Abstract
Introduction
Based on the variable benefit of taxanes in the adjuvant setting of early breast cancer in certain tumor phenotypes, especially in human epidermal growth factor receptor (HER)2-positive and triple-negative disease, and with the observation of a lesser benefit in luminal A, this research article aimed at exploring the value of docetaxel in patients with an estrogen receptor-positive, HER2-negative disease phenotype, who might not derive the same benefits as those with other phenotypes.
Patients and methods
This was a randomized prospective study comparing disease-free survival (DFS) and safety profile of sequential adjuvant three cycles Fluorouracil, Epirubicin, Cyclophosphamide followed by three cycles Docetaxel (FEC-D) versus six cycles classic Fluorouracil, Epirubicin, Cyclophosphamide (FEC)-100 in 60 Egyptian women who presented to Dar Al Fouad Hospital during the period June 2007 to July 2008 with (pT1-2 pN0-3 M0). The primary end point was DFS in a follow-up period of 4 years. The secondary end point was toxicity profile.
Results
Four-year DFS rates were comparable in both arms: 73.3% ± 8.1% in the FEC-D arm versus 76.5% ± 7.8% in the FEC-100 arm (P = 0.83). N3 and grade III subgroups achieved the worst DFS in both subgroups (P = 0.001 and P = 0.214, respectively). The rate of nausea and vomiting was higher in the FEC-100 arm (P = 0.49), while grade III–IV neutropenia and febrile neutropenia incidence was similar between both arms.
Conclusion
Sequential adjuvant chemotherapy with FEC followed by docetaxel achieved comparable DFS results to FEC alone in luminal A phenotype subgroups of breast cancer.
Keywords:
Introduction
Over the last 30 years, adjuvant chemotherapy has improved survival for women with early breast cancer. Since the 1990s, anthracycline-based chemotherapy has proved to be superior to classic cyclophosphamide, methotrexate, and fluorouracil (CMF).Citation1,Citation2 Incorporation of a taxane (paclitaxel or docetaxel) offered further improvement to patient outcomes in the adjuvant setting.Citation3,Citation4
Clinical trials incorporating taxanes in the adjuvant setting of breast cancer have not shown the same clinical outcome.Citation3,Citation4 In some studies, evaluation of taxane benefit was difficult to make because of different population sizes, inclusion of all tumor phenotypes, and biologically heterogeneous populations, and since different taxane schedules were compared with different anthracycline control regimens of often unequal duration.Citation5 In another trial, many patient subgroups gained less benefit from additional taxanes, especially in regard to overall survival (OS).Citation6 Another significant role for adjuvant taxanes sequential to anthracyclines in the adjuvant setting is reducing long-term adverse events (such as induction of leukemia and cardiotoxicity) to a minimum by reducing exposure to cumulative doses of anthracyclines.
The value of adjuvant taxane to breast cancer outcomes in luminal A patients is affected by events in the early years and further follow-up is required before a possible longer-term benefit might be witnessed or excluded in this patient population, since the risk of relapse continues for at least 15 years after diagnosis for these patients, which is partly attributed to consequences of effective endocrine therapy for patients with estrogen receptor (ER)-positive disease.
ER as a biological marker seems to have little predictive value in determining taxane responsiveness.Citation7–Citation9 The CALGB 9344 trial showed that the benefits of paclitaxel occurred mainly in patients with ER-negative or human epidermal growth factor receptor (HER)2-positive tumors, with less gain in the bigger subgroup, (ER positive or Her2 negative groups) of patients, especially those with the ER-positive/HER2-negative phenotype.Citation10 In a similar trial, docetaxel benefit was most evident in patients whose tumors were ER-negative and HER2-positive, with other subgroups deriving less or no apparent benefit.Citation10 Therefore, it is postulated that luminal A patients might not necessarily gain a clinically worthwhile benefit from taxanes. Finally, in view of the molecular diversity of breast cancer, adjuvant taxanes have provided different benefits for special patient subgroups, especially those who are HER2-positive and triple negative.Citation10 Therefore, there is a need to identify patients who would not necessarily obtain benefit from this treatment.
This study was conducted with the aim of evaluating the value of adjuvant sequential taxanes, compared to six cycles of classic anthracyclines, in patients with the luminal A phenotype in a small-sized Egyptian population.
Patients and methods
This was a randomized prospective study comparing disease-free survival (DFS) and toxicity of adjuvant taxanes in 60 females (aged >18 years) who presented at Dar Al Fouad Hospital, Egypt, during the period from June 2007 to July 2008 with stage (pT 1-2 pN 0-3 M0). Inclusion criteria were normal hematological, hepatic, and renal function. Patients were excluded if they had a history of cardiac disease, locally advanced or metastatic disease, bilateral breast cancer, pregnancy, or a previous history of cancer.
Patients were randomly allocated with closed envelope into the treatment groups at a ratio of 1:1 using a computer system to three cycles of Fluorouracil, Epirubicin, Cyclophosphamide (FEC)-100 followed by three cycles docetaxel (Arm I) or six cycles of FEC-100 (Arm II). Thirty patients were assigned to each arm.
Treatments
Patients in Arm I received three cycles of FEC, comprising cyclophosphamide 500 mg/m2, epirubicin 100 mg/m2, and 5-fluorouracil 500 mg/m2 and three cycles of Taxotere® (Sanofi-Aventis, Bridgewater, NJ, USA), comprising docetaxel 100 mg/m2.
In Arm II, patients received six cycles of FEC, comprising cyclophosphamide 500 mg/m2, epirubicin 100 mg/m2, and 5-fluorouracil 500 mg/m2.
Adjuvant treatment after chemotherapy completion
Adjuvant tamoxifen 20 mg/day to pre- or postmenopausal women or aromatase inhibitor to postmenopausal women was started after chemotherapy for patients not subjected to radiotherapy otherwise following radiotherapy and continued for 5 years.
Radiotherapy was initiated within 4 weeks after the last cycle of chemotherapy and was mandatory for all patients who had undergone breast-conserving surgery. Radiation to the chest wall, supraclavicular area, and internal mammary chain was recommended following mastectomy to indicated patients. Irradiation of the axilla was prohibited. Radiotherapy procedures were similar for both arms.
Evaluations
The tolerability of chemotherapy was evaluated before each cycle. In addition, an absolute blood count was performed on day 21, and nonhematologic toxicity was evaluated during the period between cycles. Toxicity was graded according to National Cancer Institute Common Toxicity Criteria for Adverse Events version 3.0.Citation11 The resting left ventricle ejection fraction (LVEF) was measured by echocardiographic methods at baseline. A physical examination was performed every 4 months for the first 2 years, then every 6 months for the following 2 years. Imaging studies (ie, mammography, chest X-ray, liver ultrasound, and bone scan) were performed 1 year after the initial surgery, then yearly thereafter for 5 years. Beyond this period, a mammography was performed annually.
The primary end point of the study was 4 years of DFS, which was defined as the time from randomization until first relapse (local, regional, or distant), contralateral breast cancer, or death from any cause. The secondary end point was toxicity profile.
Statistical analysis
Comparison of toxicity was done using Fisher’s exact test and survival was estimated using Kaplan–Meier and log rank for comparing curves. P-values were always two-tailed and significance was at the 0.05 level.
Results
Between June 2007 and July 2008, 60 women were enrolled at Dar Al Fouad Hospital (30 in the FEC-D arm and 30 in the FEC arm). Baseline patient information and disease characteristics are shown in .
Table 1 Baseline patient and disease characteristics of randomly assigned patients
Efficacy results
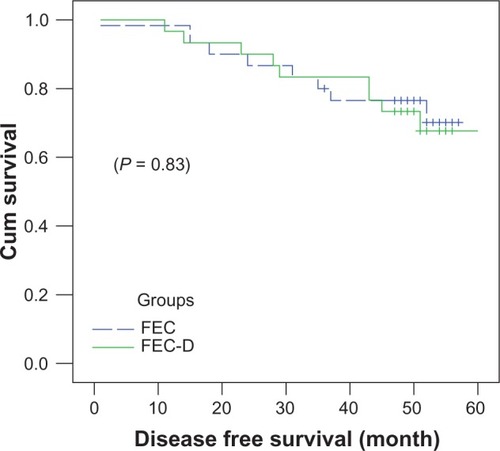
Four-year DFS rates () were similar in both arms, at 73.3% ± 8.1% in the FEC-D arm versus 76.5% ± 7.8% in the FEC-100 arm (P = 0.83).
Figure 1 Four-year disease-free survival rates in patients receiving FEC-D or FEC-100.
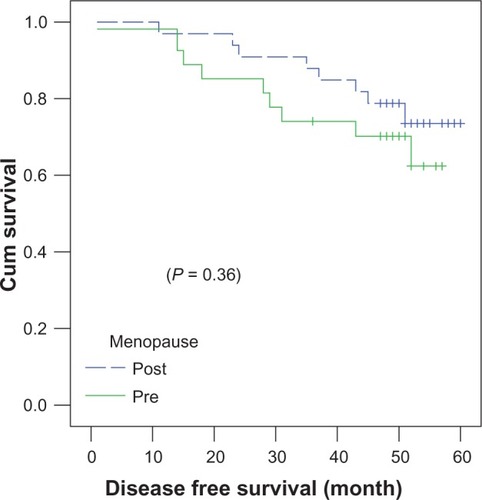
In both treatment arms, postmenopausal women () achieved slightly better 4-year DFS (78.8% ± 7.1%) compared to premenopausal women (70% ± 8.8%) (P = 0.364). The 4-year DFS was almost identical in T1 patients (71.4% ± 12%) and T2 patients (75.8% ± 6.3%) (P = 0.89).
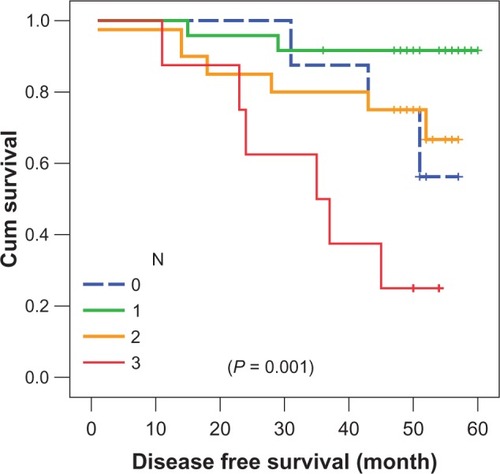
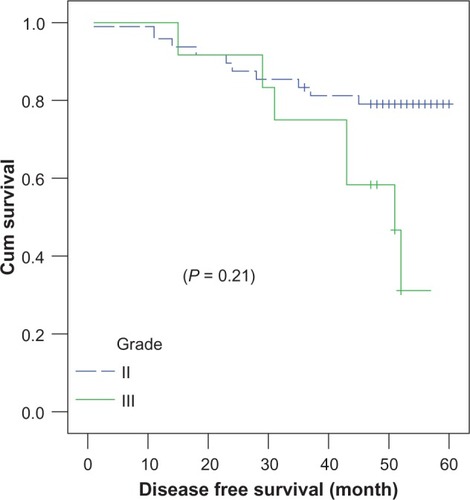
N3 patients () achieved the worst 4-year DFS, at 25% ± 1.5%, compared to those at N0–N2 (P = 0.001) in both treatment arms. Grade III patients () achieved inferior survival rates, at 58.3% ± 14.2%, compared to grade II patients (79% ± 5.8%) (P = 0.214).
Four-year DFS was almost identical in the group receiving antihormonal treatment as tamoxifen (77% ± 7.1%) as in the aromatase inhibition group (72% ± 8.9%) (P = 0.7).
Acute and delayed toxic effects
Grade III–IV drug-related toxicity according to National Cancer Institute Common Toxicity Criteria for Adverse Events version 3.0 was as follows: neutropenia grade III–IV was slightly higher, not reaching statistical significance in the FEC arm (5/30) versus the FEC-D arm (4/30) (P = 0.72). These patients required secondary prophylaxis by Granulocyte colony-stimulating factor (G-CSF) with subsequent cycles, as primary prophylaxis was not permitted from the beginning of treatment. Febrile neutropenia requiring hospital admission was noted in three of 30 patients in Arm I and three of 30 patients in Arm II (P = 1). Furthermore, the incidence of grade III–IV nausea and vomiting were higher in the FEC arm (6/30) versus the FEC-D arm (4/30) (P = 0.49). Hematological malignancy was not monitored in either arm, and cardiac events were not observed during follow-up; however, a decrease of >20% of baseline value in LVEF at the end of chemotherapy occurred in two of 30 patients in Arm II versus 0 patients in Arm I (P = 0.49). During follow-up after the end of treatment, an echocardiography was not performed.
Discussion
The benefits of adjuvant taxane need to be further explored in breast cancer phenotypes, especially in the luminal A subtype. The Cancer and Leukemia Group B (CALBG) 9344 trial demonstrated benefits from paclitaxel, especially in ER-negative or HER2-positive tumors, with less benefit in luminal A.Citation10 Other studies demonstrated improved outcomes from adjuvant sequential paclitaxel in all tumor phenotypes.Citation12,Citation13 The Taxotere as Adjuvant Chemotherapy Trial (TACT) included 4,162 patients and revealed that a docetaxel-based regimen was not superior to anthracycline chemotherapy of equivalent duration in terms of DFS in all tumor phenotypes.Citation5 Another trial demonstrated benefits of adjuvant docetaxel in the whole breast cancer population.Citation14 Of interest is the Program Action Concertée Sein-01 (PACS-01) trial,Citation6 which included 1,999 node-positive breast cancer patients and compared six cycles of FEC-100 with three cycles of FEC-100 followed by three cycles of docetaxel 100 mg/m2. The result of the PACS-01 trial showed a small but significant improvement in DFS and OS. In a preplanned subgroup analysis, no benefit was seen in patients aged less than 50 years.Citation6 Bria et al performed a pooled analysis of 15,500 patients treated in a randomized trial designed to determine if the addition of paclitaxel or docetaxel to conventional anthracycline treatment improved survival.Citation15 The authors concluded that taxane-based sequential treatment added a significant benefit to both DFS and OS. However, the magnitude of the benefit was not large, with an approximately 2%–4% absolute benefit, meaning that 35 to 45 patients need to be treated for one to benefit.Citation15 Hence, our study was designed to explore the role of the taxane docetaxel in luminal A disease (pT1-2 pN0-3 M0).
Ultimately, the present study did not show a 4-year DFS benefit from adjuvant docetaxel, coinciding with conclusions from the TACT studyCitation5 and CALBG-9344Citation10 in a luminal A subgroup of patients. The 4-year DFS between both groups in our study were similar, ranging from 73.3% ± 8.1% for Arm I to 76.5% ± 7.8% for Arm II (P = 0.83). This DFS rate is similar to that reported in the PACS-01 trial,Citation6 which demonstrated a 5-year DFS ranging from 73.2% with FEC and 78.4% with FEC-D. The PACS-01 study reported a benefit from taxanes that reached statistical significance compared to classic FEC-100 in the 5-year DFS; however, all tumor phenotypes were included. On the other hand, the TACT trial,Citation5 including all tumor phenotypes, reported 5-year DFS ranging from 74.3% to 75.6%, with no observed benefit in 5-year DFS from adjuvant taxane (P = 0.62), coinciding with the results of the present study.
In terms of toxicity profile, grade III–IV nausea and vomiting were numerically higher in the FEC-100 arm, not reaching statistical significance (P = 0.49), which coincides with the toxicity incidence data reported in the PACS-01 trial, wherein a higher rate of grade III–IV nausea and vomiting in the continuous FEC arm versus the sequential docetaxel arm was found (P = 0.001), being more statistically significant due to their larger sample size.Citation6 To the contrary, Gastrointestinal tract (GIT) toxicity, like nausea and vomiting, toxicity in the TACT trial was higher in the FEC-D sequential arm (P = 0.001).Citation5 Grade III–IV hematological toxicity was higher in our study in the FEC-100 arm, but did not reach statistical significance (P = 0.72), coinciding with the data from TACT not reaching statistical significance,Citation5 though grade III–IV hematological toxicity was statistically higher (P = 0.008) in the FEC-100 arm in the PACS trial,Citation6 possibly due to their larger sample size.
Conclusion
This study did not support the idea that “one size fits all” in terms of DFS benefit for adjuvant docetaxel in breast cancer. Our study, focusing on the luminal A subtype, demonstrated similar DFS between FEC-100 and FEC-D treatment, where the value of taxane became less than that witnessed in other adjuvant trials recruiting all patient phenotypes. The toxicity profile was comparable between both treatment arms but numerically favored the FEC-D arm.
Disclosure
The authors report no conflicts of interest in this work.
References
- [No authors listed]Polychemotherapy for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative GroupLancet19983529309429752815
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG)Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trialsLancet20053651687171715894097
- FergusonTWilckenNVaggRGhersiDNowakAKTaxanes for adjuvant treatment of early breast cancerCochrane Database Syst Rev20074CD00442117943815
- De LaurentiisMCancelloGD’AgostinoDTaxane-based combinations as adjuvant chemotherapy of early breast cancer: a meta-analysis of randomized trialsJ Clin Oncol200826445318165639
- HopwoodPRidolfiARussellSImpact on quality of life (QoL) of FEC-T compared with FEC or CMF: UK Taxotere as Adjuvant Chemotherapy Trial (TACT) 2-year follow-upJ Clin Oncol200826Suppl: abstr 548
- RochéHFumoleauPSpielmannMSequential adjuvant epirubicin-based and docetaxel chemotherapy for node-positive breast cancer patients: the FNCLCC PACS 01 TrialJ Clin Oncol2006245664567117116941
- MartinMMackeyJVogelCBenefit from adjuvant taxanes and endocrine responsiveness in breast cancerBreast200716Suppl 2S127S13117923408
- AndreFBroglioKRocheHEstrogen receptor expression and efficacy of docetaxel containing adjuvant chemotherapy in patients with node positive breast cancer: results from a pooled analysisJ Clin Oncol2008262636264318509176
- HughJHansonJCheangMCBreast cancer subtypes and response to docetaxel in node-positive breast cancer use of an immunohistochemical definition in the BCIRG 001 TrialJ Clin Oncol2009271168117619204205
- HayesDFThorADDresslerLGCancer and Leukemia Group B (CALGB) InvestigatorsHER2 and response to paclitaxel in node-positive breast cancerN Engl J Med20073571496150617928597
- Cancer Therapy Evaluation ProgramCommon terminology criteria for adverse events 3.0 (CTCAE)2006 Available from: http://www.eortc.be/services/doc/ctc/ctcaev3.pdfAccessed February 28, 2013
- HendersonICBerryDADemetriGDImproved outcomes from adding sequential paclitaxel but not from escalating doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancerJ Clin Oncol20032197698312637460
- MamounasEPBryantJLemberskyBPaclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node-positive breast cancer: results from NSABP B-28J Clin Oncol2005233686369615897552
- MartinMPienkowskiTMackeyJBreast Cancer International Research Group 001 InvestigatorsAdjuvant docetaxel for node-positive breast cancerN Engl J Med20053522302231315930421
- BriaENisticoCCupponeFBenefits of taxane as adjuvant chemotherapy for early breast cancer: pooled analysis of 15,500 patientsCancer2006106112337234416649217