Abstract
Background
The association of cancer stem cells with epithelial–mesenchymal transition (EMT) is receiving attention. We found in our previous study that EMT existed from CD24− phenotype cells to their differentiated cells. It was shown that cyclin D1 functioned in sustaining self-renewal independent of CDK4/CDK6 activation, but its effect on the EMT mechanism in ovarian cancer stem cells is unclear.
Methods
The anchorage-independent spheroids from ovarian adenocarcinoma cell line 3AO were formed in a serum-free medium. CD24− and CD24+ cells were isolated by fluorescence-activated cell sorting. Cell morphology, viability, apoptosis, and migratory ability were observed. Stem-related molecule Bmi-1, Oct-4 and EMT-related marker E-cadherin, and vimentin expressions were analyzed. Cyclin D1 expression in CD24− phenotype enriched spheroids was knocked down with small interfering RNA, and its effects on cell proliferation, apoptosis, migration ability, and EMT-related phenotype after transfection were observed.
Results
In our study, CD24− cells presented stronger proliferative, anti-apoptosis capacity, and migratory ability, than CD24+ cells or parental cells. CD24− cells grew with a scattered spindle-shape within 3 days of culture and transformed into a cobblestone-like shape, identical to CD24+ cells or parental cells at 7 days of culture. CD24− cells or spheroids highly expressed cyclin D1, Bmi-1, and vimentin, and seldom expressed E-cadherin, while CD24+ or parental cells showed the opposite expression. Furthermore, cyclin D1-targeted small interfering RNA resulted in decreased vimentin expression in spheroids. Transfected cells also exhibited an obvious decrease in cell viability and migration, but an increase in cell apoptosis.
Conclusion
Cancer stem cell-like cells possess mesenchymal characteristics and EMT ability, and cyclin D1 involves in EMT mechanism, suggesting that EMT of cancer stem cell-like cells may play a key role in invasion and metastasis of ovarian cancer.
Introduction
Epithelial ovarian carcinoma, accounting for approximately 90% of ovarian cancers, has always been the most lethal malignancy in the gynecological cancers.Citation1 In order to explore the exact biological features of epithelial ovarian carcinoma, a series of studies is focusing on cancer stem cells or cancer stem cell-like cells (CSC-LCs). Cancer stem cells or CSC-LCs have been identified in several solid tumors in breast, liver, prostate, and other sites.Citation2–Citation5 However, in light of recent studies, ovarian cancer stem cells have been found to present diverse phenotypes and functions.Citation6–Citation9 Our previous study has identified that the CSC-LCs derived from the epithelial ovarian cancer cell line 3AO have CD24− phenotype with higher capacity of tumorigenesis, drug-resistance, self-renewal, and differentiation abilities.Citation10
The epithelial–mesenchymal transition (EMT) is the well-known process whereby terminal differentiated epithelial cells convert into migratory mesenchymal cells in embryo development.Citation11 Recently, EMT phenomenon in cancer stem cells is also been paid increasing attention.Citation12 It was found that breast cancer stem cells with CD44high/CD24low phenotype existed in the circulation system of breast cancer patients, and further, they expressed epithelial–mesenchymal transition markers,Citation13 therefore this subset of cells was presumed to be the original source of breast cancer metastasis through EMT. Moreover, Mani et alCitation14 discovered that a small group of cells with stem cell features could express CD44high/CD24low phenotype by exogenous-induced EMT in the normal human and mouse mammary glands. It has been speculated that EMT may induce cancer cells to express stem cell phenotypes and thus acquire the abilities of migration and invasion.Citation15 In addition, drug-resistance of cancer cells was also regarded to be associated with EMT.Citation15 However, EMT in ovarian cancer stem cells and its effects on drug-resistance are still undiscovered. Preliminary findings in our previous study showed changed expression patterns of CK18 and Ep-CAM in 3AO spheroids and CD24− cells compared with parental cells and CD24+ cells respectively, implying the possibility that EMT existed from ovarian CSC-LCs to their differentiated cells.Citation10
Proto-oncogenic cyclin D1 helps promote cell cycle progression by regulating the G1 to S phase.Citation16 A recent study has showed that Cyclin D1 was necessary in maintenance of self-renewal in mammary stem and progenitor cells.Citation17 Cyclin D1 functioned dependently on kinase 4 or 6 (CDK4 or CDK6) and ultimately induced phosphorylation and inactivation of tumor suppressor protein Rb.Citation18 It has been revealed that cyclin D1 also existed in different human tumors.Citation19 Molenaar et al reported that neuroblastoma functionally depended on the overexpression of G1-regulating genes such as cyclin D1 to maintain its undifferentiated phenotype.Citation20 It has also been shown that cyclin D1 over-expression may be correlated with platinum-resistance in ovarian, pancreatic, and non-small-cell lung carcinoma.Citation16,Citation21 A recent study found that cyclin D1 promoted survival of anchorage-independent cells beyond CDK4 or CDK6 path-ways,Citation22 which may play a critical role in tumorigenesis and cancer metastasis. However, to our knowledge, the effect of cyclin D1 on the EMT mechanism in cancer stem cells remains undiscovered. Here, we investigated the EMT phenomenon and cyclin D1 effect in ovarian CSC-LCs, and aimed to search and discover new targets for ovarian cancer therapy.
Materials and methods
Cell culture
Ovarian adenocarcinoma cell line 3AO was obtained from the Women’s Hospital, School of Medicine, Zhejiang University. Cells were maintained in RPMI 1640 medium, supplemented with 10% fetal bovine serum. The anchorage-independent spheroids were formed in a serum-free medium composed of DMEM/F12, 10 ng/mL basic fibroblast growth factor and 20 ng/mL epidermal growth factor (PeproTech Inc, Rocky Hill, NJ, USA), 1 mg/mL insulin (Sigma-Aldrich, St, Louis, MO, USA), and 10 μL/mL B27 additive (Life Technologies, Carlsbad CA, USA) on culture dishes. All cells were maintained at 37°C in a humidified 5% CO2 incubator.
Fluorescence-activated cell sorting analysis
For fluorescence-activated cell sorting (FACS), 3AO adherent cells were washed twice with phosphate-buffered saline after 0.25% trypsin digestion. Cells were then suspended in phosphate-buffered saline and labeled with phycoerythrin-conjugated mouse anti-human monoclonal CD24 antibody (Life Technologies). This was followed by FACS using a FACSAria flow cytometer (Beckman Coulter Inc, Indianapolis, IN, USA). Isotype control was established and cells were routinely analyzed for purity.
Cell viability assay
Fresh CD24+ and CD24− cells were plated at 5000 per well onto 96-well plates with a low-serum medium (DMEM/F12 supplemented with 1% fetal bovine serum) directly post-isolation and cultured overnight for cell attachment. At daily intervals (24, 48, 72, and 96 hours), 20 μL MTT (5 mg/mL) were added per well and incubated in the dark for 4 hours. After removal of the medium, the dye crystals were dissolved in dimethyl sulfoxide for termination, and the absorbance was measured at OD490 with a Universal Microplate Reader ElX800 (BioTek Instruments Inc, Winooski, VT, USA). Three independent experiments were done in quadruplicate wells.
Cell apoptosis assay
The parental adherent cells were treated with 50 μg/mL carboplatin (Bristol-Myers Squibb Co, New York, NY, USA) for 24 hours. Then, cells were digested with 0.25% trypsin without EDTA and washed twice with phosphate-buffered saline. Cells were colabeled with FITC-Annexin V (Biouniquer Technology, Beijing, People’s Republic of China) and phycoerythrin-conjugated monoclonal CD24 antibody for 30 minutes at 4°C in the dark. Cell apoptosis of different cell subsets was analyzed using FACSAria flow cytometer. Propidium iodide was added 10 minutes before detection.
Wound-repair assay
Spheroids were trypsinized then seeded 1×106 per well in 6-well plates overnight for adherent growth with four repeats. The same operation was applied to adherent parental cells. A wound was scratched in the middle of the monolayer with the tip of a sterile 10 μL pipette, then a fresh serum-free medium was added. Ten representative fields of each group were marked and measured. Wounds were measured at 0, 4, and 8 hour intervals after scratching. The wound width was measured using a gauge tool with Photoshop CS4 software (Adobe Systems Inc, San Jose, CA, USA).
RNA interference
Small interfering RNA (siRNA) targeting cyclin D1 and nontargeting siRNA were both synthesized with the T7 RiboMAX™ Express RNAi System (Promega Corporation, Fitchburg, WI, USA). The cyclin D1-targeted mRNA sequence of cyclin D1 and the nontargeting control sequence were 5′-CCAGAGTGATCAAGTGTGA-3′ and 5′-GACTTCATAAGGCGCATGC-3′, respectivelyCitation23 DNA oligonucleotides used in siRNA synthesization are shown in . The process of siRNA synthesis was carried out strictly according to the technical bulletin. The interfering effect was routinely assessed at mRNA and protein level by quantitative reverse transcription polymerase chain reaction (qRT-PCR) and western blot. For siRNA transfection, the spheroids were trypsinized and seeded 8 × 104 cells per well in 6-well plates with complete medium and were transfected with 80 nmol/L cyclin D1 siRNA or nontargeting siRNA using siPORT NeoFX Transfection Agent as a transfection mediator according to the manufacturer’s instructions (Ambion, Carlsbad CA, USA). To analyze the effect of cyclin D1 down-regulation on cell proliferation, cyclin D1 siRNA-transfected cells and negative control cells were plated 5000 per well in 96-well plates; cell viability was assessed by MTT assay as described above at 0, 24, 48, and 72 hour intervals after transfection. Cell apoptosis and wound-repair assays of transfected spheroid-differentiated cells within 3 day cultures were carried out as described above.
Table 1 DNA oligonucleotides sequence used in synthesis of cyclin D1 siRNA with T7 RiboMAX™ Express RNAi system
Real-time RT-PCR analysis
Total RNA was extracted using a Trizol reagent (Takara Bio Inc., Shiga, Japan) and reverse-transcribed into cDNA. The SYBR® Premix ExTaq™ (Takara Bio Inc.,) was used to carry out real-time RT-PCR with an Applied Biosystems 7900HT Fast real-time PCRsystem (Life Technologies) according to the manufacturer’s protocols, and GAPDH was selected for normalization. Product specificity was confirmed by constructing a melting curve for each primer pair. All reactions were run in triplicate and no template control reactions were included in each assay run. A Δtc value means the differential value, which was derived by subtracting the ct value of each target gene from the ct value of GAPDH. The relative mRNA expression levels of all genes were calculated according to the 2−Δct method. The gene-specific primers for RT-qPCR are shown as .
Table 2 Primer used for quantitative RT-PCR
Western blot analysis
Cells were harvested into radioimmunoprecipitation assay lysate buffer containing freshly added 1% phenylmethyl-sulfonyl fluoride. Protein was subjected to 10% SDS– PAGE and then transferred to 0.45 μm transfer membranes (Millipore, Billerica, MA, USA). Membranes were blocked at room temperature for 1 hour in Tris-buffered saline with Tween-20 containing 5% nonfat milk, and then incubated with rabbit anti-cyclin D1 monoclonal antibody (1:5000), anti-N-cadherin (1:10,000) (Epitomics Inc, Burlingame, CA, USA), anti-vimentin (1:700; Abcam, Cambridge, UK), and mouse anti-β-actin monoclonal antibody (1:2000) (Sigma-Aldrich) at 4°C overnight. After incubation with appropriated secondary antibodies, goat-anti-mouse IgG (1:1000) or goat-anti-rabbit IgG (1:1000) (Sigma-Aldrich), at room temperature for 1 hour, bands were visualized by Chemiluminescence Detection Kit for HRP (Biological Industries, Kibbutz Beit Haemek, Israel). The densitometry readings of the bands were normalized to β-actin expression.
Statistical analysis
Homogeneity of variance and normal distribution were commonly tested. Appropriate statistical methods were used as needed. Student’s t-test was used for measurement data with normal distribution, while the Mann-Whitney nonparametric test was used for data with nonnormal distribution. The level of significance was set at P < 0.05.
Results
CD24− cells possess stronger proliferative capacity
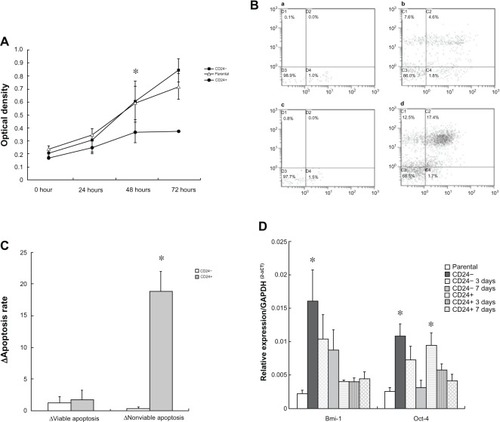
The parental 3AO cells, CD24− and CD24+ cells with high purity underwent normal proliferation when seeded in the medium supplied with 1% fetal bovine serum within 48 hours; however, the proliferation rate of CD24+ cells was obviously lower than that of parental 3AO and CD24− cells. At 48 hours after culture, CD24+ cells stopped proliferating, while the other two kinds of cells still continuously proliferated. But at 72 hours, CD24− cells grew continuously while parental cells grew slowly and entered growth plateau ().
Figure1 Cell viability, apoptosis, and stem-related genes expression in CD24− and CD24+ cells.
CD24− cells present stronger resistance to platinum
CD24− and CD24+ cells presented the obvious differences in resistance to carboplatin-induced apoptosis after 24 hours of carboplatin treatment (). The basic apoptosis level was not different between CD24− and CD24+ cells, while the nonviable apoptotic rate of CD24+ cells was significantly increased at 24 hours after carboplatin treatment compared with that of CD24− cells (Z = −3.363, P = 0.001) though the viable apoptotic rate was not found to be changed at 24 h (Z = −0.211, P = 0.878), as shown in and .
CD24− cells highly expressed stem-related gene Bmi-1
Bmi-1 mRNA expression was significantly higher in fresh CD24− cells than that in fresh CD24+ cells (t = 4.761, P = 0.001) and gradually and significantly decreased during the differentiation cultivation (F = 11.584, P = 0.001). However no similar change of Bmi-1 mRNA expression in CD24+ cells was found during the differentiation cultivation (F = 0.242, P = 0.788). Oct-4 expression was not different between fresh CD24− and CD24+ cells (t = 0.296, P = 0.774), but gradually and significantly decreased in both cells during the differentiation cultivation (FCD24− = 6.016, PCD24− = 0.016; FCD24+ = 5.426, PCD24+ = 0.021), as shown in .
CD24− cells possessed EMT characteristic
CD24− and CD24+ cells with high purity showed different growth features after isolation. CD24+ cells presented the typical cobblestone-like shape similar to parental cells, which grew as a single cell colony. But a confluence of monoclone gradually occurred following prolonged culture. Contrarily, CD24− cells grew with scattered spindle shape similar to fibroblast cells until 3 days of culture, but the cell colony grew towards the shape of parental 3AO cells following culture prolongation ().
Figure 2 Epithelial–mesenchymal transition (EMT) phenomenon in CD24− cells.
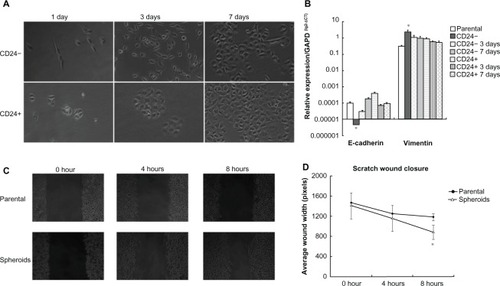
The qRT-PCR assay revealed that E-cadherin mRNA expression in CD24− cells was significantly lower than CD24+ cells (t = -4.095, P = 0.015). There was a clear, upregulated trend of E-cadherin mRNA expression from fresh isolated, 3-day cultures, to 7-day cultured CD24- cells (F = 6.459, P = 0.012), but not among CD24+ cells with different culture times. Vimentin mRNA expression in CD24− cells was significantly higher than CD24+ cells (t = 5.767, P = 0.002). There was also a clear downregulated trend of vimentin mRNA expression from fresh isolated, 3-day cultures, to 7-day cultured CD24− cells (F = 54.637, P = 0.001), but also not among CD24+ cells with different culture times ().
CD24- enriched spheroids possess stronger migratory capability
Our previous outcome has verified that an average of 80% of cells presented CD24− phenotype in spheroids cultured for 6 days.Citation10 Wound assays were performed to compare the migratory potential between adherent parental cells and spheroid-differentiated cells. The width of wound closure was shown in . Spheroid-differentiated cells presented wider width closure than parental cells, and the difference was significant at 8 hours after inflicting a scratch (Z = −4.099, P = 0.000) though was not at 4 hours after scratching (Z = −1.848, P = 0.068) as shown in and .
Spheroids highly express cyclin D1 and vimentin
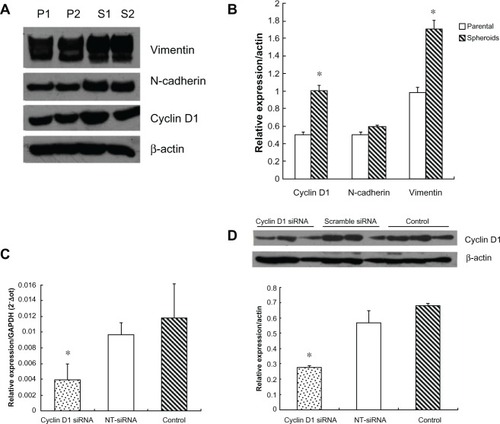
The expression of cyclin D1, N-cadherin, and vimentin in parental and spheroids was detected by western blot. The average value of cyclin D1 was 0.507 ± 0.069 in parental cells and 1.007 ± 0.132 in spheroids, with a significant difference (t = −3.351, P = 0.007). The value of N-cadherin was 0.501 ± 0.063 in parental cells and 0.599 ± 0.039 in spheroids without statistical difference (t = −1.322, P = 0.221). The average value of vimentin was 0.987 ± 0.155 in parental cells and 1.705 ± 0.245 in spheroids with a significant difference (t = −2.471, P = 0.033), as shown in and .
Figure 3 The expression pattern of cyclin D1, N-cadherin, vimentin in parental cells and spheroids, and transfection efficacy of cyclin D1 siRNA.
Abbreviations: P1, parental cell sample 1; P2, parental cell sample 2; P1, spheroids sample 1; S2, spheroids sample 2; NT-siRNA, nontargeting small interfering RNA.
Cyclin D1 knockdown alters the proliferation, apoptosis, and migration abilities of spheroid-differentiated cells
At 48 hours after cyclin D1 siRNA was transfected into spheroid-differentiated cells, the amount of cyclin D1 mRNA was decreased by 59.13% and cyclin D1 protein was reduced by 51.76% compared with the control group ( and ).
Cell viability was observed after cyclin D1 knockdown. MTT assay showed that the cell viability was not obviously changed at 0, 24, and 48 hours, but significantly decreased at 72 hours after cyclin D1 siRNA transfection (Z = −2.241, P = 0.025), as shown in .
Figure 4 Cell viability, apoptosis induced by cyclin D1 silence.
Abbreviation: NT-siRNA, nontargeting small interfering RNA.
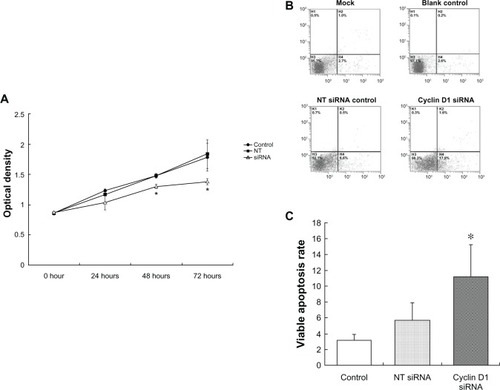
The effect of cyclin D1 knockdown on cell apoptosis was observed by FITC-Annexin V/Propidium iodide apoptosis assay. As shown in and , at 30 hours after siRNA transfection, the viable apoptosis was significantly increased in the cyclin D1 siRNA-transfected cells, compared with scramble siRNA group (Z = −2.650, P = 0.008).
The change of migration ability of spheroid-differentiated cells after cyclin D1 knockdown was further observed. Spheroid-differentiated cells were plated 8 × 104 per well in 6-well plates. Following attachment overnight, cells were transfected with cyclin D1 siRNA. At 12 h after transfection, cells were replated 3 × 105 per well in 12-well plates for attachment overnight, then wound repair assays were performed. At 16 hours after inflicting a scratch, wound width of cyclin D1 siRNA-transfected cells was significantly decreased compare to that of control (t = −3.125, P = 0.004) as shown in and .
Figure 5 Cell migration and n-cadherin, vimentin expression induced by cyclin D1 silence.
Abbreviation: NT-siRNA, nontargeting small interfering RNA.
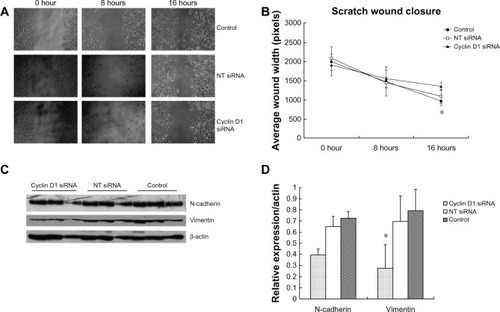
Cyclin D1 knockdown reduces the expression of vimentin and n-cadherin protein in spheroid-differentiated cells
Knockdown of cyclin D1 resulted in significantly decreased expression of vimentin protein in spheroid-differentiated cells (t = 3.748, P = 0.002). N-cadherin expression was also decreased, but the difference was not significant (Z = −1.083, P = 0.279), as shown in and .
Discussion
It has been well recognized that cancer stem cells possess the following characteristics: (a) strong clone formation ability in vitro with self-renewal capability, (b) suspension spheroid formation in vitro, (c) expression of several known stem cell-related markers, such as Oct-4, Nanog, and others, and (d) propagation in animals and recapitulation of their original phenotype.Citation6,Citation24,Citation25 As shown in our study, CD24− cells derived from ovarian epithelial adenocarcinoma cell line 3AO were able to proliferate normally under low serum conditions, suggesting that CD24− cells possess much stronger proliferative ability than parental and CD24+ cells. Previous studies have revealed that cancer stem cells only account for a very small proportion of tumor tissue, and usually stay in a state of quiescence. When damage occurs within the extracellular environment, such as starvation conditions of low or free serum and toxicity conditions of chemotherapy drugs, those maturely differentiated cells hardly tolerate such environmental pressures, presenting growth arrest and apoptosis acceleration, even death.Citation13,Citation26,Citation27 In contrast, the cancer stem cells can break through the quiescent state and into proliferation. Several studies have shown evidence that some phenotypes of cancer stem-like cells may be induced by long-term chemotherapy drugs stimulation.Citation13,Citation26,Citation27 As in our study, CD24− cells always showed continuous growth under 1% low serum, while others presented slower growth despite sustaining proliferation for a short period. Besides, parental cells appeared to possess slightly stronger proliferation ability than CD24+ cells, which may be associated with the existence of a small proportion of cancer stem cells in the parental cells. Furthermore, we found that CD24− cells showed a more significant decrease in nonviable apoptosis rate than did CD24+ cells though their basic viable apoptosis and viable apoptosis rate after dosing were the same. Thus, our results further support the findings that cancer stem cells possess stronger survival ability against extraneous damage.
The expression of undifferentiated markers is one of the most important features of cancer stem cells. We analyzed the expression of stem cell-related markers in CD24− and CD24+ cells, such as Bmi-1 and Oct-4, which have been regarded to be associated with stem and/or progenitor cells and function essential for the maintenance of an undifferentiated state.Citation28–Citation33 Bmi-1 was highly expressed in CD24− cells but gradually downregulated following cell differentiation, suggesting that Bmi-1 functioned with specificity for sustaining self-renewal characteristics in ovarian cancer stem cell-like cells. However, we found that Oct-4 was expressed in both CD24+ and CD24- cells, though it was downregulated following culture prolongation. We speculate therefore that Oct-4 may not be the specific marker for ovarian cancer stem cells, but further evidence is needed.
Epithelial cells have long been regarded as cells of terminal differentiation, but recent studies have found that epithelial cells can lose adhesion, tight junction, and cell polarity to acquire the ability of invasive migration under the action of some factors.Citation6,Citation24,Citation25 Meanwhile, these epithelial cells still present morphology and properties of mesenchyme cells. EMT is a necessary physical phenomenon of mammal embryonic development process. It has been verified that EMT is a main way by which embryonic stem cells obtain migrating ability.Citation34 Accumulated evidence has also revealed that EMT is closely related with the occurrence and development of malignant epithelial tumors,Citation35,Citation36 and plays a pivotal role in primary invasion and secondary metastasis of cancers, including breast, prostate, hepatic cancer, melanoma, and others.Citation13,Citation37–Citation39 In this study, we found that CD24− cells, freshly isolated with high purity, presented the shape of scattered spindle similar to fibroblast cells within 3 days of culture, while CD24+ and parental cells grew in the typical cobblestone-like shape. But morphology of CD24− cells changed into the shape similar to CD24+ and parental cells following cell differentiation, and part of them presented the typical cobblestone-like shape when culture continued to 7 days. Our results suggest that a differentiated culture of CD24− cells possesses the ability to transit morphologically from mesenchymal back to epithelial features.
During the EMT process, epithelial cells need to reduce or lose key proteins of epithelial cell adhesion junctions and tight junctions, and re-express or upregulate proteins originated from mesenchymal cells. E-cadherin and vimentin protein are considered two key proteins related to EMT. E-cadherin belongs to type I cadherin, which not only constitutes the powerful intercellular connection, but also maintains epithelial cell characteristics. The loss of E-cadherin is usually accompanied by the occurrence of nonepithelial cell features, including loss of cell polarity. Vimentin is one of the main components of secondary fibers in fibroblasts. Previous studies reported that the loss or downregulation of E-cadherin and upregulation of vimentin occurred almost simultaneously during the EMT process.Citation13,Citation37–Citation39 In this study, we found that fresh CD24− cells presented high expression of vimentin mRNA and protein and downregulated mRNA level following culture maintenance. Contrarily, CD24− cells expressed very low E-cadherin mRNA. Although there was a clear upregulated trend of E-cadherin mRNA expression following cell differentiation, we still could not detect E-cadherin protein by western blot. Whether ovarian epithelial cells express E-cadherin remains controversial. Some investigators reported that the original cadherin in the normal ovarian surface epithelium was N-cadherin, but not E-cadherin; however, others showed that only some clones expressed E-cadherin.Citation40–Citation42 The causation is not clear and might be associated with the existence of modified or instable expression at the protein level. Nevertheless, our results suggest that CD24− ovarian cancer stem-like cells possess mesenchymal phenotypes and EMT potential, at least at mRNA level.
Wound-repair assays can be used for measuring the migration ability of cells and have been recently shown to be useful for evaluating the mesenchymal feature. We adopted CD24− phenotype enriched spheroid cells for study and found that spheroid-differentiated cells showed significantly stronger wound-repair ability than parental cells both at 4 hours and 8 hours after inflicting a scratch; although, those cells had partly differentiated in spheroids. Hence, we deduced that undifferentiated CD24− cells should possess extraordinarily stronger migratory ability. Our result is consistent with the mesenchymal feature of cancer stem cells.
Under normal circumstances, cell survival relies on adhesion to an extracellular matrix. Loss of the extracellular matrix support usually triggers a special apoptosis, termed anoikis.Citation43 As shown in our study, cancer stem cells can survive as anchorage-independent spheroid forms under serum-free media conditions, which suggests that cancer stem cells have the capacity to acquire anoikis-resistance. It has been speculated that cyclin D1 may mediate this anoikis-resistance. Gan et alCitation22 found that the oncoprotein, cyclin D1, acted as a critical upstream inhibitor of anoikis mediated by FOXO, one of the integrin family members, through an independent pathway rather than combination with CDK4 or CDK6. Furthermore, cyclin D1 is associated with platinum resistance, growth, and metastasis in various cancers including ovarian cancers.Citation23 A recent study also found that cyclin D1 activity was required for the self-renewal of mammary stem and progenitor cells.Citation17 Thus, we speculated that cyclin D1 probably played a role in EMT in cancer stem cells. In this study, we found that spheroids expressed higher cyclin D1 and vimentin than parental cells. Cyclin D1 gene silence could downregulate the expression of vimentin and N-cadherin. Moreover, siRNA targeting-cyclin D1 transfection could decelerate cell viability and migration ability and accelerate apoptosis compared with the negative control group. Our results suggest that cyclin D1 is involved in sustaining the mesenchymal feature of ovarian cancer stem cell-like cells in EMT.
Conclusion
Taken together, our results showed that CD24− cells and CD24− phenotype enriched spheroids from the ovarian cancer cell line 3AO presented mesenchymal features, such as over-expression of stem cell-related markers, higher proliferation, increased drug-resistance, lower apoptosis, and stronger migration capacity, and showed EMT phenomenon including morphology, phenotype, and function through differentiative culture. In addition, cyclin D1 is involved in EMT. Our findings suggest that ovarian CSC-LCs possess mesenchymal characteristics and EMT ability, which may play a key role in the invasion and metastasis of ovarian cancer.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (Grant No 30973380), Zhejiang Provincial Natural Science Foundation of China (Grant No LY12H16024), Foundation of Health Department of Zhejiang Province of China (Grant No 2007A129), Specialized Research Fund for the Doctoral Program of Higher Education (Grant No 20070335060), National High-tech R&D Program (863 Program) (Grant No 2012AA02A507), and by Zhejiang Provincial Program for the Cultivation of High-level Innovative Health Talents.
J Jiao, FYe, WG Lu, and X Xie contributed to conception and design. J Jiao carried out the main parts of the study and the statistical analysis. WG Lu contributed to the revision of the article. MF Shi, L Huang, XY Wang, DX Hu and XD Cheng all participated in the data acquisition and analysis. All authors contributed to writing the manuscript and approved the final edition.
Disclosure
The authors report no conflicts of interest in this work.
References
- NossovVAmneusMSuFThe early detection of ovarian cancer: from traditional methods to proteomics. Can we really do better than serum CA-125?Am J Obstet Gynecol2008199321522318468571
- Al-HajjMWichaMSBenito-HernandezAMorrisonSJClarkeMFProspective identification of tumorigenic breast cancer cellsProc Natl Acad Sci USA200310073983398812629218
- Guzmán-RamírezNVöllerMWetterwaldAIn vitro propagation and characterization of neoplastic stem/progenitor-like cells from human prostate cancer tissueProstate200969151683169319644960
- ZhuZHaoXYanMCancer stem/progenitor cells are highly enriched in CD133+ CD44+ population in hepatocellular carcinomaInt J Cancer201012692067207819711346
- SinghSKHawkinsCClarkeIDIdentification of human brain tumour initiating cellsNature2004432701539640115549107
- ZhangSBalchCChanMWIdentification and characterization of ovarian cancer-initiating cells from primary human tumorsCancer Res200868114311432018519691
- KusumbeAPMaliAMBapatSACD133-expressing stem cells associated with ovarian metastases establish an endothelial hierarchy and contribute to tumor vasculatureStem Cells200927349850819253934
- HuCLiHLiJAnalysis of ABCG2 expression and side population identifies intrinsic drug efflux in the HCC cell line MHCC-97L and its modulation by Akt signalingCarcinogenesis200829122289229718820285
- SzotekPPPieretti-VanmarckeRMasiakosPTOvarian cancer side population defines cells with stem cell-like characteristics and mullerian inhibiting substance responsivenessProc Natl Acad Sci USA200610330111541115916849428
- ShiMFJiaoJLuWGIdentification of cancer stem cell-like cells from human epithelial ovarian carcinoma cell lineCell Mol Life Sci201067223915392520549538
- YangJWeinbergRAEpithelial-mesenchymal transition: at the crossroads of development and tumor metastasisDev Cell200814681882918539112
- SinghASettlemanJEMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancerOncogene201029344741475120531305
- AktasBTewesMFehmTHauchSKimmigRKasimir-BauerSStem cell and epithelial-mesenchymal transition markers are frequently overexpressed in circulating tumor cells of metastatic breast cancer patientsBreast Cancer Res2009114R4619589136
- ManiSAGuoWLiaoMJThe epithelial-mesenchymal transition generates cells with properties of stem cellsCell2008133470471518485877
- PolyakKWeinbergRATransitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traitsNat Rev Cancer20099426527319262571
- BiliranHJrWangYBanerjeeSOverexpression of cyclin D1 promotes tumor cell growth and confers resistance to cisplatin-mediated apoptosis in an elastase-myc transgene-expressing pancreatic tumor cell lineClin Cancer Res200511166075608616115953
- JeselsohnRBrownNEArendtLCyclin D1 kinase activity is required for the self-renewal of mammary stem and progenitor cells that are targets of MMTV-ErbB2 tumorigenesisCancer Cell2010171657620129248
- KatoJMatsushimeHHiebertSWEwenMESherrCJDirect binding of cyclin D to the retinoblastoma gene product (pRb) and pRb phosphorylation by the cyclin D-dependent kinase CDK4Genes Dev1993733313428449399
- AlaoJPThe regulation of cyclin D1 degradation: roles in cancer development and the potential for therapeutic inventionMol Cancer200762417407548
- MolenaarJJEbusMEKosterJCyclin D1 and CDK4 activity contribute to the undifferentiated phenotype in neuroblastomaCancer Res20086882599260918413728
- NoelEEYeste-VelascoMMaoXThe association of CCND1 overexpression and cisplatin resistance in testicular germ cell tumors and other cancersAm J Pathol201017662607261520395447
- GanLLiuPLuHCyclin D1 promotes anchorage-independent cell survival by inhibiting FOXO-mediated anoikisCell Death Differ200916101408141719575018
- HuangHHuYDLiNZhuYInhibition of tumor growth and metastasis by non-small cell lung cancer cells transfected with cyclin D1-targeted siRNAOligonucleotides200919215116219355812
- DalerbaPChoRWClarkeMFCancer stem cells: models and conceptsAnnu Rev Med20075826728417002552
- FongMYKakarSSThe role of cancer stem cells and the side population in epithelial ovarian cancerHistol Histopathol201025111312019924647
- AhmedNAbubakerKFindlayJQuinnMEpithelial mesenchymal transition and cancer stem cell-like phenotypes facilitate chemoresistance in recurrent ovarian cancerCurr Cancer Drug Targets201010326827820370691
- MorelAPLièvreMThomasCHinkalGAnsieauSPuisieuxAGeneration of breast cancer stem cells through epithelial-mesenchymal transitionPLoS One200838e288818682804
- MuellerTLuetzkendorfJNergerKSchmollHJMuellerLPAnalysis of OCT4 expression in an extended panel of human tumor cell lines from multiple entities and in human mesenchymal stem cellsCell Mol Life Sci200966349550319023518
- Hombach-KlonischSParanjothyTWiechecECancer stem cells as targets for cancer therapy: selected cancers as examplesArch Immunol Ther Exp (Warsz)200856316518018512024
- ChenYCHsuHSChenYWOct-4 expression maintained cancer stem-like properties in lung cancer-derived CD133-positive cellsPlos One200837e263718612434
- LiuJCaoLChenJCBmi1 regulates mitochondrial function and the DNA damage response pathwayNature2009459724538739219404261
- SangiorgiECapecchiMRBmi1 is expressed in vivo in intestinal stem cellsNat Genet200840791592018536716
- CuiHJHuBLiTBmi-1 is essential for the tumorigenicity of neuroblastoma cellsAm J Pathol200717041370137817392175
- ThieryJPAcloqueHHuangRYNietoMAEpithelial-mesenchymal transitions in development and diseaseCell2009139587189019945376
- AcloqueHAdamsMSFishwickKBronner-FraserMNietoMAEpithelial-mesenchymal transitions: the importance of changing cell state in development and diseaseJ Clin Invest200911961438144919487820
- HugoHAcklandMLBlickTEpithelial – mesenchymal and mesenchymal – epithelial transitions in carcinoma progressionJ Cell Physiol2007213237438317680632
- ChafferCLBrennanJPSlavinJLBlickTThompsonEWWilliamsEDMesenchymal-to-epithelial transition facilitates bladder cancer metastasis: role of fibroblast growth factor receptor-2Cancer Res20066623112711127817145872
- LeeTKPoonRTYuenAPTwist overexpression correlates with hepatocellular carcinoma metastasis through induction of epithelial-mesenchymal transitionClin Cancer Res200612185369537617000670
- AlonsoSRTraceyLOrtizPA high-throughput study in melanoma identifies epithelial-mesenchymal transition as a major determinant of metastasisCancer Res20076773450346017409456
- ShieldKAcklandMLAhmedNRiceGEMulticellular spheroids in ovarian cancer metastases: biology and pathologyGynecol Oncol2009113114314819135710
- PatelISMadanPGetsiosSBertrandMAMacCalmanCDCadherin switching in ovarian cancer progressionInt J Cancer2003106217217712800191
- SundfeldtKCell–cell adhesion in the normal ovary and ovarian tumors of epithelial origin: an exception to the ruleMol Cell Endocrinol20032021–2899612770736
- FrischSMRuoslahtiEIntegrins and anoikisCurr Opin Cell Biol1997957017069330874