Abstract
Background
Bevacizumab plus chemotherapy prolongs progression-free survival (PFS) and overall survival (OS) in metastatic colorectal cancer (mCRC). Although there is strong evidence to suggest that the mutational status of the K-ras oncogene has a role as a predictive factor for activity in patients treated with cetuximab and panitumumab, few data have been obtained in patients treated with bevacizumab. We conducted an additional retrospective analysis to investigate the prognostic value of K-ras mutation relative to mCRC first-line treatment with bevacizumab.
Materials and methods
A total of 108 patients were retrospectively reviewed. K-ras status was assessed in the overall population by sequencing. Statistical association for PFS and OS was analyzed using the Kaplan–Meier method, and the prognostic role of K-ras was determined using the logrank test.
Results
Median PFS was 10 months both for patients with wild-type (WT) K-ras and mutated (MT) K-ras (hazard ratio [HR] 0.94, P=0.75); neither difference in median OS was significant (27 months WT K-ras versus 26 months MT K-ras, HR 0.92; P=0.70). A further analysis was carried out in the two groups according to metastatic sites. No statistically significant difference in terms of PFS and OS was demonstrated between WT K-ras and MT K-ras with liver metastases only and in those with extrahepatic disease.
Conclusion
Although further study is required, our results seem to confirm that K-ras mutation does not have a prognostic role in mCRC patients receiving first-line treatment with bevacizumab.
Introduction
Predictive and prognostic tumor markers are playing an increasingly important role in personalized metastatic colorectal cancer (mCRC) care.Citation1,Citation2 These tags range from conventional single protein-, ribonucleic acid- or deoxyribonucleic acid (DNA)-based markers to molecular signatures based on multiplex assays, and they are used to guide clinical management of patients.Citation3 Currently, biomolecular factors with ascertained predictive and prognostic value in mCRC are the K-ras and BRAF oncogenes, respectively.Citation4–Citation10 K-ras, the human homologue of the Kirsten rat sarcoma 2 virus oncogene, encodes a small guanosine triphosphate-binding protein that acts as a self-inactivating signal transducer by cycling from guanosine diphosphate- to guanosine triphosphate-bound states in response to stimulation of a cell-surface receptor.Citation11,Citation12 K-ras can harbor oncogenic mutation that yields a constitutively active protein. Mutations (mainly at exons 2 and 3) occur early in the progression of colorectal adenoma to carcinoma and they are found in approximately 30%–50% of CRC.Citation5,Citation11,Citation13,Citation14 The identification of a biologic and a clinical role for K-ras mutation marked a turning point in the scenario of mCRC treatment. As a matter of a fact, the importance of K-ras and its downstream signaling pathways in tumor development is well established. In the last few years, K-ras mutation has acquired an essential clinical significance as a negative predictive factor in mCRC patients treated with anti-epidermal growth factor receptor (EGFR) agents, such as cetuximab and panitumumab.Citation1,Citation2 In addition, the prognostic value of tumor K-ras status has been extensively evaluated, but results have been conflicting. Some studies have demonstrated a prognostic effect,Citation1,Citation5 while others have failed.Citation6
The interaction of EGFR and vascular endothelial growth factor (VEGF) is well known.Citation15,Citation16 Bevacizumab, a humanized monoclonal antibody that binds to and neutralizes VEGF-A, has been widely used in various mCRC treatment lines, improving response rates, progression-free survival (PFS), and overall survival (OS).Citation17–Citation24 At the time, anti-VEGF predictive and prognostic factors were not found, and the role of K-ras-mutation status in patients undergoing treatment with bevacizumab remains of great interest. Furthermore, it has been ascertained that K-ras mutation is also associated with specific clinical signatures of tumor as an increased risk of metastasis in lung and brain, even if does not modify the risk of metastasis in the liver.Citation25
In a previous study, we examined the role of K-ras status as a predictive factor of response on antiangiogenic therapy, affirming that bevacizumab provides clinical benefit and objective response rate in mCRC patients independently of K-ras expression and metastatic sites.Citation26
Moving from such an appealing biological and clinical background, we planned an additional retrospective analysis aimed at the identification of a possible prognostic role of K-ras in mCRC patients treated with first-line chemotherapy plus bevacizumab. Correlations between K-ras status and PFS and OS were analyzed. Furthermore, we evaluated whether K-ras mutation could influence the outcome of patients with liver metastases only, in comparison to patients with extra-hepatic disease.
Materials and methods
Patients and study design
The design of this study has been reported previously.Citation26 In brief, this was a retrospective study enrolling consecutive 108 unresectable mCRC patients, treated in clinical practice in three Italian oncology centers (Rome, Latina, Gaeta) between September 2008 and March 2011. Patients with histologically confirmed mCRC, Eastern Cooperative Oncology Group performance status ≤2, assessment of K-ras mutation status, no previous chemotherapy for advanced disease, treated with first-line chemotherapy with either the FOLFOX (oxaliplatin, leucovorin, and fluorouracil) or FOLFIRI (folic acid, fluorouracil, and irinotecan) regimen combined with bevacizumab, not a candidate for liver metastasis resection after chemotherapy, with adequate hepatic and renal function, and no contraindications to bevacizumab therapy were included.
The primary end point of this study was to assess the prognostic value of tumor K-ras status on PFS and OS of mCRC patients treated with first-line chemotherapy plus bevacizumab; secondary end points included evaluation of how K-ras mutation could influence the outcome of patients with liver metastases only with respect to patients with extrahepatic disease, clinical response PFS and OS of bevacizumab combined with chemotherapy in the overall population, and whether these same parameters differ between patients with hepatic metastases only and those with extrahepatic metastatic sites. Response rates to treatment with bevacizumab according to K-ras expression have been reported in our previous retrospective study.Citation26 Survival results were last updated in March 2012. PFS was defined as the time from the beginning of the treatment until the first observation of disease progression or death from any cause, while OS was defined as the time from the beginning of the treatment until death from any cause. Finally, patients with extrahepatic metastatic disease were defined as patients with metastases both in the liver and in other organs or with metastases not present in the liver but in other sites.
K-ras-mutation analysis
Evaluation of K-ras status was performed retrospectively on primary tumor samples only. K-ras analysis was performed centrally using existing platforms. DNA was extracted from paraffin-embedded CRC samples after histological control for at least 50% of tumor cells. Mutations at codons 12, 13, and 61 were assessed by means of direct sequencing with a polymerase chain reaction (PCR) method.Citation27
Statistical analysis
PFS and OS curves were calculated according to K-ras tumor-mutation status using the Kaplan–Meier method. The prognostic value of the biological marker was determined using the logrank test. A P-value of less than 0.05 was considered significant.
Results
A total of 108 mCRC patients were consecutively enrolled in three Italian oncology units between September 2008 and March 2011. Tumor K-ras status was assessed in the entire population. In total, 69 (64%) patients had wild-type (WT) K-ras tumors, while 39 (36%) patients had mutated (MT) K-ras. The main patient characteristics according to K-ras status are shown in . Significant differences were observed between the two groups in primary side of origin, metastatic sites, primitive tumor resection, prior adjuvant chemotherapy, and chemotherapy first-line regimen. Regarding metastatic sites, liver metastases only were present in 53 patients (41 WT K-ras patients versus twelve MT K-ras patients), extrahepatic disease affected 55 patients (28 WT K-ras patients versus 27 MT K-ras patients); among the extrahepatic sites were reported 56% lung, 31% peritoneal, 2% brain, and 11% other sites. In particular, 61.3% and 2% of patients with lung and brain metastases, respectively, had MT K-ras tumors.
Table 1 Baseline characteristics of patients included in the K-ras analysis according to tumor K-ras status
PFS and OS were obtained from 106 of 108 patients, as two patients were lost to follow-up. In the overall population, median PFS (mPFS) and median OS (mOS) were 10 months (95% confidence interval [CI] 8.9–11.1) and 26 months (95% CI 23.5–28.5), respectively, with 86 (81%) deaths at last survival update.
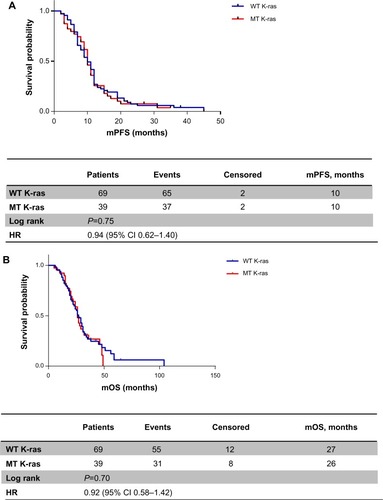
mPFS was the same between WT K-ras and MT K-ras (10 months, hazard ratio [HR] 0.94, 95% CI 0.62–1.40, P=0.75) (). No statistically significant difference was observed in mOS either: patients with WT K-ras tumors had an mOS of 27 months versus 26 months for patients with MT K-ras tumors (HR 0.92, 95% CI 0.58–1.42; P=0.70), with 55 versus 31 death events in the two groups, respectively ().
Figure 1 (A) Progression-free survival according to K-ras status; (B) overall survival according to K-ras status.
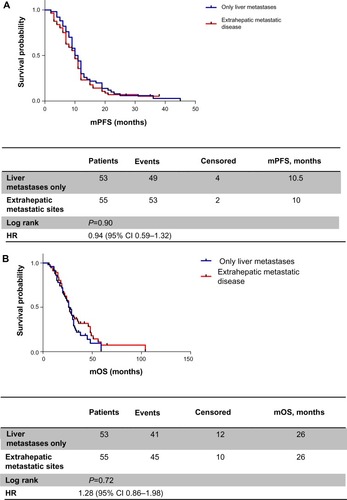
When patients were analyzed for metastatic sites, mPFS was 10.5 months in patients with liver metastases only versus 10 months in the extrahepatic disease group (HR 0.90, 95% CI 0.59–1.32; P=0.59) (); similarly, identical mOS was observed in both groups (26 months, HR 1.28, 95% CI 0.86–1.98; P=0.72) ().
Figure 2 (A) Progression-free survival according to metastatic site; (B) overall survival according to metastatic site.
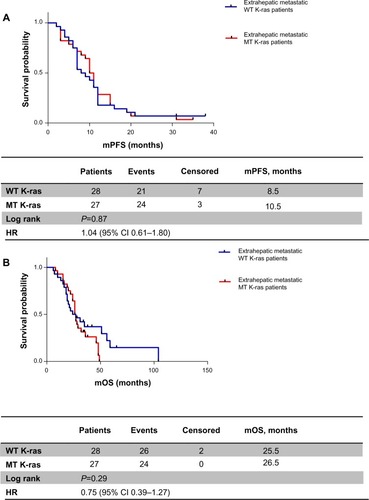
Analyzing these two subpopulations according to K-ras expression, we obtained the following results: in patients with liver metastases only, mPFS was 11 months in the WT K-ras group versus 10 months in the MT K-ras group (HR 0.88, 95% CI 0.41–1.77; P=0.70) (); mOS was greater in WT K-ras patients than in MT K-ras patients, but it was not statistically significant (27 months versus 21 months, respectively, HR 1.11, 95% CI 0.50–2.45; P=0.78) (). When metastatic disease was also extrahepatic or extrahepatic only, mPFS was 8.5 months in the WT K-ras group versus 10.5 months in the MT K-ras group (HR 1.04, 95% CI 0.61–1.80; P=0.87) (), while mOS was 25.5 versus 26.5 months, respectively (HR 0.75, 95% CI 0.39–1.27; P=0.29) ().
Figure 3 (A) Progression-free survival according to K-ras status in liver-only metastasis patients; (B) overall survival according to K-ras status in liver-only metastasis patients.
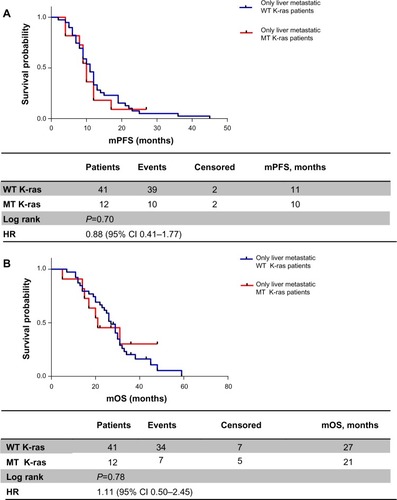
Figure 4 (A) Progression-free survival according to K-ras status in extrahepatic metastatic patients; (B) overall survival according to K-ras status in extrahepatic metastatic patients.
The administration of subsequent therapy lines after the discontinuation of first-line chemotherapy was examined. Patients treated with first-line FOLFIRI received second-line FOLFOX chemotherapy, and vice versa. All patients with WT K-ras tumors received either chemotherapy plus cetuximab in second-line therapy or one of the two anti-EGFR agents in third-line treatment (cetuximab in combination with irinotecan or monotherapy with panitumumab). However anti-EGFR agents did not induce OS difference between WT K-ras and MT K-ras tumors.
Discussion
In the overall population, the PFS and OS data regarding first-line mCRC treatment with chemotherapy and bevacizumab were modeled on literature data.Citation18,Citation21 Bevacizumab effectiveness was confirmed both in liver metastasis-only patients and in extrahepatic metastatic patients. The outcome of patients was not affected by subsequent anti-EGFR first-line chemotherapy; at the same time, several studies reported no difference between first-line chemotherapy based on the FOLFIRI regimen followed by second-line FOLFOX therapy, and vice versa.Citation28–Citation30
In this study, we addressed an important question, ie, whether K-ras status is a prognostic factor in mCRC patients receiving first-line chemotherapy plus bevacizumab, given the intimate relationship between the EGFR and VEGF signaling pathways. Our retrospective experience indicates that tumor K-ras status does not influence outcomes of patients treated with antiangiogenic therapy: patients with WT K-ras tumors had identical clinical benefit as those with MT K-ras tumors in terms of PFS (10 months) and near-identical clinical benefit in terms of OS (27 versus 26 months, respectively). A trend was observed in WT K-ras patients with liver metastases only compared with MT K-ras patients (PFS 11 versus 10 months and OS 27 versus 21 months, respectively), but this advantage was not statistically significant.
On the other hand, patients with MT K-ras tumors and extrahepatic disease appear to have a better prognosis than the WT K-ras group. Moreover, K-ras mutation is more frequently associated with extrahepatic disease, such as in the lung and brain, according to literature data.Citation25 Some studies have suggested that MT K-ras is associated with aggressive mCRC due to the presence of extrahepatic metastases,Citation31,Citation32 though in our analysis K-ras mutation was not correlated with poor prognoses.
Several studies have evaluated the role of K-ras status in mCRC patients receiving first-line treatment with bevacizumab (). Ince et al did not establish a statistically significant difference in terms of OS between WT K-ras and MT K-ras in bevacizumab-treated patients.Citation33 In a subsequent analysis by Hurwitz et al, PFS was greater for bevacizumab-treated patients with WT versus MT K-ras tumors.Citation34 Recently, Díaz-Rubio et al suggested a prognostic role for K-ras status in mCRC patients who were enrolled in the MACRO (maintenance treatment in advanced colorectal cancer) trial.Citation35 A statistically significant difference was observed in OS and PFS between WT K-ras tumors and MT K-ras tumors, with a greater benefit of bevacizumab treatment in WT K-ras patients. On the contrary, other studies have reported that K-ras mutation is not a prognostic factor for patient outcome.Citation24,Citation36–Citation39 Masi et al claimed that patients with K-ras mutations derive similar clinical benefit from bevacizumab compared with patients who have WT K-ras.Citation36 This variability in results could be a result of several factors, including the number of patients included in different trials, the proportions of patients tested for K-ras mutation status and technology used, the chemotherapy regime under investigation, and subsequent second- and third-line therapies.Citation39
Table 2 Summary of K-ras data from studies of bevacizumab + chemotherapy in mCRC patients
Our analysis seems to be in agreement with studies that did not suggest any therapeutic implication of K-ras mutation when bevacizumab was given in addition to chemotherapy,Citation24,Citation36–Citation39 but our study has several limitations. Firstly, K-ras-mutation analysis was retrospective, which is common in randomized studies examining the role of biomarkers. Secondly, the number of patients in the overall population was small, resulting in imbalance parameters between WT and MT K-ras tumors. In addition, K-ras-status evaluation was performed only on primitive tumors.
In conclusion, this retrospective analysis found that chemotherapy plus bevacizumab is a reliable first-line therapeutic approach in mCRC, regardless of whether metastases are liver only or also extrahepatic. Finally, K-ras status did not appear to affect the outcome of patients receiving antiangiogenic therapy, according to some literature reports, but not others. The reasons for the discrepancy between studies are not yet apparent or sufficiently powered. The importance of K-ras mutation status remains to be explored, and future studies will be required to answer this question.
Disclosure
The authors report no conflicts of interest in this work.
References
- LièvreABachetJBLe CorreDKRAS mutation status is predictive of response to cetuximab therapy in colorectal cancerCancer Res2006663992399516618717
- AmadoRGWolfMPeetersMWild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancerJ Clin Oncol2008261626163418316791
- McShaneLMHayesDFPublication of tumor marker research results: the necessity for complete and transparent reportingJ Clin Oncol2012304223423223071235
- KarapetisCSKhambata-FordSJonkerDJKRAS mutations and benefit from cetuximab in advanced colorectal cancerN Engl J Med20083591757176518946061
- AndreyevHJNormanARCunninghamDKirsten ras mutations in patients with colorectal cancer: the ‘RASCAL II’ studyBr J Cancer20018569269611531254
- RothADTejparSDelorenziMPrognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 TrialJ Clin Oncol20102846647420008640
- SaridakiZPapadatos-PastosDTzardiMBRAF mutations, microsatellite instability status and cyclin D1 expression predict metastatic colorectal patients’ outcomeBr J Cancer20101021762176820485284
- SouglakosJPhilipsJWangRPrognostic and predictive value of common mutations for treatment response and survival in patients with metastatic colorectal cancerBr J Cancer200910146547219603024
- TolJNagtegaalIDPuntCJBRAF mutation in metastatic colorectal cancerN Engl J Med2009361989919571295
- SamowitzWSSweeneyCHerrickJPoor survival associated with the BRAF V600E mutation in microsatellite-stable colon cancersCancer Res2005656063606916024606
- DownwardJTargeting RAS signalling pathways in cancer therapyNat Rev Cancer20033112212509763
- SchubbertSShannonKBollagGHyperactive Ras in developmental disorders and cancerNat Rev Cancer2007729530817384584
- MalumbresMBarbacidMRAS oncogenes: the first 30 yearsNat Rev Cancer2003345946512778136
- BosJLRas oncogenes in human cancer: a reviewCancer Res198949468246892547513
- RakJYuJLKerbelRSCoomberBLWhat do oncogenic mutations have to do with angiogenesis/vascular dependence of tumors?Cancer Res2002621931193411929804
- MizukamiYKohgoYChungDCHypoxia inducible factor-1 independent pathways in tumor angiogenesisClin Cancer Res20026219311934
- KabbinavarFHurwitzHIFehrenbacherLPhase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with MCRCJ Clin Oncol200321606512506171
- KabbinavarFFSchulzJMcCleodMAddition of bevacizumab to bolus fluorouracil and leucoverin in first-line metastatic colorectal cancer: results of a randomized phase II trialJ Clin Oncol2005233697370415738537
- HurwitzHFehrenbacherLNovontnyWBevacizumab plus irinotecan, fluorouracil and leucovorin for metastatic colorectal cancerN Engl J Med20043502335234215175435
- GiantonioBJCatalanoPJNealJBevacizumab in combination with oxaliplatin, fluorouracil and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200J Clin Oncol2007251539154417442997
- SaltzLBClarkeSDíaz-RubioEBevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III studyJ Clin Oncol2008262013201918421054
- WongRCunninghamDBarbachanoYA multicentre study of capecitabine, oxaliplatin plus bevacizumab as perioperative treatment of patients with poor-risk colorectal liver-only metastases not selected for up-front resectionAnn Oncol2011222042204821285134
- Díaz-RubioEGómez-EspañaAMassutíBFirst-line XELOX plus bevacizumab followed by XELOX plus bevacizumab or single agent bevacizumab as maintenance therapy in patients with metastatic colorectal cancer: the phase III MACRO TTD studyOncologist201217152522234633
- BennounaJSastreJArnoldDContinuation of bevacizumab after progression in metastatic colorectal cancer (ML18147): a randomized phase III trialLancet Oncol201314293723168366
- TieJLiptonLDesaiJKRAS mutation is associated with lung metastasis in patients with curatively resected colorectal cancerClin Cancer Res2011171122113021239505
- RossiLVeltriEZulloABevacizumab plus chemotherapy in metastatic colorectal cancer patients treated in clinical practiceFuture Oncol201281193119723030493
- SangerFNicklenFCoulsonARDNA sequencing with chain-terminating inhibitorsProc Natl Acad Sci U S A19777454635467271968
- TournigandCAndréTAchilleEFOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR studyJ Clin Oncol20042222923714657227
- ColucciGGebbiaVPaolettiGPhase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: a multicenter study of the Gruppo Oncologico Dell’Italia MeridionaleJ Clin Oncol2005234866487515939922
- BendellJCBekaii-SaabTSCohnALTreatment patterns and clinical outcomes in patients with metastatic colorectal cancer initially treated with FOLFOX-bevacizumab or FOLFIRI-bevacizumab: results from ARIES, a bevacizumab observational cohort studyOncologist2012171486149523015662
- ModestDPStintzingSLaubenderRPClinical characterization of patients with metastatic colorectal cancer depending on the KRAS statusAnticancer Drugs20112291391821795973
- NashGMGimbelMShiaJKRAS mutation correlates with accelerated metastatic progression in patients with colorectal liver metastasesAnn Surg Oncol20101757257819727962
- InceWLJubbAMHoldenSNAssociation of KRAS, b-raf, and p53 status with the treatment effect of bevacizumabJ Natl Cancer Inst20059798198915998951
- HurwitzHIYiJInceWNovotnyWFRosenOThe clinical benefit of bevacizumab in metastatic colorectal cancer is independent of KRAS mutation status: analysis of phase III study of bevacizumab with chemotherapy in previously untreated metastatic colorectal cancerOncologist200914222819144677
- Díaz-RubioEGómez-EspañaAMassutíBRole of KRAS status in patients with metastatic colorectal cancer receiving first-line chemotherapy plus bevacizumab: a TTD group cooperative studyPLoS One20127e4734523174912
- MasiGLoupakisFSalvatoreLBevacizumab with FOLFOXIRI (irinotecan, oxaliplatin, fluorouracil, and folinate) as first-line treatment for metastatic colorectal cancer: a phase 2 trialLancet Oncol20111184585220702138
- TolJKoopmanMCatsAChemotherapy, bevacizumab and cetuximab in metastatic colorectal cancerN Engl J Med200936056357019196673
- HechtJRMitchellEChidiacTA randomized phase IIIB trial of chemotherapy, bevacizumab and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancerJ Clin Oncol20092767268019114685
- PriceTJHardinghamJELeeCKImpact of KRAS and BRAF gene mutation status on outcomes from the phase III AGITG MAX trial of capecitabine alone or in combination with bevacizumab and mitomycin in advanced colorectal cancerJ Clin Oncol2011292675268221646616