarian cancer stem cell-like side populations are enriched following chemotherapy and overexpress EZH2Mol Cancer Ther201110232533521216927
- LiHBitlerBGVathipadiekalVALDH1A1 is a novel EZH2 target gene in epithelial ovarian cancer identified by genome-wide approachesCancer Prev Res (Phila)20125348449122144423
- LuCHanHDMangalaLSRegulation of tumor angiogenesis by EZH2Cancer Cell201018218519720708159
- KikuchiJKinoshitaIShimizuYDistinctive expression of the polycomb group proteins Bmi1 polycomb ring finger oncogene and enhancer of zeste homolog 2 in nonsmall cell lung cancers and their clinical and clinicopathologic significanceCancer2010116123015302420564407
- HuqunIshikawaRZhangJEnhancer of zeste homolog 2 is a novel prognostic biomarker in nonsmall cell lung cancerCancer201211861599160621837672
- ZhangHZhangHZhaoMMiR-138 inhibits tumor growth through repression of EZH2 in non-small cell lung cancerCell Physiol Biochem2013311566523343715
- PiuntiAPasiniDEpigenetic factors in cancer development: polycomb group proteinsFuture Oncol201171577521174538
- FiskusWWangYSreekumarACombined epigenetic therapy with the histone methyltransferase EZH2 inhibitor 3-deazaneplanocin A and the histone deacetylase inhibitor panobinostat against human AML cellsBlood2009114132733274319638619
- AvanACreaFPaolicchiEMolecular mechanisms involved in the synergistic interaction of the EZH2 inhibitor 3-deazaneplanocin A with gemcitabine in pancreatic cancer cellsMol Cancer Ther20121181735174622622284
- McCabeMTOttHMGanjiGEZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutationsNature2012492742710811223051747