Abstract
Introduction
Despite trastuzumab having enhanced selectivity for human epidermal growth factor receptor 2 (HER-2) overexpressing breast cancer cells, treatment is hampered by interindividual variation and tumors with high mitogenic potential. The lack of significant clinical benefit in certain patient cohorts suggests that HER-2 expression is ineffective as a sole prognostic indicator of response to therapy. Therefore, optimizing the clinical role of trastuzumab in drug combinations remains critical for clinical success.
Aim
To investigate the effects of trastuzumab in combination with either doxorubicin or geldanamycin on in vitro cell viability, cell cycling, apoptosis and relative HER-2 expression in HER-2-positive (SK-BR-3) and estrogen receptor-positive (MCF-7) breast adenocarcinoma models.
Results
HER-2-rich SK-BR-3 cells demonstrated a greater sensitivity to the effects of doxorubicin than MCF-7 cells. Concurrent trastuzumab exposure resulted in a further reduction in cell viability. This decreased cell viability induced by doxorubicin was associated with activation of executioner caspases as well as with alterations in cell-cycle kinetics, primarily promoting S-phase accumulation. Doxorubicin had no effect on surface HER-2 density expression. Geldanamycin reduced cell viability significantly greater in SK-BR-3 than MCF-7 cells, and was associated with G2 cell-cycle accumulation. The addition of trastuzumab did not augment these effects. Geldanamycin promoted substantial reductions in relative surface HER-2 density in SK-BR-3 cells.
Conclusion
The in vitro data supported the rationale for using doxorubicin in trastuzumab-based therapies. Therefore, despite the incidence of cardiotoxicity, doxorubicin could retain a fundamental role in treating HER-2-positive breast cancer. While geldanamycin is a potent cytotoxic agent, its concurrent use with trastuzumab requires further research into the transient or permanent nature of alterations in HER-2 status in cell progeny.
Introduction
Selective amplification of human epidermal growth factor receptor 2 (HER-2) is found in approximately 25%–30% of all primary breast tumor tissue.Citation1 HER-2, even when expressed at standard physiological levels, requires no ligand-binding for activation,Citation2–Citation4 and is thus the preferred dimer partner for other HER-family members (HER-1, HER-3, and HER-4) due to its constitutive activation.Citation5,Citation6 HER-2 plays an essential part in regulating cell proliferation, adhesion, motility, and survival. Therefore, when HER-2 is overexpressed, these cellular responses are exaggerated and unpredictable,Citation4,Citation7,Citation8 resulting in a unique, highly mitogenic subtype of breast cancer.Citation1
The emergence of targeted therapies directed against definitive molecular targets implicated in specific processes of neoplastic transformation has radically altered the natural progression of certain types of cancer.Citation9–Citation11 Trastuzumab (Herceptin®; Genentech, San Francisco, CA, USA), an anti-HER-2 monoclonal antibody, is one such therapy that has had a dramatic impact on the treatment of HER-2-positive breast cancer.Citation9,Citation10 However, despite having enhanced selectivity for tissues that overexpress HER-2,Citation12 use of this targeted therapy remains hampered by interindividual variation and mitogenic thresholds beyond the limitations of trastuzumab efficacy.Citation11,Citation13 Furthermore, although initial studies have suggested that HER-2 status remains stable over time, discordances of up to 30% are now being reported between primary and metastatic tumor sites.Citation13
Retrospective studies suggest that only 30%–40% of patients derive substantial clinical benefit from trastuzumab,Citation11 with a diverse set of molecular mechanisms contributing to resistance.Citation14–Citation16 Regulatory anomalies resulting in resistance could possibly produce differences in both biological and clinical behavior, further confounding this already heterogeneous disease.Citation16,Citation17 This suggests that either HER-2 expression is inadequate as a sole prognostic indicator, or that the fundamental molecular characteristics of the HER-2-positive tumor subtype may rapidly evolve in response to certain stimuli.
Optimizing trastuzumab treatment has been attempted by using sequential combinations of diverse chemotherapeutic agents,Citation16 and remains critical to elevate trastuzumab efficacy towards greater clinical success. Herewith, the authors assessed the current relevance and future potential of two cytotoxic agents to determine which model and mechanism of cytotoxicity best complements trastuzumab’s efficacy in vitro.
Doxorubicin, an anthracycline currently used in treatment regimens with trastuzumab, is considered one of the most effective antineoplastic agents used to treat breast cancer.Citation9 DNA–topoisomerase II-á complex interactions are considered the primary triggering event for both growth arrest and apoptotic event signaling.Citation18,Citation19 Although trends towards greater doxorubicin sensitivity have been seen in HER-2 overexpressing cells, it has generally been deemed statistically insignificant.Citation19 Furthermore, an undesirable incidence of cumulative dose-dependent cardiotoxicity has become a limiting side effect of this treatment approach.Citation20,Citation21
Geldanamycin, a benzoquinone ansamycin, is capable of disrupting the chaperone heat-shock protein 90 (HSp90).Citation22 Chaperone proteins are merely mediators in the assembly of polypeptides, and are not ultimately integrated into the assembled structure.Citation23,Citation24 Ubiquitous HSp90 protein is constitutively expressed at higher levels in tumor cells compared to normal cell counterparts, suggesting that HSP90 may be a potential target in neoplastic tissue.Citation25
HSP90 association is required for the functioning of, amongst others, tumor-suppressor protein p53, transmembrane tyrosine kinases including the HER family, casein kinase II, and Cdk4/cyclin D complexes.Citation24 The degree to which HSP90 is involved in polypeptide assembly correlates with the extent to which the protein and downstream clients are adversely influenced by geldanamycin, and it may therefore only have minimal influence on HER-2.
The aim of this study was to determine which of these two cytotoxic agents best complements the efficacy of trastuzumab in vitro, in order to support the use of doxorubicin with concurrent trastuzumab in HER-2-positive breast adenocarcinoma, and to determine the extent to which geldanamycin reduces downstream HER-2 targets, thereby potentially nullifying its use in trastuzumab-containing regimens.
Materials and methods
This project was approved by the Faculty of Health Science Student Research Ethics Committee of the University of pretoria.
Cells and experimental agents
Adherent breast adenocarcinoma cell lines MCF-7 (American Type Culture Collection HTB-20) expressing normal levels of HER-2 and SK-BR-3 cells (American Type Culture Collection HTB-30) overexpressing HER-2 were maintained in Dulbecco’s Modified Eagle’s Medium and Roswell park Memorial Institute 1640 medium (Sigma-Aldrich, St Louis, MO, USA), respectively. Medium was supplemented with 10% heat-inactivated fetal bovine serum (PAA Laboratories, pasching, Austria) and 1% (v/v) penicillin-streptomycin (BioWhittaker; Lonza, Basel, Switzerland). For experimental purposes, cells were seeded at 1 × 104 cells per well or at 1.5 × 105 cells per 25 cm2 flask and maintained in a humidified atmosphere containing 5% CO2 at 37°C.
Cell culture-compatible doxorubicin (Sigma-Aldrich) and geldanamycin (Tocris Bioscience, Bristol, UK) were dissolved in dimethyl sulfoxide (Merck, Darmstadt, Germany), stored at -80°C, and reconstituted in the appropriate medium prior to use.
Trastuzumab (Herceptin), reconstituted with bacteriostatic water as per the manufacturer’s instructions, was kindly donated by Roche pharmaceuticals (Johannesburg, South Africa). Final drug concentrations were as follows: 0.17 ìM doxorubicin, 0.35 ìM geldanamycin, and 25–100 ìg/mL trastuzumab.
Tetrazolium conversion assay
After exposure to experimental agents for 96 hours, cell viability was determined using a quantitative colorimetric tetrazolium conversion assay using a 96-well plate format. Vehicle-treated controls, cell-free medium controls, and cell-free drug controls were included to ensure culture sterility and to discern potential spontaneous reactivity of 3-(4,5- dim ethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT). Cells were incubated with 20 ìL MTT solution (5 mg/mL; Sigma-Aldrich) for 4 hours, washed, dried, and the formazan product solubilized in dimethyl sulfoxide (Merck). plates were read spectrophotometrically on an ELx800uv universal microplate reader (BioTek Instruments, Winooski, VT, USA) at a dual wavelength of 570 and 630 nm.
Cell-cycle analysis
Analysis of cell-cycle kinetics was conducted after 24, 48, and 72 hours using intercalating fluorescent propidium iodide with flow-cytometric detection by means of a Cytomics FC500 (Beckman Coulter, Brea, CA, USA). Medium decanted from flasks along with all the cells was fixed in 70% ethanol and stored overnight at 4°C. prior to analysis, the cell pellet was resuspended in 1 mL of staining solution (propidium iodide [40 ìg/mL], Triton X-100 [0.1% v/v], DNase-free RNase [100 ìg/mL]; [Sigma-Aldrich]) and incubated at 37°C for 40 minutes. Analysis of histograms was conducted using MultiCycle version 3.0 for Windows deconvolution software (phoenix Flow Systems, San Diego, CA, USA).
Caspase 3 and 7 assay
Activated executioner caspases 3 and 7 hydrolyze Ac-DEVD-AMC substrates, releasing a fluorescent product (AMC). Cells were exposed for a series of time points between 4 and 30 hours, in a 96-well plate format, followed by removal of medium and addition of 25 ìL lysis buffer (10 mM HEPES, 1 mM PMSF, 5mM CHAPS [Merck Chemicals, Darmstadt, Germany], 2 mM EDTA and 5 mM P-mercaptoethanol [Labchem; Johannesburg, South Africa]).
After 40 minutes of incubation on ice, 125 ìL assay buffer (20 mM HEPES, 2 mM EDTA, and 5 mM P-mercaptoethanol) containing 5 ìM of Ac-DEVD-AMC substrate (Sigma- Aldrich) was added to each well. plates were incubated overnight at 37°C to facilitate complete cleavage of Ac-DEVD-AMC substrates and read using a Fluostar Optima (BMG Labtech, Ortenberg, Germany) with excitation-emission wavelengths of 350 and 450 nm, respectively. The fluorescence intensity was standardized to that of the untreated control.
Apoptosis/necrosis
Later apoptotic hallmarks were assessed using fluorescein isothiocyanate (FITC)-conjugated annexin V after exposure for 48 or 72 hours. Cells were washed and resuspended in annexin V binding buffer (10 mM HEPES, 150 mM NaCl, 5 mM KCL, 1.8 mM CaCl2, and 1 mM MgCl2; Merck) followed by staining with 2.5 μL annexin V FITC (Sigma-Aldrich). Samples were incubated in the dark for 10 minutes. prior to analysis, 3 μL propidium iodide (3 mM) was added as a counterstain for membrane integrity, and data were acquired using the Cytomics FC500 (Beckman Coulter).
Relative HER-2 density
Relative HER-2 density was determined at 12, 24, and 48 hours using an FITC-conjugated anti-HER-2 affibody molecule (Abcam, Cambridge, UK). Cells were labeled with the affibody molecule to a final concentration of 3.7 μg/mL and incubated on ice for 30 minutes. Samples were washed once before conducting flow-cytometry protocols using the Cytomics FC500 (Beckman Coulter), and the mean relative fluorescence intensity was compared to untreated samples.
Statistics
A minimum of three independent interday repeats were conducted, with a minimum of three intraday repeats where required. The Kruskal–Wallis nonparametric test was conducted to compare the mean of trastuzumab versus doxorubicin or geldanamycin alone versus the respective combinations. Dunn’s multiple-comparison test was used for post hoc analysis, with significance set at P < 0.05. Statistical analysis was conducted using Graphpad prism version 5.0 for Windows (Graphpad Software, San Diego, CA, USA).
Results and discussion
The relationship between HER-2 status and the success of anthracycline-based regimens in patients was initially assessed in the Cancer and Leukemia Group B 8541 study in 1994.Citation26 Compilation of retrospective data suggested that HER-2-positive tumors were associated with high overall response rates to high-dose anthracyclines.Citation27
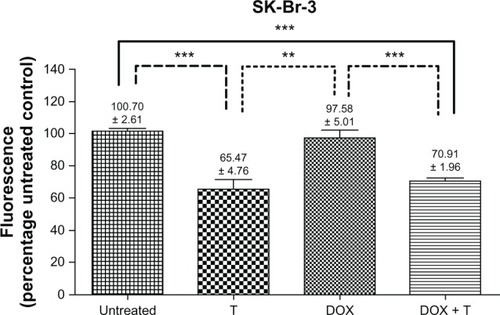
Campiglio et al established that SK-BR-3 cells, which also amplify topoisomerase II-á, possessed greater sensitivity to the effects of doxorubicin than MCF-7 cells. However, a direct correlation between topoisomerase II-á amplification and doxorubicin sensitivity is lacking. Furthermore, doxorubicin sensitivity has been strongly associated with the rate of cellular replication.Citation19 Doxorubicin (0.17 μM) exposure dramatically decreased cell viability in MCF-7 cells and even more dramatically in HER-2-rich SK-BR-3 cells, which is consistent with the data by Campiglio et al.Citation19 Concurrent trastuzumab resulted in a further reduction in cell viability.
This suggests that trastuzumab and doxorubicin possess a relationship that allows each agent to produce an effect without mechanistic interferences by the other (). Doxorubicin plays a role in apoptotic cell death during DNA synthesis primarily by influencing the ability of topoisomerase II-á to adequately cut and reanneal DNA.Citation19,Citation28 It is known to induce activation of executioner caspases 3 and 7.Citation29 These were detected in this study between 12 and 30 hours in both SK-BR-3 and MCF-7 cells (data not shown). Concurrent trastuzumab did not significantly alter the presence or onset of executioner-caspase activity.
Figure 1 Cell viability in MCF-7 and SK-BR-3 cells expressed as a percentage of the untreated controls. Statistically significant differences were found between trastuzumab (T; 100 μg/mL) alone versus doxorubicin (DOX; 0.17 μM) alone and the DOX-T combination. The combination decreased cell viability more than either agent used alone in both cell lines.
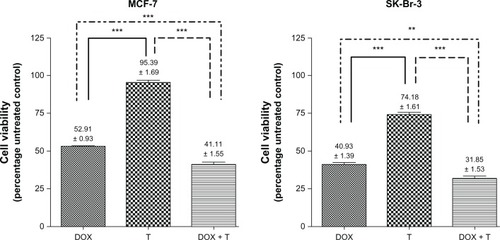
Doxorubicin-induced apoptosis was confirmed in both cell lines by detecting the translocation of phosphatidylserine using annexin V binding. Concurrent trastuzumab resulted in a similar distribution of living and apoptotic cells compared to doxorubicin alone. The presence of trastuzumab did not potentiate additional increases in cell death (). Thus, the differences in cell viability observed between doxorubicin and the combination may have been due to the antiproliferative effects of trastuzumab complementing the cytotoxic nature of doxorubicin.
Figure 2 Cell viability in MCF-7 and SK-Br-3 cells expressed as a percentage of the untreated controls. Statistically significant differences were found between trastuzumab (T; 100 μg/mL) alone versus geldanamycin (GLD; 0.35 μM) alone and the GLD-T combination.
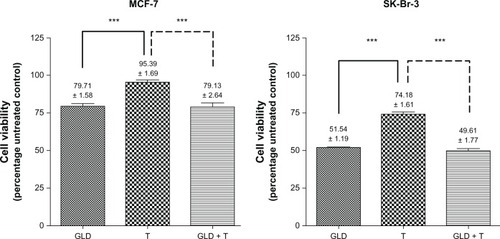
Figure 3 (A–F) Flow-cytometry histograms analyzed with deconvolution software dividing phases into G1 (left), S (center), and G2 (right). (A) Untreated MCF-7 cells in Dulbecco’s Modified Eagle’s Medium with 10% fetal bovine serum; (B) MCF-7 cells exposed to trastuzumab, resulting in G1 accumulation at 72 hours; (C) MCF-7 cells exposed to doxorubicin, resulting in late S-phase accumulation at 48 hours; (D) MCF-7 cells exposed to the doxorubicin-trastuzumab combination, resulting in G1 and late S-phase accumulation at 48 hours; (E) MCF-7 cells exposed to geldanamycin, resulting in G2 accumulation at 48 hours; (F) MCF-7 cells exposed to the geldanamycin-trastuzumab combination resulting in G2-phase accumulation at 48 hours. Similar trends in phase accumulation were observed in the SK-BR-3 graphs. However, the percentages differed.
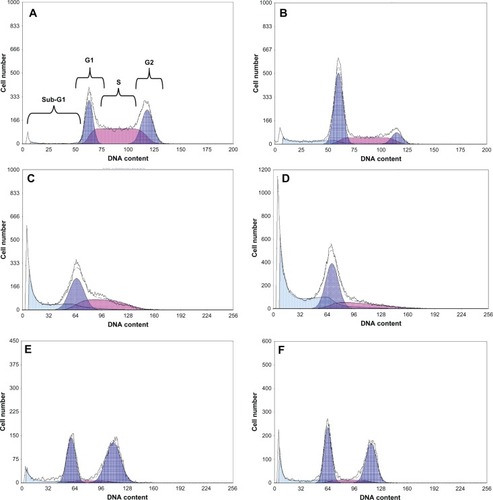
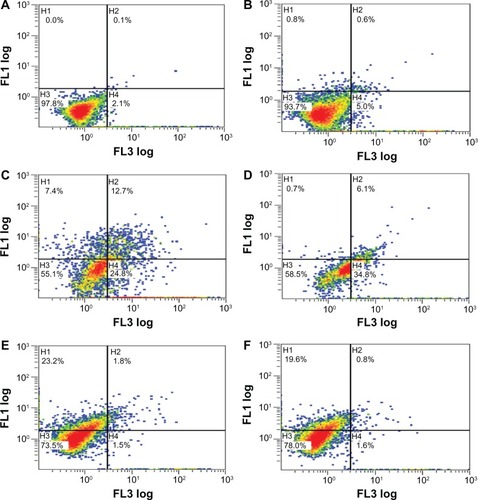
Figure 4 (A–F) Apoptosis/necrosis plots for MCF-7 cells at 72 hours. (A) Untreated control; (B) cells exposed to trastuzumab at 72 hours; (C) cells exposed to doxorubicin at 72 hours; (D) cells exposed to the doxorubicin-trastuzumab combination at 72 hours; (E) cells exposed to geldanamycin at 48 hours; (F) cells exposed to the geldanamycin-trastuzumab combination at 48 hours. Similar trends were observed in the SK-BR-3 graphs. However, higher percentages for necrosis as opposed to early apoptosis were observed.
Late S-phase accumulation occurred in MCF-7 cells exposed to doxorubicin alone. While exposure to the doxorubicin-trastuzumab combination maintained a high S-phase accumulation, it also yielded a statistically significant difference in the G1 phase and an insignificant increase in the sub-G1 phase. These alterations in cell-cycle kinetics support the concept of an antiproliferative effect induced by trastuzumab. Similar trends were observed in SK-BR-3 cells (). It is known that doxorubicin has the potential to differentially alter cell-cycle kinetics. Cell type-specific interactions result in G1/S and G2/M checkpoint arrest in some cell types and only G2/M checkpoint arrest in others.Citation30 Cells in the G2/M phase are thought to be the most susceptible to doxorubicin,Citation31 which could explain the increase in the percentage of cells in the S phase in both cell lines.
As a single agent, doxorubicin had no influence on surface HER-2 density. Furthermore, trastuzumab resulted in an equivalent decrease in receptors when used alone and in combination with doxorubicin (). pegram et al demonstrated that HER-2 expression remains unaltered following exposure to doxorubicin, and considered the possibility that cytotoxic drugs such as doxorubicin modify HER-2 functional activity as opposed to altering expression levels.Citation32 The consistent decrease in HER-2 by trastuzumab in combination with doxorubicin may have ultimately aided in significantly reducing cell viability. Therefore, the induction of apoptosis by doxorubicin coupled with the cytostatic effects of trastuzumab appeared to provide an effective and complementary treatment modality in vitro.
Figure 5 Relative human epidermal growth factor receptor 2 density at 24 hours in SK-BR-3 cells expressed as a percentage of the fluorescence of untreated controls (standardized to 100%).
Abbreviations: DOX, doxorubicin (0.17 μM); T, trastuzumab (100 μg/mL).
Concentration-dependent growth inhibition has also been noted in vivo for doxorubicin-trastuzumab combinations.Citation33 Enhancing antitumor activity of targeted therapies remains important, and therefore, despite the limitations of cardio toxicity, these in vitro results strongly support the use of doxorubicin in trastuzumab-based therapies provided that HER-2 density is maintained throughout disease progression.
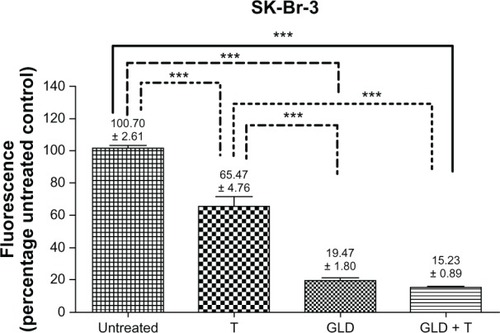
Secondly, the authors set out to determine the influence of geldanamycin on HER-2, and its ability to maintain sufficient surface-expression levels to promote an effective combination with trastuzumab. In these experiments, geldanamycin exposure reduced cell viability in MCF-7 cells. Concurrent trastuzumab had negligible effects on these HER-2 normal cells. While cell viability was dramatically lower, the trend for SK-BR-3 cell viability was similar ().
Geldanamycin decreases prosurvival factors, which include Akt1, cyclic adenosine monophosphate-dependent protein kinases, and Raf-1, in tumor cell lines.Citation25 Raf-1, an integral part of a protein kinase cascade of the mitogen-activated protein kinase signaling pathways, is strongly implicated in reductions in cell proliferation, with cell type-specific recovery from inhibition or depletion being evident.Citation25,Citation34 Alternatively, in SK-BR-3 cells, the remarkable decrease in cell viability could have been due to alterations in HER-2 receptors, which are known chaperone clients.
Geldanamycin and a variety of modified analogs, such as herbimycin A and tanespimycin, are suspected inhibitors of normal maturation of tyrosine kinase receptors that include HER-1, insulin-like growth factor receptor, platelet-derived growth factor receptor, and HER-2.Citation35 Therefore, the extent of HER-2 depletion on the cell surface was assessed.
Trastuzumab-exposed SK-BR-3 cells illustrated a statistically significant decrease in HER-2 compared to untreated controls. An even greater decrease in HER-2 was observed for geldanamycin alone and the geldanamycin-trastuzumab combination from as early as 12 hours. This trend was sustained at 24 and 48 hours (). These results confirmed that HER-2 is a prominent client protein for HSp90,Citation24 and that geldanamycin disrupts the ability of HER-2 to accumulate on the cell surface.
Figure 6 Relative human epidermal growth factor receptor 2 density at 24 hours in SK-BR-3 cells expressed as a percentage of the fluorescence of untreated controls (standardized to 100%).
Abbreviations: GLD, geldanamycin (0.35 μM); T, trastuzumab (100 μg/mL).
Despite the potential complexities, the kinetics of differential molecular responses of a particular cell subtype can be cytostatic or apoptotic.Citation34 No executioner caspase 3 or 7 activation was observed in geldanamycin-exposed cells, although apoptosis has previously been observed using the terminal deoxynucleotidyl transferase dUTP nick-end labeling assay, poly(adenosine diphosphate ribose) polymerase cleavage, and proteolytic activation of caspase 3.Citation36 Others have observed time-dependent induction of caspase 3 after 48 hours of geldanamycin exposure.Citation37 The inability of our assay to accurately quantify executioner-caspase activity may have been an artifact of timing, as our assessments were only carried out until 30 hours post-exposure.
Another possibility was that geldanamycin caused alterations in cell-cycle kinetics prior to executioner caspases being present.Citation38 This theory is supported by observations of downregulation of cyclin D1 and cyclin-dependent kinase (CDK)-4 with an accompanying upregulation in CDK inhibitors (p27), prior to detection of apoptosis using annexin V.Citation39 Later hallmarks of apoptosis using annexin V showed increasing apoptosis between 48 and 72 hours in both cell lines. Significant differences were apparent between untreated controls or trastuzumab alone (no effect) versus geldanamycin and the geldanamycin-trastuzumab combination ().
Wee1 kinase, a nuclear tyrosine kinase and an integral player in controlling the duration of the sequential G2 cell-cycle phase via CDK2 phosphorylation, is a potential target of HSp90 inhibitors.Citation40 Furthermore, antagonizing HSP90 function may result in G1-phase accumulation due to altered CDK4 sequestration and posttranslational destabilization.Citation41 Statistically significant G2-phase accumulation was apparent in geldanamycin and geldanamycin-trastuzumab exposed MCF-7 cells from 24 hours. While G2 accumulation was the most prominent, increases in the G1 phase were also evident. Similarly in SK-BR-3 cells, geldanamycin promoted comparable perturbations in cell-cycle kinetics. The exception occurred at 72 hours, when differences between geldanamycin alone and the geldanamycin-trastuzumab combination were evident, suggesting a potential influence of trastuzumab on cell-cycle kinetics ().
However, the alteration was not translated into differential responses in apoptosis or cell viability, which suggests that the difference was perhaps merely an experimental anomaly due to the deconvolution, or a transient, nontranslatable effect of the combination. Others have shown that geldanamycin-induced decreases in cell viability occur secondary to cell-cycle arrest at both the G1/S and G2/M boundaries,Citation36 which may be unob-servable at 72 hours.Citation34 Nonetheless, even with two potential points of cell-cycle alterations induced by geldanamycin, there was no potentiation of effects in the presence of trastuzumab. Due to the decrease in HER-2 density, geldanamycin probably functions independently of trastuzumab.
Geldanamycin remains an attractive chemotherapeutic agent, considering its ability to antagonize HSP90 functions crucial for the maintenance of normal housekeeping duties. Differential cell-type responses may be due to different preferential proliferative signaling pathways and the necessity of HSP90 for regulating these functions. The ability of geldanamycin to rapidly and dramatically reduce relative surface HER-2 density indicates that combinatorial regimens with trastuzumab would not have any clinical benefit. However, the ability to regain HSP90 function and for receptor density to be restored upon removal of geldanamycin was not assessed.
Concluding remarks
The in vitro data support the use of doxorubicin in trastuzumab-based therapies. Despite the enhanced efficacy, the incidence of cardiotoxicity in anthracycline-trastuzumab combinations still casts a shadow over this apparently effective treatment modality. Therefore, if mechanisms for cardioprotection can be identified, this combination could retain its role as an integral component of HER-2-positive breast cancer treatment. Geldanamycin-induced removal of HER-2 as a target for trastuzumab could theoretically result in negligible effects of this targeted therapy in tumor reduction. However, if geldanamycin can be shown to produce only transient alterations in receptor density, and if HER-2 density is regained or maintained in cell progeny, there may still be a place for HSP90 inhibitors as potent cytotoxic agents used subsequently to trastuzumab in treatment regimens.
Acknowledgments
The authors would like to acknowledge Roche pharmaceuticals for the kind donation of trastuzumab and the Cancer Association of South Africa (CANSA) as well as the Research and Development program (RDP), University of pretoria, for providing their generous funding, and to Dr AD Cromarty and Dr JJ van Tonder without which this project would not have been possible.
Disclosure
The authors have no financial disclosures and declare no conflict of interest with regard to this research.
References
- BursteinHJThe distinctive nature of HER2-positive breast cancersN Engl J Med20053531652165416236735
- KlapperLGlatheSVaismanNThe ErbB-2/HER2 oncoprotein of human carcinomas may function solely as a shared coreceptor for multiple stroma-derived growth factorsProc Natl Acad Sci U S A1999964995500010220407
- CitriASkariaKBYardenYThe deaf and the dumb: the biology of ErbB-2 and ErbB-3Exp Cell Res2003284546512648465
- RossJSSlodkowskaEASymmansWFPusztaiLRavdinPMHortobagyiGNThe HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicineOncologist20091432036819346299
- TzaharEWatermanHChenXA hierarchical network of interreceptor interactions determines signal transduction by Neu differentiation factor/neuregulin and epidermal growth factorMol Cell Biol199616527653878816440
- YardenYSliwkowskiMXUntangling the ErbB signalling networkNature Rev Mol Cell Biol2001212713711252954
- LeahyBDJStructure and function of the epidermal growth factor (EGF/ErbB) family of receptorsAdv Protein Chem20046812715500857
- HudisCATrastuzumab – mechanism of action and use in clinical practiceN Engl J Med2007357395117611206
- PerezEASumanVJDavidsonNECardiac safety analysis of doxorubicin and cyclophosphamide followed by paclitaxel with or without trastuzumab in the North Central Cancer Treatment Group N9831 adjuvant breast cancer trialJ Clin Oncol2008261231123818250349
- KruserTJWheelerDLMechanisms of resistance to HER family targeting antibodiesExp Cell Res20103161083110020064507
- MountziosGSanoudouDSyrigosKNClinical pharmacogenetics in oncology: the paradigm of molecular targeted therapiesCurr Pharm Des2010162184219320459386
- TaiWMahatoRChengKThe role of HER2 in cancer therapy and targeted drug deliveryJ Control Release201014626427520385184
- LowerEEGlassEBlauRHarmanSHER-2/neu expression in primary and metastatic breast cancerBreast Cancer Res Treat200911330130618273700
- NahtaRYuDHungMCHortobagyiGNEstevaFJMechanisms of disease: understanding resistance to HER2-targeted therapy in human breast cancerNat Clin Pract Oncol2006326928016683005
- FriedländerEBarokMSzöllosiJVerebGErbB-directed immunotherapy: antibodies in current practice and promising new agentsImmunol Lett200811612614018201769
- CallahanRHurvitzSHuman epidermal growth factor receptor-2-positive breast cancer: current management of early, advanced, and recurrent diseaseCurr Opin Obstet Gynecol201123374321500375
- WeigeltBGeyerFCReis-FilhoJSHistological types of breast cancer: how special are they?Mol Oncol2010419220820452298
- GewirtzDAA critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicinBiochem Pharmacol19995772774110075079
- CampiglioMSomenziGOlgiatiCRole of proliferation in HER2 status predicted response to doxorubicinInt J Cancer200310556857312712452
- DangCFornierMSugarmanSThe safety of dose-dense doxorubicin and cyclophosphamide followed by paclitaxel with trastuzumab in HER-2/neu overexpressed/amplified breast cancerJ Clin Oncol2008261216122218323546
- FerencPSolárPMikešJKoval’JFedoročkoPBreast cancer and current therapeutic approaches: from radiation to photodynamic therapyGunduzMGunduzEBreast Cancer: Current and Alternative Therapeutic ModalitiesRijeka, CroatiaInTech20116387
- DeboerCMeulmanPAWnukRJPetersonDHGeldanamycin, a new antibioticJ Antibiot1970234424475459626
- ToftDORecent advances in the study of hsp90 structure and mechanism of actionTrends Endocrinol Metab1998923824318406275
- NeckersLSchulteTWMimnaughEGeldanamycin as a potential anti-cancer agent: its molecular target and biochemical activityInvest New Drugs19991736137310759403
- BishtKSBradburyCMMattsonDGeldanamycin and 17-allylamino-17-demethoxygeldanamycin potentiate the in vitro and in vivo radiation response of cervical tumor cells via the heat shock protein 90-mediated intracellular signaling and cytotoxicityCancer Res2003638984899514695217
- MussHBThorADBerryDAc-erbB-2 expression and response to adjuvant therapy in women with node-positive early breast cancerN Engl J Med1994330126012667908410
- PetitTBorelCGhnassiaJNew therapeutic targets in the intrinsic apoptotic pathway in neuroblastomaClin Cancer Res200171577158111410493
- WangSKonorevEAKotamrajuSJosephJKalivendiSKalyanaramanBDoxorubicin induces apoptosis in normal and tumor cells via distinctly different mechanismsJ Biol Chem2004279255352554315054096
- GamenSAnelaAPérez-GalánPDoxorubicin treatment activates a Z-VAD-sensitive caspase, which causes Δψm loss, caspase-9 activity, and apoptosis in Jurkat cellsExp Cell Res200025822323510912804
- Bar-OnOShapiraMHershkoDDDifferential effects of doxorubicin treatment on cell cycle arrest and Skp2 expression in breast cancer cellsAnticancer Drugs2007181113112117893511
- PotterAJGollahonKAPalancaBJAFlow cytometric analysis of the cell cycle phase specificity of DNA damage induced by radiation, hydrogen peroxide and doxorubicinCarcinogenesis20022338940111895853
- PegramMHsuSLewisGInhibitory effects of combinations of HER-2/neu antibody and chemotherapeutic agents used for treatment of human breast cancersOncogene1999182241225110327070
- BaselgaJNortonLAlbanellJKimYMendelsohnJRecombinant humanized anti-HER2 antibody (Herceptin) enhances the antitumor activity of paclitaxel and doxorubicin against HER2/neu overexpressing human breast cancer xenograftsCancer Res199858282528319661897
- HosteinIRobertsonDDistefanoFWorkmanPClarkePAInhibition of signal transduction by the Hsp90 inhibitor 17-allylamino-17-demethoxygeldanamycin results in cytostasis and apoptosisCancer Res2001614003400911358818
- HelmbrechtKZeiseERensingLChaperones in cell cycle regulation and mitogenic signal transduction: a reviewCell Prolif20003334136511101008
- ShimamuraTLowellAMEngelmanJAShapiroGIEpidermal growth factor receptors harboring kinase domain mutations associate with the heat shock protein 90 chaperone and are destabilized following exposure to geldanamycinsCancer Res2005656401640816024644
- KimSKangJHuWEversBMChungDHGeldanamycin decreases Raf-1 and Akt levels and induces apoptosis in neuroblastomasInt J Cancer200310335235912471618
- ParkJYehMWWongMGThe heat shock protein 90-binding geldanamycin inhibits cancer cell proliferation, down-regulates oncoproteins, and inhibits epidermal growth factor-induced invasion in thyroid cancer cell linesJ Endocrinol Metab20038833463353
- GeorgakisGVLiYYounesAThe heat shock protein 90 inhibitor 17-AAG induces cell cycle arrest and apoptosis in mantle cell lymphoma cell lines by depleting cyclin D1, Akt, Bid and activating caspase 9Br J Haematol2006135687116925576
- AligueRAkhavan-NiakHRussellPA role for Hsp90 in cell cycle control: Wee1 tyrosine kinase activity requires interaction with Hsp90EMBO J1994136099601067813446
- StepanovaLLengXParkerSBHarperJ WMammalian p50Cdc37 is a protein kinase-targeting subunit of Hsp90 that binds and stabilizes Cdk4Genes Dev199610149115028666233