Abstract
Objective
Resveratrol is a phytoestrogen with various antiproliferative and proapoptotic effects. This in vitro study aimed to analyze the effect of resveratrol on the viability and expression of modulators of apoptosis in GH3 pituitary adenoma cells of the rat.
Methods
GH3 cells were incubated with resveratrol concentrations from 20 to 100 μM for 48–72 hours. Cell viability was quantified using a hemocytometer. We assessed the ability of resveratrol to kill GH3 cells by an enzyme-linked immunosorbent assay (ELISA) of nucleosome liberation and by DNA degradation (unidimensional gel electrophoresis). Relative messenger RNA (mRNA) expression of survivin, B-cell lymphoma-2 protein (BCL-2) and BCL-2-associated X protein (BAX) normalized to β2 microglobulin was measured using quantitative real-time polymerase chain reaction (qRT-PCR).
Results
GH3 cell survival significantly decreased with increasing concentrations of resveratrol. In GH3 cells treated with 100 μM resveratrol, ELISA demonstrated a significant rise of nucleosome liberation, which typically occurs during apoptosis. In parallel, gel electrophoresis showed degradation of DNA into random fragments, pointing to a necrotic mode of cell death in most GH3 cells. In GH3 cells treated with 100 μM resveratrol, qRT-PCR detected a significant decrease of BCL-2 mRNA expression and a decrease of survivin mRNA expression, whereas a change of BAX mRNA expression could not be found. The BAX/BCL-2 ratio was significantly increased in GH3 cells after resveratrol treatment.
Conclusions
Resveratrol reduces GH3 cell viability in a dose-dependent manner by inducing nonapoptotic cell death and apoptosis. Apoptosis in GH3 cells is probably mediated by resveratrol-dependent downregulation of apoptosis inhibitors, namely BCL-2 and possibly survivin. Further investigation of the potential effects of resveratrol on pituitary adenoma cells is warranted.
Introduction
Pituitary adenomas carry the risks of endocrine dysfunction and cranial nerve deficiencies. Control of hormonally active, symptomatic pituitary adenomas of any size is necessary to achieve stable function of the endocrine system. Macroadenomas of the pituitary gland may exert mechanical pressure on the pituitary gland itself as well as on cranial nerves II–VI, which may result in hypopituitarism, facial numbness, loss of eye-motor function, loss of visual acuity, visual field cuts, and even blindness. Control of macroadenomas of the pituitary gland is necessary in cases of growth, hormonal disorders, and visual disturbances. Therapeutic options consist of surgery, radiation, and pharmacotherapy. The possible extent of surgical resection is limited in pituitary adenomas invading the skull base, and may be limited in recurrent tumors. Radiation therapy is limited by the inherent risks of damage to neighboring tissues and tumor induction. Pharmacotherapy in pituitary adenomas is confined to certain tumor entities, as its feasibility depends greatly on the hormones and receptors expressed in the adenomatous cell clone. Currently, valid options comprise dopamine agonists for tumors producing prolactin and somatostatin (growth hormone-inhibiting hormone [GHIH]) analogues for tumors expressing GHIH receptors.Citation1 New options in pharmacotherapy for pituitary adenomas are highly desirable.
Resveratrol is a phytoestrogen with various antiproliferative and proapoptotic effects.Citation2 Underlying mechanisms include downregulation of apoptosis inhibitors, such as survivinCitation2 and B-cell lymphoma-2 protein (BCL-2),Citation2 as well as upregulation of apoptosis inductors, such as BCL-2-associated X protein (BAX).Citation2 An estradiol-induced increase in the BAX/BCL-2 ratio leading to apoptosis in the anterior pituitary gland of the rat has been demonstrated.Citation3 In human pituitary adenoma cells, survivin expression is increased compared to healthy pituitary gland tissue.Citation4
This in vitro study aims to investigate the effect of resveratrol on viability, expression of BAX, BCL-2, and survivin as modulators of apoptosis in GH3 pituitary adenoma cells of the rat.
Materials and methods
Cell culture
GH3 cells (LGC/American Type Culture Collection [ATCC], Wesel, Germany) were cultivated in F12-K medium (LGC/ATCC) containing 2.5% fetal bovine serum (PAA, Linz, Austria), 15% heat-inactivated horse serum (Life Technologies, Carlsbad, CA, USA), and 1% penicillin/streptomycin at 37°C in 95% air and 5% carbon dioxide. The culture medium was changed every 2–3 days. Cells grew as a loosely adherent suspension.
Cell viability
Two passages of GH3 cells were incubated at 37°C in 95% air and 5% carbon dioxide with 0, 20, 50, and 100 μM resveratrol (Sigma-Aldrich, Taufkirchen, Germany) in a twelve-well plate containing three wells per treatment, 2 mL culture medium per well, and 100,000 cells per well. After 72 hours, cells were trypsinized. Cell suspensions with a resulting total volume of 4.5 mL each were centrifuged. Cells were resuspended in 450 μL culture medium and counted twice using a hemocytometer.
Free nucleosome-specific ELISA
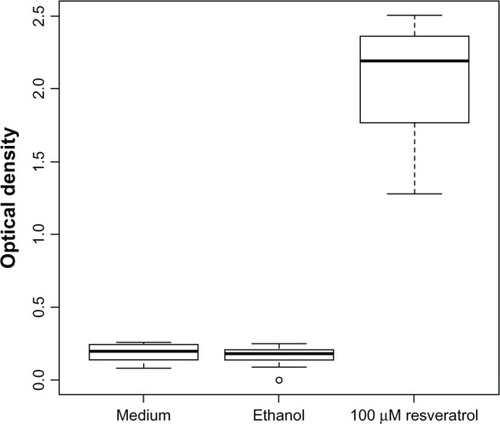
Two passages of GH3 cells were incubated at 37°C in 95% air and 5% carbon dioxide in a 96-well plate containing 36 wells per passage, 100 μL culture medium per well, and 12,000 cells per well. After 24 hours of incubation, the culture-medium volume was increased to 200 μL per well. Thereafter, twelve wells each per passage contained culture medium only, medium with 100 μM resveratrol, or medium with the solvent ethanol in a concentration corresponding to resveratrol treatment (0.1%). After another 48 hours of incubation, apoptosis was quantified using the Cell Death Detection enzyme-linked immunosorbent assay (ELISA) Plus kit (Roche Diagnostics Deutschland, Mannheim, Germany), which detects free nucleosomes in the cytoplasm. The ELISA was applied with cell lysates according to the shipped manual, including internal blank and positive controls. Optical density was measured photometrically at 405 nm (reference wavelength 490 nm) using a NanoQuant infinite M200 reader (Tecan, Männedorf, Switzerland) with Magellan 6 software (Tecan).
DNA-fragmentation assay
Two passages of GH3 cells were incubated at 37°C in 95% air and 5% carbon dioxide in a six-well plate containing three wells per passage, 2 mL culture medium per well, and 106 cells per well. After 24 hours of incubation, the culture-medium volume was increased to 4 mL per well. Thereafter, one well each per passage contained culture medium only, medium with 100 μM resveratrol, or medium with the solvent ethanol in a concentration corresponding to resveratrol treatment (0.1 %). After another 48 hours of incubation, cells were trypsinized centrifuged and stored at −20°C. Genomic DNA was extracted from proteinase K-treated cell pellets with conventional phenol/chloroform extraction, avoiding shear forces by gentle pipetting. Ten micrograms DNA per lane were separated electrophoretically on an agarose gel (1%), which contained 1 μg/mL ethidium bromide (Carl Roth, Karlsruhe, Germany) as fluorescent labeling agent. λ-DNA, digested with the restriction enzyme Hind III (New England Biolabs, Ipswich, MA, USA) served as a molecular weight marker. Electronic pictures (black and white) of the fluorescence were taken from the gel positioned on an ultraviolet illuminator. The whole pictures, but not individual lanes, were adjusted for brightness and contrast using Photoshop CS3 (Adobe Systems, San Jose, CA, USA), in order to allow a simultaneous reproduction of the bright fluorescence of intact high-molecular-weight DNA and of the moderate fluorescence of degraded DNA. One of the coauthors (EK) and three technicians inspected the gel visually for the presence of discrete bands of higher fluorescence (apoptotic DNA laddering) in the lanes with a smear of degraded DNA.
qRT-PCR
Two passages of GH3 cells were incubated at 37°C in 95% air and 5% carbon dioxide in six-well plates containing six wells per passage with 2 mL culture medium per well and 106 cells per well. After 24 hours of incubation, the culture-medium volume was increased to 4 mL per well. Thereafter, two wells each per passage contained culture medium only, medium with 100 μM resveratrol, or medium with the solvent ethanol in the appropriate concentration. After another 48 hours of incubation, RNA was extracted using Trizol reagent (Life Technologies).Citation5 One microgram of RNA, 4 μL random hexamer primers (Bioline, Luckenwalde, Germany) and diethylpyrocarbonate (DEPC)-treated water comprised a volume of 10 μL per tube; 4 μL buffer, 0.4 μL nucleotide mix, 0.125 μL reverse transcriptase and 5.475 μL DEPC water were added per tube to generate complementary DNA (cDNA). cDNA was stored at −20°C.
We investigated the changes in relative expression of BAX, BCL-2, and survivin using quantitative real-time PCR (qRT-PCR). β2 microglobulin (B2MG) was used as a housekeeping gene. Primers for B2MG, BAX, BCL-2, and survivin (www.biomers.net) were designed by coauthor EK. Primers for real-time PCR (anneal temperature 55°C) were as follows: B2MG – TGAGCTACTGAAGAATGGAAAG (forward) and GTGTTTAACTCTGCAAGCATATAC (reverse); BAX – AAACTGGTGCTCAGGGCCCT (forward) and AGCAGCCGCTCACGGAG (reverse); BCL-2 – GTCGCGACTTTGCAGAGATG (forward) and CTGAAGAGTTCCACCACC (reverse); survivin – TGCCTTACGCTGAGCCTTTG (forward) and CCTGGAAAGCTG GGACAAGT (reverse). SYBR Green I (SensiMix SYBR, Bioline) served as detector. A 96-well plate was set up with 8 μL SYBR Green I, 1 μL forward primer, 1 μL backward primer, 1 μL cDNA, and 5.3 μL fluorescence-free water per cDNA sample. An ABI Prism 7000 sequence detection system (SDS) with SDS software (Life Technologies) was employed.
Statistical analysis and figures
Statistical analysis was conducted using NeoOffice 3.1.2 patch 9 (Planamesa, Santa Clara, CA, USA) and the R software package 2.10.1 (R Foundation, Vienna, Austria) on a Mac OS X 10.6.8 (Apple, Cupertino, CA, USA). After two-way analysis of variance, R’s post-hoc pairwise t test served to assess differences in multiple group means, and P< 0.05 was considered to be statistically significant.
Figures were created using the R software package 2.10.1, the GNU Image Manipulation Program (GIMP) 2.6.8 (http://www.gimp.org) and Photoshop CS3 (Adobe).
Results
Cell viability
In wells treated with 0, 20, 50, and 100 μM resveratrol for 72 hours, the respective mean numbers of viable cells counted in passage 8 were 186.83 (equal to 210,190 cells per well, 100%), 87.83 (equal to 98,810 cells per well, 47.01%), 50.67 (equal to 57,000 cells per well, 27.12%), and 32.50 (equal to 36,560 cells per well, 17.40%). In wells treated with 0, 20, 50, and 100 μM resveratrol for 72 hours, the respective mean numbers of viable cells counted in passage 10 were 144.50 (equal to 162,560 cells per well, 100%), 70.33 (equal to 79,130 cells per well, 48.67%), 35.17 (equal to 39,560 cells per well, 24.34%) and 17.50 (equal to 19,690 cells per well, 12.11%). All differences in cell counts were statistically significant between treatment groups (), whereas a statistically significant difference between passages could not be found (P = 0.18).
Figure 1 After 72 hours of treatment, viability in two passages of GH3 cells significantly decreased with growing concentrations of resveratrol (0 μM versus 20 μM resveratrol, P < 2 × 10−16; 20 μ M versus 50 μ M resveratrol, P = 5.8 × 10−6; 50 μ M versus 100 μM resveratrol, P = 0.012; passage 8 [white] versus passage 10 [gray], P=0.18). °Outlier.
![Figure 1 After 72 hours of treatment, viability in two passages of GH3 cells significantly decreased with growing concentrations of resveratrol (0 μM versus 20 μM resveratrol, P < 2 × 10−16; 20 μ M versus 50 μ M resveratrol, P = 5.8 × 10−6; 50 μ M versus 100 μM resveratrol, P = 0.012; passage 8 [white] versus passage 10 [gray], P=0.18). °Outlier.](/cms/asset/6a7a9198-65c1-4301-b676-e95b20ab6f9b/dott_a_45154_f0001_b.jpg)
Free nucleosome-specific ELISA
In wells treated with 100 μM resveratrol for 48 hours, the mean optical density was 2.05 (passage 10; ) and 1.96 (passage 13). In wells treated with medium as control, the mean optical density was 0.19 (passage 10; ) and 0.16 (passage 13). In wells treated with ethanol as control, the mean optical density was 0.17 (passage 10; ) and 0.17 (passage 13). The differences in optical densities between wells treated with resveratrol as compared to controls were highly statistically significant (P< 2 × 10−16). Statistically significant differences between passages or between medium and ethanol controls could not be found. Since free single nucleosomes or oligonucleosomes in the cytoplasm, as measured in this assay, occur typically during apoptosis, the result suggested at least a participation of this process. However, ELISA is hugely sensitive and can measure unmasked cytoplasmic nucleosomes even in a small percentage of cells among an overwhelming majority of cells, which may die by necrosis. It is also possible to identify apoptosis by fluorescence staining of the DNA, because apoptosis leads to a liberation of discrete DNA fragments, which represent multiples of the DNA length twisted around a nucleosome core. Although this “DNA-laddering assay” is by far less sensitive for the detection of chromatin degradation into nucleosomal units, it gives an impression about the dominating mode of cell death – apoptosis versus necrosis. For this reason, it was performed next.
DNA gel electrophoresis
In DNA obtained from cells treated with 100 μM resveratrol for 48 hours, unidimensional gel electrophoresis showed a continuous faint staining (smear) in the whole range of molecular weights between 2 kb and 23.1 kb, ie, degradation of DNA into fragments of random molecular weights (passage 8; ). DNA “laddering,” ie, degradation of DNA into fragments of specific lengths, was not observed after resveratrol treatment. DNA degradation did not occur in the medium or ethanol controls, which showed a bright spot-like staining, corresponding to fragments clustering around 20 kb, which is expected after gentle preparation of genomic DNA with standard procedures. While the smear on the corresponding lane clearly identified DNA degradation during resveratrol-induced cell death, the absence of DNA laddering indicated that most of the cells had died by a nonapoptotic mechanism.
Figure 3 Ethidium bromide-stained agarose gel (1%) showing electrophoretic mobility of 10 μg genomic DNA, which had been freshly isolated from GH3 cells treated with 100 μM resveratrol (R) for 48 hours, or with the corresponding concentration of 0.1% of the solvent ethanol (E) or solely with cell-culture medium (M). λ-DNA digested with the restriction endonuclease Hind-III served as a molecular weight standard ranging from 2 to 23.1 kb. It can be seen that the DNA isolated from the medium or solvent controls migrates as a bright spot of high-molecular-weight genomic DNA of approximately 20 kb, while this spot disappeared in resveratrol-treated cells and was replaced by a more continuous faint staining along the whole lane, thus indicating severe DNA degradation. The continuous staining of this lane was clearly visible under the ultraviolet illumination. It cannot be seen ideally on the photograph, due to the large differences in fluorescence/cm2 compared to the high-molecular-weight DNA in lanes E and M. Four independent observers confirmed this homogeneous stain, but did not detect any laddering. This result indicated that most of the cells had died by a nonapoptotic mode of cell death.

qRT-PCR
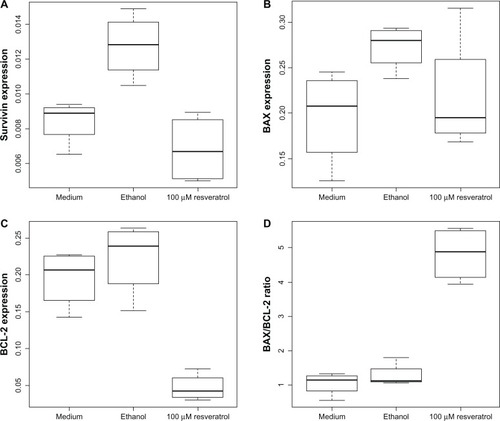
In the ethanol control, we detected a statistically significant increase in survivin expression compared to medium control (P = 0.00652, ). In wells treated with 100 μM resveratrol for 48 hours, we detected a statistically significant decrease in survivin expression compared to the ethanol control (P = 0.00094, ). In wells treated with 100 μM resveratrol for 48 hours, we detected a statistically significant decrease in BCL-2 expression compared to medium and ethanol controls (resveratrol versus medium, P = 0.00041; resveratrol versus ethanol, P = 0.00012; ). Statistically significant changes in BAX expression could not be found (resveratrol versus medium, P = 0.557; resveratrol versus ethanol, P = 0.164; ). This resulted in a highly significant increase in the BAX/BCL-2 ratio in GH3 cells treated with 100 μM resveratrol compared to the ethanol control (P = 7 × 10−6) and the medium control (P = 4.1 × 10−6, ). Statistically significant differences between passages or between medium and ethanol controls could not be found.
Figure 4 (A) Relative expression of survivin compared to β2 microglobulin significantly increased in the ethanol control (P = 0.00652) and significantly decreased after 48 hours of incubation with 100 μM resveratrol compared to the ethanol control (P=0.00094). (B) Relative expression of B-cell lymphoma-2 associated X protein (BAX) as compared to β2 microglobulin did not change significantly after treatment with 100 μM resveratrol (P = 0.164 and above). (C) Relative expression of B-cell lymphoma-2 protein (BCL-2) as compared to β2 microglobulin significantly decreased after treatment with 100μM resveratrol (P = 0.00041 and less). (D) A statistically significant shift in the B-cell lymphoma-2 X-associated protein/B-cell lymphoma-2 protein (BAX/BCL-2) ratio was observed after 48 hours of incubation with 100 μM resveratrol (P = 7 × 10−6 and less).
Summary of results
GH3 cell viability significantly decreased with growing concentrations of resveratrol. In GH3 cells treated with 100 μM resveratrol, the ELISA demonstrated a significant increase of nucleosome liberation, and unidimensional gel electrophoresis showed severe degradation of DNA. In GH3 cells treated with 100 μM resveratrol, qRT-PCR detected a statistically significant decrease of BCL-2 mRNA expression and a decrease of survivin expression, whereas there was no change in BAX mRNA expression. The BAX/BCL-2 ratio was significantly increased after resveratrol treatment.
Discussion
Cell viability
Wang et alCitation6 attempted to prove a dose-dependent effect of resveratrol on GH3 cell counts using an MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) assay. This colorimetric assay was intended to measure the number of viable cells as a correlate of the reduction of MTT (yellow) to formazan (blue).Citation7 The authors observed a significantly slower increase of MTT signals over 3 days in cell cultures treated with 50 μM resveratrol. They interpreted these data as inhibition of cell proliferation by the drug. However, the reduction of MTT to formazan occurs at least in part within the mitochondria, and depends on reducing enzymes within these organelles.Citation8 One may therefore argue that the well-known interference of resveratrol with the mitochondrial electron-transport chainCitation9 might compromise the validity of MTT assays to assess the number of viable cells following resveratrol treatment. As recently published by Chan et al,Citation10 MTT assays may underestimate the effect of cytotoxic drugs, since the number of mitochondria may still increase in arrested cells. Moreover, a major prerequisite for MTT assays is cell adherence, since loosely adherent or floating cells will not withstand the required washing procedures. This is well known to the scientific community, and MTT assays can thus even be exploited to measure cell adherence.Citation11 We found that GH3 cells, in accordance with the supplier’s (ATCC) description, were loosely adherent. We therefore abstained from relying on the MTT assay in GH3 cells. Using a hemocytometer, we were able to demonstrate a significant dose-dependent effect of resveratrol on GH3 cell viability. The slower increase of MTT reduction over time in resveratrol-treated cellsCitation6 may at least in part be caused by its metabolic effect on mitochondria, rather than by inhibited cell proliferation.
To support an inhibition of cell proliferation by resveratrol, Wang and colleaguesCitation6 demonstrated a significant increase of cells in the G0/G1 fraction by fluorescence-activated cell sorting (FACS), but the observed shift was only moderate. Although sub-G0 fractions were not communicated, no relevant levels of dead cells seemed to have occurred in propidium iodide-stained cells used for FACS.
In contrast to these results, we observed an absolute decline of the microscopically counted cell number under resveratrol over 3 days (). Compared to the initially seeded number of cells per well, the cell numbers declined to 48.67% and 12.11% for resveratrol concentrations of 20 and 100 μM, respectively. Therefore, in our hands, resveratrol did not only inhibit proliferation, but actually killed up to 88% of GH3 cells, which has as far as we know not yet been reported. From preliminary experiments, we had learned that resveratrol does not significantly decrease viability in GH3 cells during the first day of treatment.
Free nucleosome-specific ELISA
We next questioned whether the significant dose-dependent effect of resveratrol on GH3 cell counts is at least in part due to induction of apoptosis. Using an ELISA that detects free nucleosomes, we were able to demonstrate that resveratrol is likely to induce apoptosis in GH3 cells (). Wang et alCitation6 demonstrated resveratrol-induced apoptosis in GH3 cells using an annexin V FACS assay. This type of assay allowed an estimation of the percentage of apoptotic cells, which was 16.1% after 3 days under 50 μM resveratrol, compared to 7.4% in controls. This suggested a rather limited induction of apoptosis (2.2-fold), which would correspond well to the observation that the treated cells still showed an increment of MTT signal over time. Although not allowing a precise quantification of apoptosis rate, our ELISA data, ie, a greater-than-tenfold increment of nucleosome liberation by 100 μM resveratrol, suggested induction of apoptosis at least to some extent. The specificity of the employed ELISA with regard to apoptosis is limited, and – most of all – it gives no clue regarding the dominating mode of cell death. We therefore assessed to what degree non-apoptotic cell death contributed to the observed cell loss.
DNA gel electrophoresis
In a unidimensional gel electrophoresis of DNA obtained from GH3 cells treated for 48 hours with 100 μM resveratrol, we observed a continuous faint staining, ie, a severe degradation of DNA into random fragments (), which is indicative of nonapoptotic cell death, whereas there was no evidence of a “laddering” of DNA fragments of specific lengths, which would be expected in apoptotic cells. DNA degradation did not occur in medium and ethanol controls. We conclude that nonapoptotic cell death largely contributed to the reduced viability in GH3 cells treated with 100 μM resveratrol for 48 hours. This effect has, as far as we know, not yet been reported. We did not assess whether apoptosis would have dominated reduction of viability in GH3 cells at earlier points in time or after treatment with lower doses of resveratrol.
qRT-PCR
Since the marked nucleosome liberation we observed in the ELISA and the results from Wang et alCitation6 suggest that apoptosis does to some extent occur in resveratrol-treated GH3 cells, we next questioned how the effect of resveratrol on apoptosis in GH3 cells is mediated. Induction of apoptosis dependent on downregulation of survivinCitation2 as well as an estrogen receptor-dependent shift in BAX/BCL-2 ratioCitation3,Citation6,Citation12 leading to apoptosis were taken into consideration. qRT-PCR was employed for quantitative analysis of survivin, BAX, and BCL-2 expression. Due to its constant expression, B2MG was considered suitable as a housekeeping gene. From preliminary experiments we had learned that actin, which we favored initially, is inconstantly expressed and therefore useless as a housekeeping gene in resveratrol treated GH3 cells.
We found a statistically significant increase in survivin expression in the ethanol control at a very low ethanol concentration of 0.1% as compared to medium control, whereas apoptosis was neither induced in the ethanol control nor in medium control according to the results from the nucleosome-specific ELISA and from the DNA gel electrophoresis. A protective effect of survivin on ethanol susceptibility at ethanol concentrations of 1%–5% was found by Jones et al in gastric endothelial and epithelial cells of the rat.Citation13,Citation14 We found a statistically significant decrease in survivin expression after resveratrol treatment compared to the ethanol control.
We draw the following conclusions from our measurements of survivin expression: GH3 cells exposed to ethanol increase survivin expression even at ethanol concentrations as low as 0.1%, possibly to protect themselves against potentially toxic effects of ethanol. The resveratrol-dependent decrease of survivin expression in GH3 cells may at least in part be masked by an increase in survivin expression due to the effect of the solvent ethanol. Apoptosis in GH3 cells may possibly be mediated by resveratrol-dependent downregulation of survivin. Further investigation of the effects of ethanol on modulators of apoptosis in GH3 cells may be promising.
We found a significant decrease in BCL-2 expression after resveratrol treatment, whereas a significant change in BAX expression was not observed. This resulted in a significant increase in the BAX/BCL-2 ratio after resveratrol treatment. The changes we detected in relative expression of BCL-2 (about eightfold) are very unlikely to be false-positive. We conclude that resveratrol-dependent downregulation of BCL-2 with a subsequent rise of the BAX/BCL-2 ratio plays a major role in inducing apoptosis in resveratrol-treated GH3 cells. Other pathways that mediate resveratrol-induced apoptosis in GH3 cells are of course conceivable.
We must point out that our findings result from in vitro experiments in rat cells. As far as we know, these findings have yet to be confirmed in human pituitary adenoma cells and in vivo before any conclusion as to potential therapeutic options may be drawn. A major obstacle to studying resveratrol-induced cell death in pituitary adenoma tissue in vivo is that due to rapid intestinal and hepatic metabolism,Citation15 resveratrol concentrations achievable in humans after oral or intravenous administration usually do not exceed 2 μM,Citation16 whereas we, as well as other authors,Citation6 found a significant impact of resveratrol on GH3 cell viability to occur at higher micromolar concentrations. Therefore, in vivo studies aiming to demonstrate an effect of resveratrol on cell viability in pituitary adenoma tissue would require prodrugs or implants delivering resveratrol at micromolar concentrations.
However, since resveratrol is known to induce cell death,Citation17,Citation18 to increase radiation sensitivity,Citation19,Citation20 and to act synergistically with other chemotherapeutic agentsCitation21,Citation22 in a variety of tumor cells, further investigation of the impact of resveratrol and of other phytoestrogens on GH3 cells is warranted.
Conclusion
Resveratrol, a phytoestrogen, decreases GH3 cell viability in a dose-dependent manner by inducing nonapoptotic cell death, and to some extent apoptosis. Apoptosis in GH3 cells is probably mediated by resveratrol-dependent downregulation of apoptosis inhibitors, namely BCL-2 and possibly survivin. Further investigation of the potential effects of resveratrol and other phytoestrogens on pituitary adenoma cells is warranted.
Disclosure
The authors report no conflicts of interest in this work.
References
- FahlbuschRBuchfelderMPituitary surgeryMelmedSThe Pituitary3rd edLondonAcademic Press2011703719
- AtharMBackJHKopelovichLBickersDRKimALMultiple molecular targets of resveratrol: anti-carcinogenic mechanismsArch Biochem Biophys20094869510219514131
- ZaldivarVMagriMLZárateSEstradiol increases the Bax/Bcl-2 ratio and induces apoptosis in the anterior pituitary glandNeuroendocrinology20099029230019684383
- JankowskaAWaskoRWaligorska-StachuraJSurvivin products in pituitary tumorsNeuroendocrinol Lett2008291033103719112393
- ChomczynskiPSacchiNSingle-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extractionAnal Biochem19871621561592440339
- WangCHuZQChuMResveratrol inhibited GH3 cell growth and decreased prolactin level via estrogen receptorsClin Neurol Neurosurg201211424124822104698
- MossmannTRapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assaysJ Immunol Methods19836555636606682
- BerridgeMVTanASCharacterization of the cellular reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT): subcellular localization, substrate dependence, and involvement of mitochondrial electron transport in MTT reductionArch Biochem Biophys19933034744828390225
- ZiniRMorinCBertelliABertelliAATillementJPEffects of resveratrol on the rat brain respiratory chainDrugs Exp Clin Res199925879710370869
- ChanGKKleinheinzTLPetersonDMoffatJGA simple high-content cell cycle assay reveals frequent discrepancies between cell number and ATP and MTS proliferation assaysPLoS One20138e6358323691072
- MikiIIshiharaNOtoshiMKaseHSimple colorimetric cell-cell adhesion assay using MTT-stained leukemia cellsJ Immunol Methods19931642552618370931
- TanaseCOgrezeanuIBadiuCMolecular Pathology of Pituitary AdenomasLondonElsevier20124552
- JonesMKPadillaORWebbNANorngMThe anti-apoptosis protein, survivin, mediates gastric epithelial cell cytoprotection against ethanol-induced injury via activation of the p34(cdc2) cyclin-dependent kinaseJ Cell Physiol200821575076418181150
- JonesMKPadillaORZhuESurvivin is a key factor in the differential susceptibility of gastric endothelial and epithelial cells to alcohol-induced injuryJ Physiol Pharmacol20106125326420610854
- WalleTBioavailability of resveratrolAnn N Y Acad Sci2011121591521261636
- WalleTHsiehFDeLeggeMHOatisJEJrWalleUKHigh absorption but very low bioavailability of oral resveratrol in humansDrug Metab Dispos2004321377138215333514
- LinXWuGHuoWQZhangYJinFSResveratrol induces apoptosis associated with mitochondrial dysfunction in bladder carcinoma cellsInt J Urol20121975776422607368
- ZhouJHChengHYYuZQHeDWPanZYangDTResveratrol induces apoptosis in pancreatic cancer cellsChin Med J (Engl)20111241695169921740780
- FangYDeMarcoVGNichollMBResveratrol enhances radiation sensitivity in prostate cancer by inhibiting cell proliferation and promoting cell senescence and apoptosisCancer Sci20121031090109822417066
- TakJKLeeJHParkJWResveratrol and piperine enhance radiosensitivity of tumor cellsBMB Rep20124524224622531135
- HuangHLinHZhangXLiJResveratrol reverses temozolomide resistance by downregulation of MGMT in T98G glioblastoma cells by the NF-κB-dependent pathwayOncol Rep2012272050205622426504
- GuptaSCKannappanRReuterSKimJHAggarwalBBChemosensitization of tumors by resveratrolAnn N Y Acad Sci2011121515016021261654