Abstract
Primary primitive neuroectodermal tumors (PNETs) are rare and high-grade malignant tumors that mostly occur in children and young adults. The most common sites are the trunk, limbs, and retroperitoneum. Herein, we present a case of a PNET involving the cervix uteri in a 27-year-old woman. The lesion showed characteristic histologic features of a PNET and was positive for the immunohistochemical markers cluster of differentiation (CD) 99, vimentin, neuron-specific enolase, neural cell adhesion molecule 1 (CD56), and CD117 (c-kit), further defining the tumor while helping to confirm PNET. The clinical Stage IIIB tumor was treated with chemotherapy and radiotherapy.
Introduction
Primitive neuroectodermal tumors (PNETs) are a type of small round cell tumor that originate from the primitive neuroectoderm and most commonly arise in the chest wall and paraspinal regions. Although PNETs can occur in a number of organs, such as in the kidneys,Citation1 brain,Citation2 and lungs,Citation3 they also occur very rarely in the cervix. As far as we are aware, only nine cases have been reported. PNETs are high-grade malignant, invasive tumors. Currently, there is no uniform standard treatment protocol. Herein, we present a case of primary cervical PNET and describe the diagnosis and treatment process to increase awareness of this condition.
Materials and methods
We used 4 μm sections of formalin-fixed, paraffin-embedded tissue for routine light microscopy examination and immunohistochemical (IHC) analysis. Antibodies against cluster of differentiation (CD) 99, pan-cytokeratin, vimentin, neuron-specific enolase (NSE), neural cell adhesion molecule 1 (CD56), CD117, thyroid transcription factor 1 (TTF-1), terminal deoxynucleotidyl transferase (TdT), leukocyte common antigen (CD45), common acute lymphoblastic leukemia antigen CD10, CD38, myogenic markers (desmin and myogenin), markers of malignant melanoma (S-100 and HMB-45), and Ki-67 (ready to use, Fuzhou Maixin Biotechnology Development, Fuzhou, People’s Republic of China) were used for IHC analysis (). The sections were incubated with the primary antibody at 4°C overnight before being treated with 3% H2O2 and 5% rabbit serum at 37°C for 1 hour. Next, the sections were incubated with the secondary antibody and streptavidin-peroxidase (SP) complex for 30 minutes using an SP kit (Fuzhou Maixin Biotechnology Development). Slides were visualized using 3,3-diaminobenzidine. Sections were stained with nonspecific antibodies to provide a negative control for each reaction.
Table 1 Antibodies used
Case description
A 27-year-old woman presented with contact bleeding, which she had experienced more than 3 months. At first, the bleeding was slight, guttate, scarlet, and would stop on its own, so the patient paid no attention to it. Further, 10 days before presenting to the hospital, she experienced yellow vaginal discharge with no special odor. She also suffered from occasional lower abdominal pain, without waist pain. Therefore, she visited the Department of Obstetrics and Gynecology, Shengjing Hospital of China Medical University.
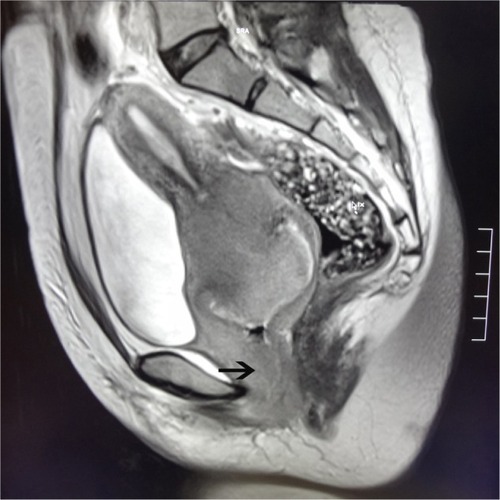
Pelvic examination revealed that the vulva had developed normally, but there was evidence of vaginal stenosis. Many nodular lesions were observed on the vagina anterior wall. These extended to the lower third of the vagina and were dark grey and hard. The cervix had lost its normal form and could not be exposed completely. The surface of the cervix was not smooth and had necrotic tissue, which showed evidence of contact bleeding. Tri-manual examination revealed bilateral cardinal ligament thickening and shortening with poor elasticity, which reached the pelvic wall. The values for serum tumor markers such as carcinoembryonic antigen (0.4 ng/mL), alpha-fetoprotein (0.7 ng/mL), carbohydrate antigen 125 (6.5 U/mL), cancer antigen 15–3 (0.5 U/mL), carbohydrate antigen 19–9 (4.7 U/mL), and squamous cell carcinoma antigen (0.8 ng/mL) were all within the normal range. Magnetic resonance imaging showed a space-occupying lesion arising from the cervix with a diameter of approximately 5.5 cm. The lesion extended into the vagina and parametrium (). The tumor was classified as clinical Stage IIIB. A cervical biopsy specimen of the tumor, obtained for confirmation of the tumor type, was prepared and analyzed by a pathologist using IHC analysis.
Pathologic findings
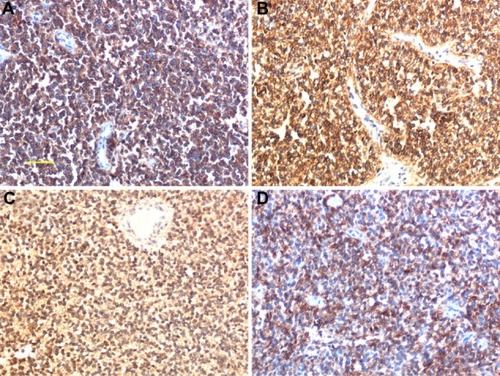
Microscopically, hematoxylin and eosin (HE) staining of sections showed that the tumor was composed of diffuse, small, round blue-staining cells. The cells were fairly uniform and contained round nuclei with fine chromatin. Nucleoli were small and inconspicuous (). Mitotic figures and Homer Wright rosettes were occasionally encountered (). IHC staining for CD99 (), vimentin, CD117 (), NSE (), and CD56 () was positive. Staining for all the other tested markers including pan-cytokeratin, TTF-1, TdT, CD45, CD38, desmin, myogenin, S-100, and HMB45 was negative. The Ki-67 labeling index was approximately 80%.
Figure 2 Histological findings. (A) Tumor composition – a diffused distribution of small round cells, with scattered Homer Wright rosettes (indicated by the arrow); (B) high-power microscopy revealed that the small round cells with round nuclei contained fine chromatin and there was limited clear or eosinophilic cytoplasm.
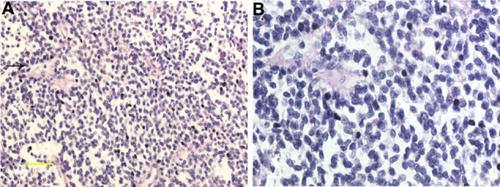
Figure 3 Immunohistochemical findings. (A) Strong reactivity in tumor cells with anti-cluster of differentiation (CD) 99. (B) CD117; membranous and cytoplasmic staining. (C) Neuron-specific enolase, cytoplasmic staining (×200). (D) CD56; membranous staining.
Discussion
PNET is a relatively uncommon tumor that mainly occurs in the trunk, limbs, and retroperitoneum.Citation4 Primary cervical PNETs are extremely rare and, to date and as far as we are aware, only nine cases have been reported in the literature.Citation5–Citation12 In contrast to patients with PNETs at other sites, which usually include children and young adults, the age of patients with primary cervical PNETs, as per these previous reports, ranged from 21 to 50 years (mean, 34 years). Of the nine patients, six who originally presented with localized disease were alive without evidence of recurrence or metastatic disease at an average follow-up time of 19 months (range, 5–50 months). Two patients died of disease, one 7 days following surgery and the other 4.2 years after surgery. Survival details are not available for one patient. The patient presented in this paper was 27 years old, which is consistent with the literature.
PNET is mainly diagnosed based on histopathological morphology, IHC examination, and genetic testing. In the present case, conventional HE staining revealed that the tumor was composed of small round cells. Homer Wright rosettes, a characteristic manifestation of PNETs, are seen in approximately 30% to 70% of cases.Citation13 While the presence of such rosettes can be used as an important basis to diagnose the tumor, IHC analysis is the primary means to diagnose PNETs. CD99 and vimentin staining is very characteristic and has a high sensitivity for PNET diagnosis. Occasionally, epithelial markers and desmin might show focal positivity, whereas the expression of neural markers such as NSE, S-100, neurofilaments, glial fibrillary acid protein, and CgA is uncertain. A characteristic chromosomal translocation t(11;22)(q24;q12), resulting in EWS–FLI1 fusion, is observed in 85% of PNET cases;Citation14,Citation15 most of the remaining cases (10%–15%) show fusion of the EWS gene to a second member of the ETS family of genes, namely ERG. EWS–ERG fusion occurs as a result of the chromosomal translocation t(21;22)(q22;q12).Citation12
PNETs share characteristics with other small round blue-cell cancers including intra-abdominal desmoplastic small round cell tumor (IDSRCT), rhabdomyosarcoma, lymphoblastic lymphoma, endometrial stromal sarcoma or undifferentiated sarcoma, and small cell carcinoma.Citation16 Membranous CD99 staining and focally positive NSE staining may be noted for PNETs. However, CD99 is not specific for PNETs; it can also be expressed in lymphoblastic lymphoma.Citation17 In this case, based on negative staining for CD45 and TdT together with no evidence of lymphoepithelial lesions on histopathology, lymphoid system disease was excluded. IDSRCT is characterized by a nested pattern with prominent desmoplastic stroma. The tumor nests often show peripheral palisading with central necrosis.Citation16 IDSRCT is diffusely positive for cytokeratin, desmin, and NSE. Rhabdomyosarcoma, in contrast to PNET, is strongly positive for desmin, myogenin, and myoD1. Endometrial stromal sarcoma and undifferentiated sarcoma are mainly composed of short spindle cells. Thick-wall spiral arteries are visible in the interstitial. In the present case, IHC analysis showed CD10 negativity and CD99 positivity, thereby excluding the diagnosis of stromal sarcoma. Negative staining for pan-cytokeratin and TTF-1 and positive staining for vimentin and CD117 also ruled out the possibility of small cell carcinoma.Citation18,Citation19
The most effective treatment for a PNET is surgery combined with chemotherapy and partial or systemic high-dose radiotherapy.Citation20 In the case of tumors without metastasis, surgical resection combined with chemotherapy is the treatment of choice. In the literature, five patients previously received only chemotherapy, one received radiotherapy alone, and the other three received a combination of chemotherapy and radiotherapy postoperatively. For chemotherapy regimens, Sato et alCitation5 used cisplatin, cyclophosphamide, etoposide, and vincristine for six cycles. Horn et alCitation6 used cisplatin and fluorouracil for 3 years after the initial therapy (for pulmonary metastasis). Snijders-Keilholz et alCitation10 used six cycles of doxorubicin, ifosfamide, mesna, and etoposide preoperatively and vincristine, ifosfamide, and actinomycin for five cycles. Tsao et alCitation13 used cyclophosphamide, doxorubicin (Adriamycin® Pfizer, New York, NY, USA), and vincristine and ifosfamide and etoposide for two cycles before surgery and two cycles after surgery.
In the present case, the clinical stage of the tumor was IIIB. The tumor had invaded the vaginal wall and was therefore too large for surgical resection. We used comprehensive treatment including partial radiotherapy (radiation dose, 45–55 Gy) combined with chemotherapy. We alternated between cyclophosphamide, Adriamycin, and vincristine with ifosfamide and etoposide chemotherapy every 3 weeks. After undergoing radiotherapy and chemotherapy, the patient experienced significant relief. At time of writing, 6 months after treatment, the patient is alive.
Conclusion
PNETs rarely occur as primary cervical neoplasms and have a poor prognosis. They can be diagnosed based on histopathological morphology, IHC examination, and genetic testing. Combined therapy including surgery, chemotherapy, and radiotherapy is an often-used treatment strategy. Here, we have described a case of PNET involving the cervix uteri in a 27-year-old woman. The patient was treated with chemotherapy and radiotherapy and remains alive without disease after 6 months. However, further follow-up is needed to evaluate the prognosis of the patient.
Disclosure
The authors declare no conflicts of interest in this work.
References
- BartholowTParwaniARenal primitive neuroectodermal tumorsArch Pathol Lab Med2012136668669022646279
- von BuerenAOCNS PNET molecular subgroups with distinct clinical featuresLancet Oncol201213875375422691719
- AndreiMCramerSFKramerZBZeidanAFaltasBAdult primary pulmonary primitive neuroectodermal tumor: molecular features and translational opportunitiesCancer Biol Ther2013142758023114712
- ShimadaHNewtonWAJrSouleEHQualmanSJAoyamaCMaurerHMPathologic features of extraosseous Ewing’s sarcoma: a report from the Intergroup Rhabdomyosarcoma StudyHum Pathol19881944424533284809
- SatoSYajimaAKimuraNNamikiTFuruhashiNSakumaHPeripheral neuroepithelioma (peripheral primitive neuroectodermal tumor) of the uterine cervixTohoku J Exp Med199618021871959111767
- HornLCFischerUBilekKPrimitive neuroectodermal tumor of the cervix uteri. A case reportGen Diagn Pathol19971423–42272309065588
- CenacchiGPasquinelliGMontanaroLPrimary endocervical extraosseous Ewing’s sarcoma/PNETInt J Gynecol Pathol199817183889475198
- PauwelsPAmbrosPHattingerCPeripheral primitive neuroectodermal tumour of the cervixVirchows Arch20004361687310664164
- MalpicaAMoranCAPrimitive neuroectodermal tumor of the cervix: a clinicopathologic and immunohistochemical study of two casesAnn Diagn Pathol20026528128712376920
- Snijders-KeilholzAEwingPSeynaeveCBurgerCWPrimitive neuroectodermal tumor of the cervix uteri: a case report – changing concepts in therapyGynecol Oncol200598351651915979131
- MasouraSKourtisAKalogiannidisIPrimary primitive neuroectodermal tumor of the cervix confirmed with molecular analysis in a 23-year-old woman: A case reportPathol Res Pract2012208424524922365564
- FarzanehFRezvaniHBoroujeniPTRahimiFPrimitive neuroectodermal tumor of the cervix: a case reportJ Med Case Rep2011548921962148
- TsaoASRothLMSandlerAHurteauJACervical primitive neuroectodermal tumorGynecol Oncol200183113814211585426
- DedeurwaerdereFGianniniCSciotRPrimary peripheral PNET/Ewing’s sarcoma of the dura: a clinicopathologic entity distinct from central PNETMod Pathol200215667367812065782
- TeicherBABagleyRGRouleauCKrugerARenYKurtzbergLCharacteristics of human Ewing/PNET sarcoma modelsAnn Saudi Med201131217418221422656
- Hayes-JordanAAndersonPMThe diagnosis and management of desmoplastic small round cell tumor: a reviewCurr Opin Oncol201123438538921577112
- OzdemirliMFanburg-SmithJCHartmannDPPrecursor B-Lymphoblastic lymphoma presenting as a solitary bone tumor and mimicking Ewing’s sarcoma: a report of four cases and review of the literatureAm J Surg Pathol19982277958049669342
- PisickESkarinATSalgiaRRecent advances in the molecular biology, diagnosis and novel therapies for various small blue cell tumorsAnticancer Res20032343379339612926079
- TsokosMAlaggioRDDehnerLPDickmanPSEwing sarcoma/peripheral primitive neuroectodermal tumor and related tumorsPediatr Dev Pathol201215Suppl 110812622420726
- SaifMWNgJChangBRussoSIs there a role of radiotherapy in the management of pancreatic neuroendocrine tumors (PNET)?JOP201213217417622406594