?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
The effects of inductive hyperthermia on lung cancer have yet to be fully investigated. Magnetic nanoparticles used in inductive hyperthermia are made-to-order and expensive. This study was performed to investigate the use of ferucarbotran in inductive hyperthermia and to clarify whether inductive hyperthermia using ferucarbotran promotes antitumor effects in vivo using a lung cancer cell line.
Methods
We injected A549 cells subcutaneously into the right thighs of BALB/c nu/nu nude mice. Forty mice with A549 xenografts were then classified into three groups. Group 1 was the control group. All mice in groups 2 and 3 had ferucarbotran injected into their tumors, and mice in group 3 were then subjected to alternating magnetic field irradiation. We evaluated tumor temperature during the hyperthermic procedure, the time course of tumor growth, histologic findings in tumors after hyperthermic treatment, and adverse events.
Results
Intratumor temperature rose rapidly and was maintained at 43°C–45°C for 20 minutes in an alternating magnetic field. Tumor volumes in groups 1 and 2 increased exponentially, but tumor growth in group 3 was significantly suppressed. No severe adverse events were observed. Histologic findings for the tumors in group 3 revealed mainly necrosis.
Conclusion
Inductive hyperthermia using ferucarbotran is a beneficial and promising approach in the treatment of lung cancer. Ferucarbotran is a novel tool for further development of inductive hyperthermia.
Introduction
Hyperthermia is a promising approach in the treatment of cancer, and various methods have been adopted, including whole body hyperthermia,Citation1 radiofrequency hyperthermia,Citation2 and induced hyperthermia using a microwave antennaCitation3 or implantable needles.Citation4 However, an inevitable technical problem with hyperthermia is the difficulty of uniformly heating only the tumor region to the required temperature without damaging healthy tissues. Therefore, some researchers have proposed inductive hyperthermia using submicron magnetic particles.Citation5,Citation6
The dextran magnetite complex is a submicron magnetic particle that consists mainly of magnetic nanoparticles coated with dextran which generate heat in an alternating magnetic field by both Brownian and Néel relaxation.Citation7,Citation8 These magnetic nanoparticles have been widely studied to improve adsorption activity, heat-generating efficiency, and accumulation in tumor cells due to electrostatic interaction with the cell membrane.Citation6,Citation9–Citation11 Although excellent magnetic nanoparticles overcoming these difficulties have emerged, they are made-to-order and expensive.Citation6 Therefore, more practical magnetic nanoparticles are currently in demand.
In this study, we used ferucarbotran, a magnetic resonance imaging contrast agent reported to be able to generate heat in an alternating magnetic field.Citation12–Citation15 Although there have been numerous studies on the effects of inductive hyperthermia in various cancers,Citation10,Citation16,Citation17 the effects on lung cancer have yet to be investigated fully. In this study, we performed animal experiments using nude mice with lung cancer xenografts for the following purposes: to reconfirm that commercially available ferucarbotran can generate heat in an alternating magnetic field and to clarify whether inductive hyperthermia using ferucarbotran exerts antitumor effects on lung cancer xenografts.
Materials and methods
Ferucarbotran
Ferucarbotran consists mainly of a hydrophilic colloidal solution of superparamagnetic iron oxide coated with carboxydextran. It is a complex composed of ultrafine (7 nm diameter) magnetite particles and alkali-treated dextran.Citation6 Ferucarbotran, which is inexpensive and readily available (Nihon Schering KK, Osaka, Japan) is typically used in medical practice as a magnetic resonance imaging contrast agent (Resovist® one vial, 1.6 mL; ferucarbotran, 540 mg/mL; 27.9 mg Fe/mL or 496 μmol Fe/mL).Citation18
Cell line
The human A549 non-small cell lung cancer cell line was used in this study. The A549 cells were obtained from the American Type Culture Collection, maintained in RPMI-1640 medium supplemented with 10% fetal bovine serum, 5 mM nonessential amino acids, and antibiotics (penicillin 100 U/mL and streptomycin 100 μg/mL) at 37°C in a 5% CO2 and 95% air incubator, and subcultured by harvesting with trypsin-EDTA.
Development of xenografts
All animal experiments were approved by the Animal Care and Use Committee of Kanazawa University. Forty BALB/c nu/nu athymic female nude mice aged 4 weeks and weighing approximately 20 g were used in this study, and were purchased from Sankyo Laboratory Service (Toyama, Japan). All mice were injected with cell suspensions, consisting of approximately 1 × 10Citation7 cells in 0.1 mL of phosphate-buffered saline, subcutaneously into the right thigh. When the maximum diameter of the tumors reached 10 mm after transplantation, the hyperthermic experiments were started. All mice were randomly divided into three groups. Group 1 (n = 16) was the control group without treatment. Mice in group 2 (n = 8) had ferucarbotran injected into their tumors without alternating magnetic field irradiation. Mice in group 3 (n = 16) were injected with ferucarbotran and subjected to alternating magnetic field irradiation once only. A smaller number of mice were assigned to group 2 because the safety of ferucarbotran alone has already been established.Citation13,Citation18–Citation20 Tumor volumes (V) were measured three times per week and calculated using the formula: V = π/6 × larger diameter × (smaller diameter)Citation2, as reported previouslyCitation21 Tumor volumes were evaluated for one month after the start of hyperthermic treatment.
Inductive hyperthermia
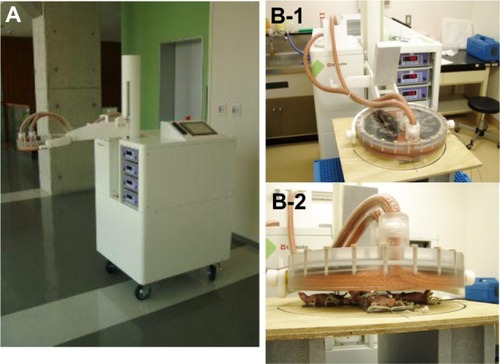
All procedures in animals were performed under anesthesia using 2% pentobarbital sodium (Nembutal®, Dainippon Sumitomo Pharma, Osaka, Japan) administered by intraperitoneal injection (50 mg/kg). Reportedly, the lethal dose of ferucarbotran solution in mice is more than 10 mmol Fe/kg (558 mg Fe/kg; Resovist 20 mL/kg).Citation13 Ferucarbotran was injected into the core of the tumors in all mice only once, immediately before alternating magnetic field irradiation at a dose of 150 ìL, which is equivalent to Resovist 7.5 mL/kg. A newly developed powerful induction heating device comprising a high-frequency power source and an applicator ()Citation13 was then used to generate an alternating current 142 kHz magnetic field from an electric current (maximum 440 amps) in a pancake coil (diameter 240 mm; five turns). The power consumption of this device is 3.5 kW with use of a single-phase alternating current 200 V (). For safe use in humans, this device adheres to the guidelines of the International Commission on Non-Ionizing Radiation Protection for limiting exposure to time-varying electric, magnetic, and electromagnetic fields at a distance of 1.5 m from the applicator.Citation22 We set the surface of the applicator 2 cm from the center of the tumor in each mouse. The strength of the magnetic field is 20–24 mTesla at this position (). Under these conditions, temperatures in the peripheral regions of the tumors (distant side to rectum) were measured using an optical fiber thermometer (FL-2000, Anritsu Meter Co, Ltd, Tokyo, Japan) during the hyperthermic procedures. At the same time, rectal temperatures were monitored to reflect normal tissue temperature. The rationale for hyperthermia is based on the direct cell-killing effects observed at temperatures above 42.5°C,Citation23 so intratumor temperature was adjusted to 43°C–45°C for 20 minutes in the alternating magnetic field to avoid overheating or underheating. We evaluated tumor size and observed any adverse events of inductive hyperthermia for 28 days after the start of hyperthermic treatment.
Figure 1 Portable induction heating device. (A) Dimensions are height 173 cm, width 73 cm, and length 83 cm; weight is 150 kg, and it has a wheel carriage. (B-1) It is composed of a high-frequency power source and an applicator, which creates an alternating current 142 kHz magnetic field from an electric current in a pancake coil. (B-2) The center of the tumor in each mouse was 2 cm distant from the surface of the applicator.
Histologic examination
On day 7 following hyperthermic treatment, two mice in each group were euthanized for histologic examination. The tumors were resected, fixed with 10% formalin solution, and sectioned longitudinally. Tumor specimens were stained with hematoxylin and eosin, and the condition of the cells around ferucarbotran was subsequently evaluated. Following staining with hematoxylin and eosin, tumor specimens from all groups were examined for apoptosis by a TUNEL (TdT-mediated dUTP-biotin nick-end labeling) assay using the DeadEnd™ Colorimetric TUNEL system (Promega, Madison, WI, USA), in accordance with the manufacturer’s instructions. Apoptosis was evaluated using the apoptotic index, as reported previously.Citation24 Briefly, 500 cells were counted in each specimen, and the apoptosis index was defined as:
Statistical analysis
The statistical analysis was performed using Statview version 5.0 software (SAS Institute Inc, Cary, NC, USA). The statistical significance of tumor growth between the three groups was evaluated using the Mann–Whitney U test. P < 0.05 was considered to be statistically significant.
Results
Antitumor effects of inductive hyperthermia
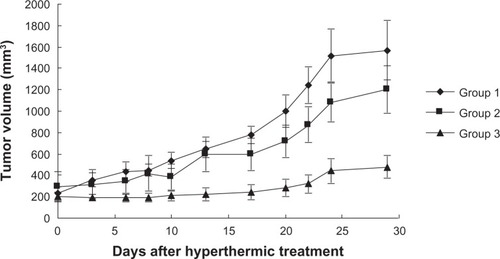
The time course of tumor growth in each group is shown in . Tumor volumes in groups 1 and 2 increased exponentially with no regression, and no significant differences were observed between tumor volumes on day 28 in groups 1 and 2 (P = 0.517). Tumor volume on day 28 after treatment in group 3 was significantly suppressed when compared with that in groups 1 and 2 (group 1 versus group 3, P = 0.0002; group 2 versus group 3, P = 0.016). However, complete tumor suppression was not observed in group 3.
Measurement of temperature
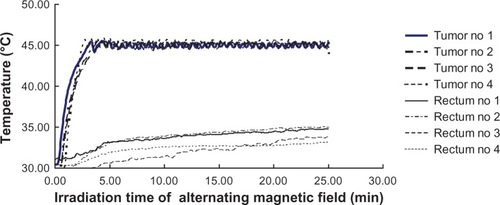
To evaluate the selectivity of heating in our hyperthermic treatment, the temperatures in both tumor and normal tissue (rectum) were measured. Intratumor and rectal temperatures during the hyperthermic procedures are shown in . Intratumor temperature in group 3 rose rapidly and reached over 43°C within 2.5 minutes. The temperature was maintained at 43°C–45°C for 20 minutes in an alternating magnetic field. In contrast, rectal temperatures did not increase in an alternating magnetic field.
Histologic findings
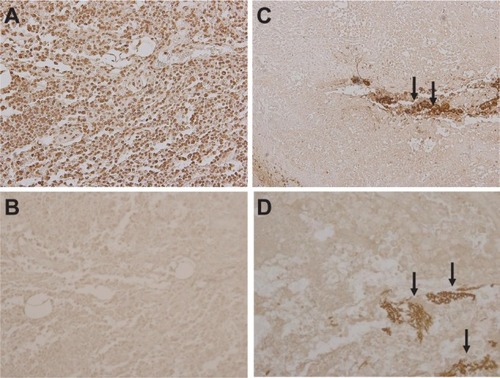
Representative microscopic findings for the tumor cells in each group are shown in . Hematoxylin and eosin staining of tumor cells around ferucarbotran in group 3 revealed broad necrosis after hyperthermic treatment (), whereas tumor cells in groups 1 and 2 showed no necrosis ( and B). Representative TUNEL staining of tumor cells in each group is shown in . The positive control for TUNEL staining (tumor cells treated with DNase) is shown in . Few tumor cells were positive for TUNEL staining in groups 1 and 2 ( and C). The apoptotic index in groups 1 and 2 was 1.78% ± 0.66% and 1.72% ± 0.79%, respectively. Further, the apoptotic index in group 3 was 2.42% ± 0.85%, similar to groups 1 and 2 in TUNEL staining (). There was no significant difference between groups 1, 2, and 3 (P > 0.05). These findings suggest that inductive hyperthermia using ferucarbotran causes mainly necrosis but not apoptosis in tumor cells.
Figure 4 Microscopic appearances of tumor specimens. (A and B) Necrosis was not induced in tumors in group 1 or 2 (hematoxylin and eosin staining, ×200). (C) Ferucarbotran was injected into tumors (arrows) and tumor cells around ferucarbotran in group 3 showed broad necrosis after inductive hyperthermia (arrowheads, hematoxylin and eosin staining, ×200 field).
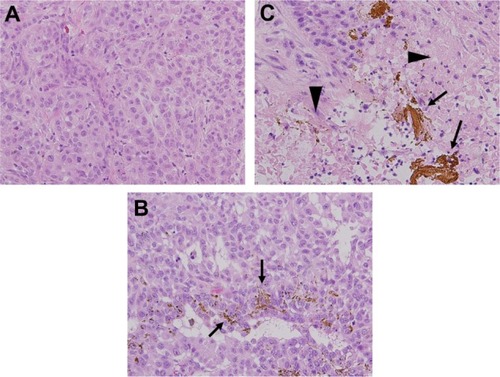
Figure 5 TUNEL staining of tumors in each group. (A) Positive control for TUNEL staining. Brown staining cells indicate TUNEL-positive cells, (B) group 1, and (C) group 2. Irradiation of alternating magnetic field alone or injection of ferucarbotran alone did not induce apoptosis in lung cancer cells. (D) In group 3, inductive hyperthermia did not increase apoptotic cells around ferucarbotran (arrows) compared with groups 1 or 2 (TUNEL staining ×200 field).
Adverse events
No treatment-related deaths or toxicities related to injection of ferucarbotran were reported. Three of 14 mice in group 3 showed a transient decrease in body weight, but no severe adverse events, such as ulcers in the region of heating, growth impairment, or loss of a leg, were observed. No adverse events were seen in animals from groups 1 or 2.
Discussion
We demonstrated the activity and safety of inductive hyperthermia using clinically available ferucarbotran in the treatment of lung cancer xenografts in an in vivo model. Our results indicate that inductive hyperthermia using ferucarbotran stably heated the whole tumor to approximately 45°C during most of the treatment period (), and subsequently induced mainly necrosis of tumor cells, as reported previously.Citation25,Citation26 Hyperthermia at temperatures above 44°C has been reported to cause necrosis of tumor cells.Citation25,Citation26 Further similar results have been reported in inductive hyperthermia models in vivo. Takamatsu et alCitation13 reported that induction heating using ferucarbotran did not increase apoptosis in treated tumor cells, and Wang et alCitation27 reported that most tumor cells heated to a higher temperature became necrotic in response to inductive hyperthermia, as we demonstrated in this study. In contrast, normal tissues without ferucarbotran were not heated in an alternating magnetic field. These findings indicate stable, efficient, and selective heating by inductive hyperthermia using ferucarbotran.
The safety and permissible dose of ferucarbotran in medical practice has already been established.Citation18–Citation20 Although there are numerous magnetic nanoparticles used in inductive hyperthermic experiments, most of them are not approved for human use, and only a few safe magnetic nanoparticles, such as ferumoxides (Feridex®, AMAG Pharmaceuticals Inc, Lexington, MA, USA), have been developed for medical practice. Although the ferumoxides approved as a magnetic resonance imaging contrast agent produce heat in an alternating magnetic field, we confirmed that the heat-generating efficiency of ferucarbotran in an alternating magnetic field was superior to that of ferumoxides in a preliminary in vitro study (data not shown). Therefore, we consider that inductive hyperthermia using ferucarbotran is a beneficial and feasible hyperthermic approach.
Experimental and clinical studies have indicated that heat plays a crucial role in human lung cancer.Citation28–Citation30 However, despite promising results, hyperthermia has yet to be established as a routine clinical treatment due to lack of availability of safe magnetic nanoparticles and techniques for clinical hyperthermia. The effects of inductive hyperthermia on lung cancer xenografts have been investigated, and reported to be promising by Hu et al.Citation28,Citation29 However, the magnetic nanoparticles used in their studies were approved for laboratory use, but not for human use. Inductive hyperthermia using clinically available ferucarbotran had marked antitumor effects on lung cancer xenografts, and the effectiveness of our hyperthermia strategy was similar to that of previous studies using magnetic nanoparticles for laboratory use.
Moreover, we used not only ferucarbotran in this study, but also a powerful and safe induction heating machine developed for human use.Citation13 Our results strongly suggest that inductive hyperthermic treatment using a combination of clinically available and safe ferucarbotran and machines has the potential for application in clinical settings. To develop inductive hyperthermia for lung cancer further, we need to deliver ferucarbotran to target tumor sites selectively using clinically available techniques. One possible solution to this problem is delivery of ferucarbotran to the feeding arteries of tumors using transcatheter arterial embolization, as previously reported.Citation13 Furthermore, computed tomography-guided percutaneous injection of ferucarbotran or endobronchial ultrasound-guided transbronchial injection into target tumors using bronchoscopy can be used to assess the exact location of the ferucarbotran injection. These techniques may enable delivery of ferucarbotran selectively to target tumor sites.
Finally, despite the satisfactory antitumor effects and many advantages of ferucarbotran, complete tumor suppression was not observed in this study. In previous studies, tolerable hyperthermia alone was reported to be insufficient for complete suppression of tumors,Citation27,Citation31 so combination therapy with hyperthermia is necessary for complete tumor suppression. We are continuing our search for suitable drugs to use concomitantly with inductive hyperthermia and ferucarbotran to enhance the antitumor effects.
This study has several limitations. First, we only heated the tumors to 45°C to avoid thermal damage to other organs, particularly in an animal as small as the nude mouse. Therefore, the effects of induction heating at higher temperatures are unknown. Second, the surface of the induction heating applicator was set at 2 cm from the target lesions, which is too superficial to refect deep organ cancers in adult humans in clinical practice. Further investigation is needed to confirm the effectiveness and safety of this method in the treatment of lung cancer in humans.
Conclusion
Inductive hyperthermia using ferucarbotran is a beneficial and promising approach in the treatment of lung cancer. Ferucarbotran is a novel tool needing further development in inductive hyperthermia.
Disclosure
The authors report no conflicts of interest in this work.
References
- BabaHStephensLCStrebelFRProtective effect of ICRF-187 against normal tissue injury induced by adriamycin in combination with whole body hyperthermiaCancer Res19915113356835771905199
- IkedaNHayashidaOKamedaHItoHMatsudaTExperimental study on thermal damage to dog normal brainInt J Hyperthermia19941045535617963810
- LinJCWangYJInterstitial microwave antennas for thermal therapyInt J Hyperthermia19873137473559297
- StaufferPRCetasTCFletcherAMObservations on the use of ferromagnetic implants for inducing hyperthermiaIEEE Trans Biomed Eng198431176906724613
- DeNardoSJDeNardoGLMiersLADevelopment of tumor targeting bioprobes ((111)In-chimeric L6 monoclonal antibody nanoparticles) for alternating magnetic field cancer therapyClin Cancer Res200511197087s7092s16203807
- JordanAWustPFählingHJohnWHinzAFelixRInductive heating of ferrimagnetic particles and magnetic fluids: physical evaluation of their potential for hyperthermiaInt J Hyperthermia19939151688433026
- FanninPCMagnetic spectroscopy as an aide in understanding magnetic fluidsJ Magn Magn Mater20022525964
- MaMWuYZhouJSunYZhangYGuNSize dependence of specific power absorption of Fe3O4 particles in AC magnetic fieldJ Magn Magn Mater20042683339
- ShinkaiMYanaseMHondaHWakabayashiTYoshidaJKobayashiTIntracellular hyperthermia for cancer using magnetite cationic liposomes: in vitro studyJpn J Cancer Res19968711117911839045948
- MitsumoriMHiraokaMShibataTTargeted hyperthermia using dextran magnetite complex: a new treatment modality for liver tumorsHepatogastroenterology19964312143114378975944
- HilgerIFruhaufKAndraWHiergeistRHergtRKaiserWAHeating potential of iron oxides for therapeutic purposes in interventional radiologyAcad Radiol20029219820211918373
- MitsumoriMHiraokaMShibataTDevelopment of intra-arterial hyperthermia using a dextran-magnetite complexInt J Hyperthermia19941067857937533813
- TakamatsuSMatsuiOGabataTSelective induction hyperthermia following transcatheter arterial embolization with a mixture of nano-sized magnetic particles (ferucarbotran) and embolic materials: feasibility study in rabbitsRadiat Med200826417918718509717
- YamadaKOdaTHashimotoSMinimally required heat doses for various tumour sizes in induction heating cancer therapy determined by computer simulation using experimental dataInt J Hyperthermia201026546547420377361
- MuraseKOonokiJTakataHSimulation and experimental studies on magnetic hyperthermia with use of superparamagnetic iron oxide nanoparticlesRadiol Phys Technol20114219420221667079
- JohannsenMGneveckowUEckeltLClinical hyperthermia of prostate cancer using magnetic nanoparticles: presentation of a new interstitial techniqueInt J Hyperthermia200521763764716304715
- ItoATanakaKHondaHAbeSYamaguchiHKobayashiTComplete regression of mouse mammary carcinoma with a size greater than 15 mm by frequent repeated hyperthermia using magnetite nanoparticlesJ Biosci Bioeng200396436436916233538
- ReimerPBalzerTFerucarbotran (Resovist): a new clinically approved RES-specific contrast agent for contrast-enhanced MRI of the liver: properties, clinical development, and applicationsEur Radiol20031361266127612764641
- HammBStaksTTaupitzMContrast-enhanced MR imaging of liver and spleen: first experience in humans with a new superparamagnetic iron oxideJ Magn Reson Imaging1994456596687981510
- HirohashiSIchikawaTTanimotoADose investigation of superparamagnetic iron oxide (SPIO) SH U 555 A in liver MR imagingNippon Igaku Hoshasen Gakkai Zasshi2003639539550 Japanese14699862
- CiardielloFBiancoRDamianoVAntitumor activity of sequential treatment with topotecan and antiepidermal growth factor receptor monoclonal antibody C225Clin Cancer Res19995490991610213228
- International Commission on Non-Ionizing Radiation ProtectionGuidelines for limiting exposure to time-varying electric, magnetic, and electromagnetic fields (up to 300 GHz); ICNIRP guidelinesHealth Phys1998744945229525427
- DeweyWCHopwoodLESaparetoSAGerweckLECellular responses to combinations of hyperthermia and radiationRadiology19771232463474322205
- MagistrelliPCoppolaRToniniGApoptotic index or a combination of Bax/Bcl-2 expression correlate with survival after resection of pancreatic adenocarcinomaJ Cell Biochem20069719810816173075
- ItoAHondaKKobayashiTCancer immunotherapy based on intracellular hyperthermia using magnetite nanoparticles: a novel concept of “heat-controlled necrosis” with heat shock protein expressionCancer Immunol Immunother200655332032816133113
- YonezawaMOtsukaTMatsuiNHyperthermia induces apoptosis in malignant fibrous histiocytoma cells in vitroInt J Cancer19966633473518621256
- WangLDongJOuyangWWangXTangJAnticancer effect and feasibility study of hyperthermia treatment of pancreatic cancer using magnetic nanoparticlesOncol Rep201227371972622134718
- HuRMaSLiHEffect of magnetic fluid hyperthermia on lung cancer nodules in a murine modelOncol Lett2011261161116422848282
- HuRZhangXLiuXHigher temperature improves the efficacy of magnetic fluid hyperthermia for Lewis lung cancer in a mouse modelThorac Cancer201233439
- JiangZYa nWMingJDocetaxel weekly regimen in conjunction with RF hyperthermia for pretreated locally advanced non-small cell lung cancer: a preliminary studyBMC Cancer2007718917919338
- HeiTKHallEJKushnerSOsmakRSHyperthermia, chemotherapeutic agents and oncogenic transformationInt J Hyperthermia1986233113202432136