Abstract
Background
The present study was designed to determine whether Allium ursinum L (ramson) could inhibit the proliferation of human AGS gastric cancer cells. Furthermore, we attempted to determine whether this inhibition could occur by targeting regulatory elements of the cell cycle.
Methods
Flow cytometry was used to observe apoptosis and the cell cycle in AGS cell lines treated or not treated with ramson watery extract. Proteins related to the cell cycle were detected by Western blotting. Caspase activity was measured using a colorimetric assay kit according to the manufacturer’s instructions.
Results
Ramson watery extract induced apoptosis and G2/M phase arrest in AGS cells. Western blotting showed that cyclin B was inhibited by ramson watery extract. However, G1 phase-related proteins remain unchanged after treatment.
Conclusion
Our results indicate that ramson effectively sup pressed proliferation and induced apoptosis and G2/M arrest in AGS cells by regulating elements of the cell cycle.
Introduction
Gastric cancer is one of the most common malignancies, not only in the People’s Republic of China but also in the rest of the world.Citation1,Citation2 Worldwide, 989,600 new cases of stomach cancer and 738,000 deaths are estimated to have occurred in 2008, accounting for 8% of total cancer cases and 10% of total deaths.Citation3
It has been demonstrated that dietary intake of Allium vegetables may lower the risk of several types of malignancies, including stomach, esophageal, and prostate cancer.Citation4–Citation6 Epidemiologic research has shown that the risk of prostate cancer is significantly lower in men consuming >10 g/day of Allium vegetables than in men with a total Allium vegetable intake <2.2 g/day.Citation6 Allium ursinum L (ramson, wild garlic) is a perennial plant, and widely distributed throughout Europe.Citation7 Ramson is used as a spice and as a traditional medicine, lowering blood pressure, and being effective against arteriosclerosis, diarrhea, and indigestion.Citation8 Ramson leaves have been used to treat cardiovascular disease since the Middle Ages.Citation9 High amounts of volatile compounds, such as sulfides and disulfides, which had been identified in ramson, have a direct impact on the quality of ramson as a medicinal plant and as a spice.Citation10
All parts of the ramson plant are known to have antioxidant properties,Citation8 but the chemical compounds in the leaves, stalks, and seeds of the plant have not been fully identified. To our knowledge, until now, no studies have investigated the effects of ramson on gastric cancer. In this study, we demonstrated that ramson significantly prevents growth and induces apoptosis and G2/M arrest in AGS cells, and have confirmed the mechanism of G2/M arrest induced by ramson.
Methods and materials
Cell culture
AGS cells were obtained from the American Type Culture Collection (ATCC, Bethesda, MD, USA) and grown in RPMI-1640 medium (HyClone, Logan, UT, USA) supplemented with 10% fetal bovine serum and antibiotics (100 U/mL penicillin and 100 μg/mL streptomycin). Cells were maintained in a humidified incubator with 5% CO2 at 37°C.
Preparation of ramson extract and spectrophotometry
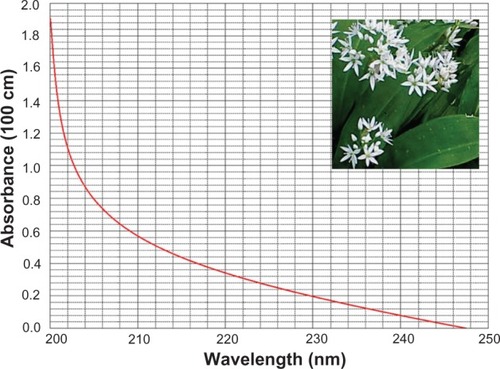
Powdered samples of whole ramson plants were obtained from Fei-fei Mou, Shijiazhuang Medical College, Shijiazhuang, People’s Republic of China. The powder samples (2 g) were extracted with 50 mL of methanol acidified with 1% acetic acid and then dissolved in distilled water to obtain a working solution. Using the methods described by Putnoky et al,Citation11 the ultraviolet absorption spectrum of the working solution was obtained against distilled water in a 1 cm quartz cuvette. The absorbance report measured at 240–250 nm was read according to the literature specifications to confirm the identity of the absorbing compound (Allicin; Xian Linhe Biotechnology Co. Ltd Xi’an, People’s Republic of China).
Cell viability assay
AGS cells were seeded into 96-well plates at a density of 1 × 103 cells per well, 24 hours before treatment. For the inhibition test, the cells were treated with different concentrations of ramson for 48 hours. Next, 20 μL (5 mg/mL) of 3-(4,5 dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma-Aldrich, Carlsbad CA, USA) was added to each well, and after incubation at 37°C for four hours, the MTT solution was removed and 200 μL of dimethylsulfoxide 0.2% was added to dissolve the crystals. Absorbance in each well at 570 nm was measured using a microplate reader (Bio-Rad Hercules, CA, USA). The IC50 value for ramson was determined and used in the subsequent experiments.
Cell apoptosis assay
Apoptosis was determined using an apoptosis detection kit (KeyGEN, Nanjing, People’s Republic of China). Briefly, the cells were collected washed twice in ice-cold phosphate-buffered solution, and then resuspended in binding buffer at a density of 1 × 106 cells/mL. The cells were incubated simultaneously with fluorescein-labeled Annexin V and propidium iodide for 20 minutes. The mixture was then analyzed using a FACScan flow cytometer (BD Biosciences, Baltimore, MD, USA).
Detection of cell cycle changes
The cells were removed from plates by trypsinization and pooled with cell culture supernatant containing nonadherent cells. The cells were washed once with phosphate-buffered solution, fixed in cold 70% ethanol, and stored at −20°C until analysis. For staining, 1 × 106 cells were washed in phosphate-buffered solution and then stained in phosphate-buffered solution with 50 μg/mL propidium iodide (KeyGEN), 200 μg/mL RNase A, and 0.1% Triton X-100. Analyses were performed using the FACScan flow cytometer.
Western blot assays
Protein extract samples (each weighing 45 μg) were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes. The membranes were blocked with 5% (w/v) nonfat milk in Phosphate-Buffered Saline with Tween 20, then probed with primary antibodies at 4°C overnight. The primary antibodies used were cyclin D1, cyclin E, cyclin B, and β-actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA). After washing, the membranes were incubated with the appropriate secondary antibodies for two hours at room temperature. Bound antibody was detected using an enhanced chemiluminescence kit (Amersham Biosciences, Westborough, MA, USA) according to the manufacturer’s instructions.
Measurement of activity of caspases 3, 8, and 9
Caspase activity was measured using a colorimetric assay kit according to the manufacturer’s instructions. After harvesting, the cells were washed in ice-cold phosphate-buffered solution and lysed. Proteins were extracted and stored at −80°C until use. Next, 20 μL of cell lysate was added to buffer containing a p-nitroaniline (pNA)-conjugated substrate (80 μL) for caspase 3 (Ac-DEVD-pNA), caspase 8 (Ac-IETD-pNA), or caspase 9 (LEHD-pNA, KeyGEN). Incubation was performed at 37°C with shaking at 500 rpm for one minute and then at room temperature for two hours. The pNA released in each well was measured using a plate-reading luminometer (Thermo Scientific, Shanghai, People’s Republic of China). Data were collected from three independent experiments.
Statistical analysis
All numerical data are expressed as the mean ± standard deviation. Differences between mean values were evaluated using the Student’s t-test. All statistical analyses were conducted using the Statistical Package for the Social Sciences version 17.0 software (SPSS Inc, Chicago, IL, USA). P values < 0.05 were considered to be statistically significant.
Results
Spectrophotometric results
The ultraviolet absorption spectrum for the ramson solution, obtained against distilled water in a 1 cm quartz cuvette, is shown in . The profile of the absorption spectrum is significant in the areas of highest and lowest absorption, and distinct profiles indicate the effects of different molecules. Using the values in the literature for specific absorption at 240 nm, the Allicin content in the fresh raw sample was found to be 0.58 μg/mL.
Effects of ramson on AGS cells
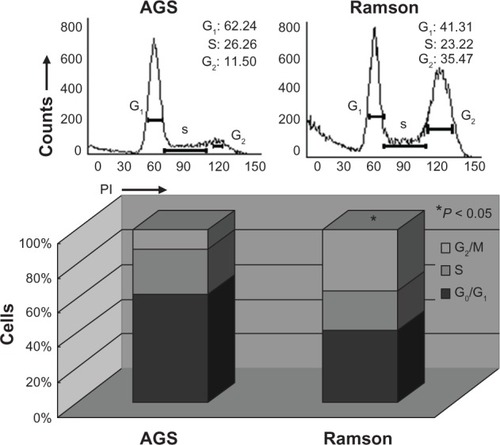
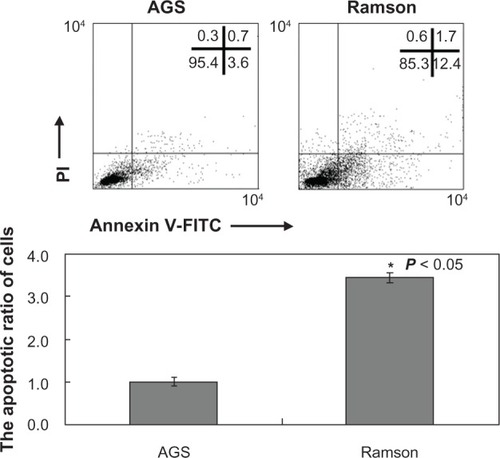
Cell viability was monitored by MTT assay. shows the growth inhibition curves generated. The IC50 value for ramson was 16.2 μM. Proliferation of the AGS gastric cancer cells was inhibited by ramson. We used double-staining with Annexin V-fluorescein isothiocyanate and propidium iodide to detect apoptotic cells. The apoptotic ratio of cells increased significantly after treatment with ramson. As shown in , the ratio of treated cells was 3–4 times higher than that of untreated cells (P < 0.05). We then detected changes in the cell cycle using propidium iodide staining. As shown in , the ratio of cells in G0/G1 phase was decreased in treated cells compared with untreated cells, but numbers in G2/M phase increased (P < 0.05). The amount of cells in G2 phase after treatment with ramson was 3–3.5 times higher than that of untreated cells.
Figure 2 Growth curve for cell lines measured using the MTT assay. The experiments were repeated three times.
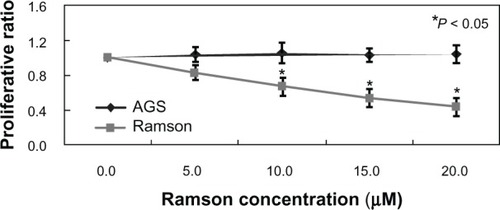
Figure 3 Apoptotic ratio of AGS cells treated with ramson analyzed by double-staining with Annexin V-FITC (fluorescein isothiocyanate) and propidium iodide. *Ramson-treated cells showed a higher apoptotic ratio than untreated cells (P < 0.05).
Mechanism of apoptosis and G2/M arrest in AGS cells
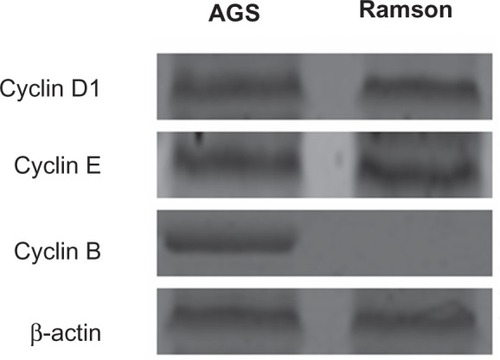
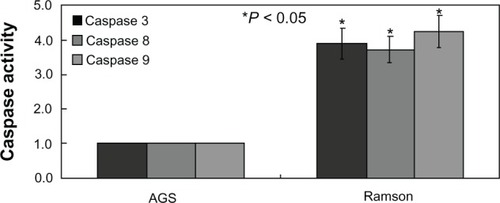
No changes in cyclin D1 or E levels were identified in treated cells using Western blotting (). The main G2 phase-related protein detected was cyclin B, which almost disappeared in treated cells (). The results were consistent with the changes in the cell cycle reported above. Further, the activity of caspases 3, 8, and 9 was significantly increased in treated cells compared with untreated cells (P < 0.05, ).
Discussion
In recent decades, much research attention has been focused on the molecular basis of oncogenic formation and transformation.Citation12 However, drug resistance in cancer is still a challenge when attempting to cure the disease, and current therapeutic combinations used to treat gastric cancer are not fully effective. The A. ursinum plant has been reported to have antioxidative, cytostatic, and antimicrobial activity.Citation13 However, the mechanism of the antitumor activity of ramson has been unclear. In this work, we confirmed that ramson can inhibit proliferation and induce apoptosis in AGS cells. To our knowledge, limited data are available on the antitumor activity of ramson. However, diallyl disulfide, an extract of garlic, has been shown to inhibit the growth of many cancers.Citation14,Citation15 Xiao et alCitation16 reported that diallyl trisulfide inhibited the viability of human lung cancer cells. Further, suppression of cancer cell growth in association with G2/M phase cell cycle arrest and/or induction of apoptosis mediated by organosulfur compounds, ie, diallyl disulfide and diallyl trisulfide, has been demonstrated in human colon, neuroblastoma, and prostate cancer cells.Citation17–Citation22 Consistent with previous studies, our research confirmed that ramson induced G2/M arrest in AGS cells. These results collectively indicate that extracts from Allium vegetables share the common feature of antitumor activity.
To determine the mechanism(s) of action of ramson in AGS cells, we identified the proteins relevant to the cell cycle by Western blotting. There were no changes in cyclin D1 or E levels between the treated and untreated cells. Cyclins D1 and E are important factors in the conversion from G1 phase to G2 phase. Zurlo et alCitation23 also confirmed that decreased levels of cyclins D1 and E could induce arrest of G1 phase in HT-29 human colon carcinoma cells. In subsequent experiments, we found that cyclin B, the main protein involved in G2 phase, disappeared on treatment with ramson, which explains why the cell cycle was obstructed in G2 phase. Similarly, Gao et alCitation24 found that downregulation of cyclin B by aspirin (a nitric oxide donor) can induce arrest of G2/M phase.
Overall, in our experiments, we identified that ramson had an antitumor effect in AGS gastric cancer cells. Apoptosis and arrest of G2/M phase were observed in cells after treatment with ramson. Our research offers a novel insight into the treatment of gastric cancer. In future studies, we plan to observe further the effects of ramson on gastric cancer.
Acknowledgments
This study was supported by grants from Shenyang Science and Technology (F12-277-1-75), the National Natural Scientific Foundation of China (81001093), a Project Supported by Scientific Research Fund of Liaoning Provincial Education Department (L2010649), and the Specialized Research Fund for the Doctoral Program of Higher Education (20102104120026). We thank Pu Xia and Fei-fei Mou for their valuable comments and excellent technical assistance.
Disclosure
The authors report no conflicts of interest in this work.
References
- XinYLiXLWangYPRelationship between phenotypes of cell-function differentiation and pathobiological behavior of gastric carcinomasWorld J Gastroenterol20017535911819733
- WeidnerNIntratumor microvessel density as a prognostic factor in cancerAm J Pathol19951479197541613
- JemalABrayFCenterMMFerlayJWardEFormanDGlobal cancer statisticsCA Cancer J Clin201161699021296855
- YouWCBlotWJChangYSAllium vegetables and reduced risk of stomach cancerJ Natl Cancer Inst1989811621642909758
- GaoCMTakezakiTDingJHLiMSTajimaKProtective effect of allium vegetables against both esophageal and stomach cancer: a simultaneous case-referent study of a high-epidemic area in Jiangsu Province, ChinaJpn J Cancer Res19999061462110429652
- HsingAWChokkalingamAPGaoYTAllium vegetables and risk of prostate cancer: a population-based studyJ Natl Cancer Inst2002941648165112419792
- IciekMKwiecieńIWłodekLBiological properties of garlic and garlic-derived organosulfur compoundsEnviron Mol Mutagen20095024726519253339
- StajnerDPopovićBMCanadanović-BrunetJStajnerMAntioxidant and scavenger activities of Allium ursinumFitoterapia20087930330518313233
- RietzBIsenseeHStrobachHMakdessiSJacobRCardioprotective actions of wild garlic (Allium ursinum) in ischemia and reperfusionMol Cell Biochem19931191431508455576
- PreussHGClouatreDMohamadiAJarrellSTComparative greater effect than regular garlic on blood pressure and blood chemistries of ratsInt Urol Nephrol19923252553011989540
- PutnokySCauniiAButnariuMStudy on the stability and antioxidant effect of the Allium ursinum watery extractChem Cent J201372123369571
- CaswellPTVadrevuSNormanJCIntegrins: masters and slaves of endocytic transportNat Rev Mol Cell Biol20091084385319904298
- IvanovaAMikhovaBNajdenskiHTsvetkovaIKostovaIChemical composition and antimicrobial activity of wild garlic Allium ursinum of Bulgarian originNat Prod Commun200941059106219768983
- HongJYWangZYSmithTJInhibitory effects of diallyl sulfide on the metabolism and tumorigenicity of the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) in A/J mouse lungCarcinogenesis1992139019041587006
- ReddyBSRaoCVRivensonAKelloffGChemoprevention of colon carcinogenesis by organosulfur compoundsCancer Res199353349334988339252
- XiaoDZengYHahmERKimYARamalingamSSinghSVDiallyl trisulfide selectively causes Bax- and Bak-mediated apoptosis in human lung cancer cellsEnviron Mol Mutagen20095020121218800351
- FilomeniGAquilanoKRotilioGCirioloMRReactive oxygen species-dependent c-Jun NH2-terminal kinase/c-Jun signaling cascade mediates neuroblastoma cell death induced by diallyl disulfideCancer Res2003635940594914522920
- HosonoTFukaoTOgiharaJDiallyl trisulfide suppresses the proliferation and induces apoptosis of human colon cancer cells through oxidative modification of β-tubulinJ Biol Chem2005280414874149316219763
- KnowlesLMMilnerJADiallyl disulfide inhibits p34 (cdc2) kinase activity through changes in complex formation and phosphorylationCarcinogenesis2000211129113410837000
- SundaramSGMilnerJADiallyl disulfide induces apoptosis of human colon tumor cellsCarcinogenesis1996176696738625476
- XiaoDChoiSJohnsonDEDiallyl trisulfide-induced apoptosis in human prostate cancer cells involves c-Jun N-terminal kinase and extracellular-signal regulated kinase-mediated phosphorylation of Bcl-2Oncogene2004235594560615184882
- XiaoDHerman-AntosiewiczAAntosiewiczJDiallyl trisulfide-induced G2-M phase cell cycle arrest in human prostate cancer cells is caused by reactive oxygen species-dependent destruction and hyperphosphorylation of Cdc25COncogene2005246256626815940258
- ZurloDLeoneCAssanteGCladosporol a stimulates G1-phase arrest of the cell cycle by up-regulation of p21 (waf1/cip1) expression in human colon carcinoma HT-29 cellsMol Carcinog20135211722025467
- GaoLWilliamsJLNitric oxide-donating aspirin induces G2/M phase cell cycle arrest in human cancer cells by regulating phase transition proteinsInt J Oncol20124132533022552812