Abstract
Interleukin (IL)-12 has emerged to be a prospective molecule for antitumor therapies with many types of cancers. Here we examine the effect of IL-12 treatment in preventing the colorectal cancer liver metastasis in a mice model. At different doses, we found that IL-12 treatment decreased the formation of liver metastasis sites and the percentage of metastasis volume in the liver. Additionally, this treatment leads to improved survival function of mice after tumor cell transplantation. We believe that IL-12 based therapy provided a novel treatment to colorectal cancer patients suffering from liver metastasis.
Introduction
Colorectal cancer has been one of the leading causes of mortality by cancer in recent years.Citation1–Citation4 Liver metastases are common for colon cancer patients even after surgery, with poor prognosis.Citation4–Citation7 The treatments for existing liver metastasis or prevention of in vivo cancer metastasis are therefore important to improve the life quality and survival of these patients.
Interleukin (IL)-12 has emerged to be a prospective molecule for antitumor therapies.Citation8–Citation11 IL-12 showed its effects in antagonizing or negatively modulating different types of cancers, as diagnosing factors;Citation10–Citation14 while its potential in treating the colorectal cancer liver metastasis has not been fully explored.Citation8,Citation15–Citation17 In the present study we investigated the possibility of IL-12 based treatment to the colorectal cancer liver metastasis in a mice model.
Materials and methods
Animals
130 Balb/c mice (male, 9–10 weeks) were obtained from and housed in the animal center of Guangzhou Medical College (Guangzhou, People’s Republic of China). This study was approved by the Ethics Committee of Animal Research in Guangzhou Medical College.
Colorectal cancer liver metastasis model
CT-26-murine colorectal adenocarcinoma cells (Shengao Shengwu, Shanghai, People’s Republic of China) were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) with 10% fetal bovine serum (FBS) (Shenggong Biotech, Shanghai, People’s Republic of China) as previously described in Xu et al,Citation15 at 37°C in 95% O2 and 5% CO2.
The animals were injected with 0.2 mL of 1 × 106 per mL CT26 cells via peritoneal cavity spleen transplant. Every 4 days (starting on the day of transplantation), the mice received 0.9% saline (control group, 20 mice), or 0.2 μg (40 mice), 0.5 μg (35 mice), 1 μg (35 mice) of recombinant murine IL-12 (Sigma and Shenggong Biotech, Shanghai, People’s Republic of China) injection.
Measurements
10 days after treatment, 3 mL blood samples were obtained from the tail vein. The serum was immediately isolated with the centrifuge and the circulating interferon-gamma (IFN-γ) levels were detected by ELISA kits (Shunhe Biotech, Shanghai, People’s Republic of China).Citation15
20 days after the injection of CT26 cells, the animals (10 from each group) were sacrificed and the number of hepatic metastases were counted. The ratio of metastasis volume over the whole liver was calculated.
For other groups of animals, the treatment lasted until the death of the animals (maximum 90 days) for survival rate calculation.
Statistics
The data were represented as mean ± standard deviation (SD), and analyzed with SPSS 16.0 software (SPSS Inc, Chicago, IL, USA). The Student’s t-test was used to compare intergroup differences and P < 0.05 was determined as statistically significant.
Results
IL-12 treatment lead to decreased metastasis
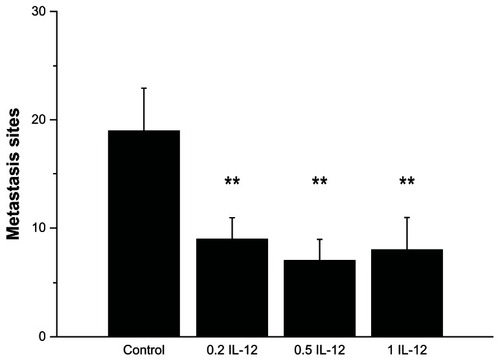
We found that IL-12 treatment decreased the number of liver metastasis sites in the liver as shown in . In the control group there were 19 ± 4 sites, while in the 0.2 μg, 0.5 μg or 1 μg IL-12 treatment groups there were 9 ± 2, 8 ± 2, and 7 ± 3 sites respectively (P < 0.01 to control) ().
Figure 1 0.2 μg, 0.5 μg or 1 μg IL-12 treatment decreased the number of metastasis sites in the liver.
Abbreviation: IL, interleukin.
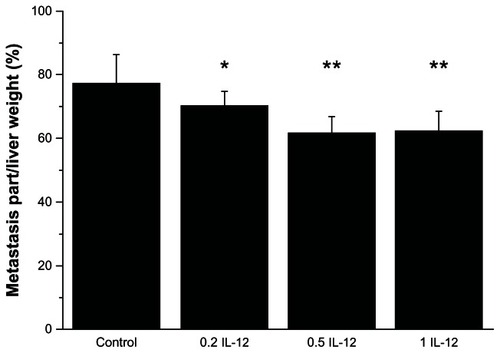
IL-12 treatment also decreased the percentage of the overall liver with metastasis volume (). In the control group the percentage was 77.2% ± 9.2%, while in the 0.2 μg, 0.5 μg or 1 μg IL-12 treatment groups were 70.4% ± 4.3%, 61.9% ± 4.8%, and 62.3% ± 6.4%, respectively (P < 0.05, 0.01 and 0.01 to control).
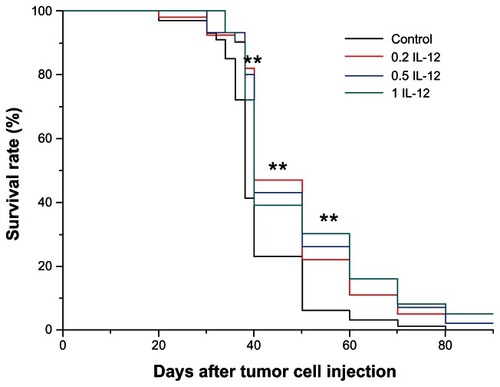
IL-12 treatment increased the survival rate of animals
We found that IL-12 treatment significantly increased the survival time length of the animals (). Black: control, Red: 0.2 μg, Blue: 0.5 μg, and Cyan: 1 μg ().
IL-12 treatment increased the blood circulating level of IFN-γ
We found that IL-12 treatment significantly increased the circulating IFN-γ level. In control group: 16.2 ± 4.1 μM/LS; 0.2 μg group: 36.1 ± 6.7 μM/LS (P < 0.01); 0.5 μg group: 38.0 ± 2.9 μM/LS (P < 0.01); 1 μg group: 30.3 ± 5.6 μM/LS (P < 0.01).
Discussion
Many colorectal cancer patients demonstrated liver metastasis after the surgery, and contributed to the major cause of death in patients at advanced stages. The management of liver metastasis often includes surgical removal, chemotherapy and other biological treatments. Different types of molecules, such as immunomodulating cytokines, have been adopted to treat liver metastasis.Citation18–Citation21 In the present study we demonstrated that IL-12 treatment could effectively decrease the formation of liver metastasis sites and the percentage of metastasis volume in the liver. Additionally, this treatment leads to improved survival function of mice after tumor cell transplantation.
IL-12 is involved in the differentiation of naïve T cells into Th1 cells, and stimulates the growth and function of T cells. IL-12 also stimulates the production of tumor necrosis factor-alpha (TNF-α) and IFN-γ,Citation8,Citation22,Citation23 which is consistent with our present findings. This might mediate the beneficial effects of IL-12 treatment in preventing liver metastasis as IFN-γ could be anti-angiogenic and block new blood vessel formation through inducible protein-10.Citation22,Citation24,Citation25 These are potential molecules with combined pharmacological effects when targeted together with IL-12.
It should be noted that IL-12 administration could also lead to some side effects and adverse results. Several solutions are possible: (1) combined treatments could be taken to prevent these adverse reactions; (2) controlled release of IL-12 in a slow manner might be of some help;Citation16 (3) targeting the upstream/downstream signaling of IL-12 might lead to more physiological regulation of IFN-γ, yet the therapeutic effects are to be tested.
In conclusion, here we reported that IL-12 administration could be effective for colorectal cancer liver metastasis in a mice model. We expect to see the use of controlled release IL-12 for clinical treatment of colorectal cancer liver metastasis patients in the future.
Acknowledgments
The authors were supported by the Department of General Surgery. The authors were supported by the Guangdong Provincial Science & Technology Project (2009B060700019). All authors designed the study together and performed the experiment together; GZ, WG, LJ and BX analyzed the data and wrote the paper; all authors approved the final manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- JinKGaoWLuYMechanisms regulating colorectal cancer cell metastasis into liverOncol Lett20123111522740847
- TomimaruYSasakiYYamadaTLiver metastasis originating from colorectal cancer with macroscopic portal vein tumor thrombosis: a case report and review of the literatureJ Med Case Rep2010438221110841
- OzturkEKilicturgaySYilmazlarTZorluogluAOzenYFactors affecting the prognosis of patients with colorectal liver metastasisActa Chir Belg201011018919420514831
- KulaylatMNGibbsJFRegional treatment of colorectal liver metastasisJ Surg Oncol201010169369820512945
- NamSJChoJYLeeHSChemotherapy-associated hepatopathy in korean colorectal cancer liver metastasis patients: oxaliplatin-based chemotherapy and sinusoidal injuryKorean J Pathol201246222923109974
- ZhangYPengZChenMElevated neutrophil to lymphocyte ratio might predict poor prognosis for colorectal liver metastasis after percutaneous radiofrequency ablationInt J Hyperthermia20122813214022335227
- ChiricaMLeconteMOberlinODoussetBSurgical treatment of liver metastasis in patients with colorectal cancerPresse Med201241586722138292
- YuzhalinAEKutikhinAGInterleukin-12: clinical usage and molecular markers of cancer susceptibilityGrowth Factors20123017619122515181
- BubenikJInterleukin 12 in cancer treatmentFolia Biol (Praha)2011571221457647
- TamandaniDMShekariMSuriVInterleukin-12 gene polymorphism and cervical cancer riskAm J Clin Oncol20093252452819487916
- NagashimaNNakayamaYInoueYPrognostic significance of the local expression of interleukin-12 in patients with advanced gastric cancerAnticancer Res2008281277128318505066
- HorinagaMHarschKMFukuyamaRHestonWLarchianWIntravesical interleukin-12 gene therapy in an orthotopic bladder cancer modelUrology20056646146616040105
- NikitinaEYDesaiSAZhaoXVersatile prostate cancer treatment with inducible caspase and interleukin-12Cancer Res2005654309431915899823
- YoshimuraCNomuraSKanazawaSComparison of interleukin-12 with lung cancer and malignant lymphoma undergoing autologous peripheral blood stem cell transplantationJ Cancer Res. Clin Oncol2002128293611862469
- XuQGuoLGuXPrevention of colorectal cancer liver metastasis by exploiting liver immunity via chitosan-TPP/nanoparticles formulated with IL-12Biomaterials2012333909391822374455
- Hallaj-NezhadiSLotfipourFDassCNanoparticle-mediated interleukin-12 cancer gene therapyJ Pharm Pharm Sci20101347248521092717
- CemazarMJarmTSersaGCancer electrogene therapy with interleukin-12Curr Gene Ther20101030031120560875
- EvenoCPocardMVEGF levels and the angiogenic potential of the microenvironment can affect surgical strategy for colorectal liver metastasisCell Adh Migr2012656957323257830
- DexiangZLiRYeWOutcome of patients with colorectal liver metastasis: analysis of 1,613 consecutive casesAnn Surg Oncol2012192860286822526903
- MarutaMMaedaKTrends in the treatment for liver metastasis of colorectal cancer in JapanRozhl Chir20119066967322509653
- ValadaoMReverse approach: a new paradigm in the treatment of synchronous liver metastasis from colorectal cancerRev Col Bras Cir20103731431521180994
- SangroBMeleroIQianCPrietoJGene therapy of cancer based on interleukin 12Curr Gene Ther2005557358116457647
- BrundaMJGatelyMKInterleukin-12: potential role in cancer therapyImportant Adv Oncol19953187672811
- NakayamaYInoueYNagashimaNRelationships between local and systemic expression of interleukin-12 and plasma levels of vascular endothelial growth factor in patients with gastric cancerAnticancer Res2004243289329415515423
- RakhmilevichALHooperATHicklinDJSondelPMTreatment of experimental breast cancer using interleukin-12 gene therapy combined with anti-vascular endothelial growth factor receptor-2 antibodyMol Cancer Ther2004396997615299079