Abstract
An epithelioid trophoblastic tumor (ETT) is a rare trophoblastic tumor originating from chorionic-type intermediate trophoblasts. Vaginal involvement in ETT in the form of isolated lesions has not been previously reported. A 43-year-old woman presented with vaginal wall cysts and was diagnosed with ETT by pathological examination after cystectomy. No clinical evidence of uterine involvement was found at diagnosis or during follow-up. The patient was treated with chemotherapy and surgery after the first recurrence and underwent follow-up for 8 months. The serum human chorionic gonadotropin titer remained at undetectable levels.
Keywords:
Introduction
Epithelioid trophoblastic tumor (ETT) was first described by Shih and Kurman in 1998.Citation1 It is an uncommon gestational trophoblastic tumor originating from the chorionic-type intermediate trophoblast. The disease is often misdiagnosed as choriocarcinoma, placental site trophoblastic tumor (PSTT) or cervical squamous cell carcinoma. According to published reports, fewer than 100 cases of ETT have been diagnosed to date.Citation2 ETT can present as isolated uterine/cervical disease, as isolated extrauterine disease, or as a primary uterine tumor with metastasis. A 43-year-old female was diagnosed with ETT after vaginal wall cystectomy, and no clinical evidence of uterine involvement was found at diagnosis or during follow-up.
Clinical summary
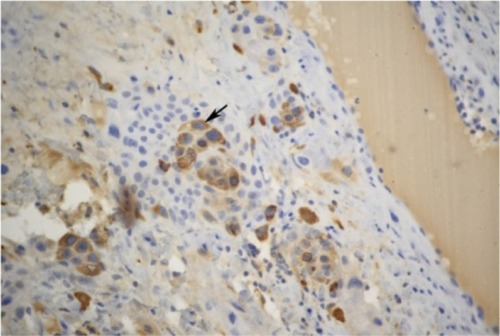
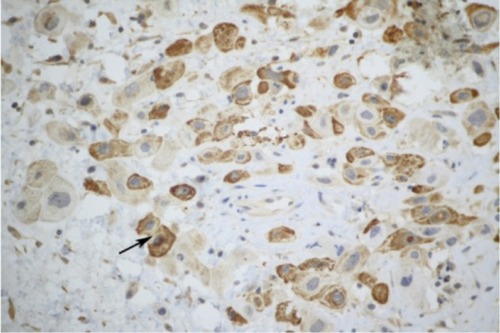
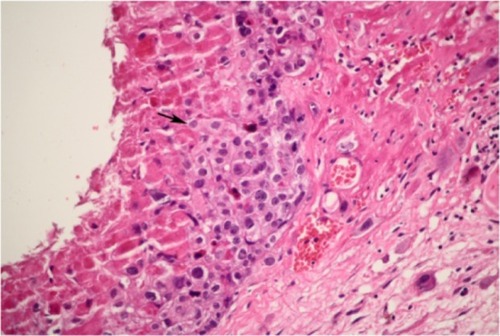
A 43-year-old female patient, gravida 2, para 0, had undergone two induced abortions, one in June 2007 and another in March 2009. In December 2011, she complained of soft tissue blocking her vagina, and cystectomy was performed in the Beijing Obstetrics and Gynecology Hospital. Vaginal wall ETT was identified with pathological consultation in our hospital after hematoxylin-eosin staining () immunohistochemical analysis as follows ( and ): tumor cells were found to be positive for anti-cytokeratin (AE1/AE3), human chorionic gonadotropin (hCG), human placental lactogen (hPL), placental alkaline phosphatase (PLAP), tumor protein 63 (P63), and antibodies to cytokeratin-8 (CAM5.2); and negative for carcinoembryonic antigen (CEA), the multifunctional protein actin, melanoma antigen melan-A, the Down syndrome marker α-inhibin, the antibody HBM45, the protein E-cadherin, and the cyclin D1 protein. The reactive antigen Ki-67 labeling index was approximately 15%. Enhanced magnetic resonance imaging (MRI) of the pelvic area and chest computed tomography (CT) showed no further lesions. The patient declined a hysterectomy and underwent follow-up for 5 months until serum β-hCG gradually rose to 15.5 mIU/mL in July 2012. A gynecological examination showed recurrence at the primary location with a 0.5 cm, light blue nodule in the lower part of the vaginal anterior wall. A pelvic MRI and chest CT showed no evidence of lesions. The patient then received three courses of chemotherapy with a 3-week regimen of vincristine (VCR; 2 mg intravenously, day 1), floxuridine (FUDR; 800 mg/m2/day, intravenously, days 1–5), dactinomycin (Act-D, 200 kg/m2/day, intravenously, days 1–5), and etoposide (VP-16, 100 mg/m2/day, intravenously, days 1–5). Vaginal lesion resection and curettage were performed during the third course after serum β-hCG levels had decreased to within the normal range. After the operation, one course of chemotherapy was carried out. Outpatient follow-up is ongoing. The patient’s serum hCG titer has remained at undetectable levels.
Figure 1 Tumor cells containing large amounts of eosinophilic cytoplasm in the interstitium of vaginal squamous epithelium (squamous epithelium lacking lesions) presents a pattern of nodular, nested, single cell growth with central cystic degeneration. There is a large amount of eosinophilic basement membrane-like material surrounding the tumor cells.
Discussion
In 1998, Shih and KurmanCitation1 described ETT as distinct from PSTT and choriocarcinoma (CC). Most cases of ETT have been reported in women aged 15 to 48 years old, although there are a few reported cases in perimenopausal and postmenopausal women.Citation3,Citation4 The uterus is the most common primary site for ETT (40%), but it also occurs in the cervix (31%).Citation2 Extra-uterine cases of ETT are rare, and it is not known how these tumors originate. ETT has been reported as originating in the broad ligaments, small bowel, lungs, fallopian tube, and ovaries.Citation1,Citation5–Citation8 Ohira et alCitation9 reported a case presenting with vaginal metastasis as the primary symptom of a patient with ETT. But using MRI, a solid lesion 15 mm in diameter was observed in the posterior myometrium near the fundus after the excision of the vaginal tumor. It is clear that this uterine lesion was the primary lesion. Shen et alCitation10 performed a retrospective analysis of 9 patients with ETT. In two of these cases, uterine tumors had metastasized to the vagina or vaginal fornix. The patient described in the present work was 43 years old, and the lesion was located in the vaginal wall. No clinical evidence of uterine involvement or other metastatic lesion was found at diagnosis or during follow-up.
The mechanism of ETT is unknown. The most commonly reported associated factors are preterm deliveries (43%), molar pregnancies (39%), and abortions (18%), all occurring 2–300 months (mean 76 months) after the antecedent gestational event.Citation2 Most patients with ETT present with abnormal vaginal bleeding, and many of them present with metastasis, which frequently occurs in the lungs.Citation2 Only three cases of patients with ETT metastatic to the vagina have been reported.Citation9,Citation10 The patient in this report had a history of two previous embryonic diapause events but no abnormal vaginal bleeding or uterine lesions were found after two curettage procedures. Vaginal ETT was defined as having occurred three-and-a-half years after the antecedent gestation.
Histologically, ETT is composed of nests of uniform mononucleated chorionic type intermediate trophoblastic cells with eosinophilic or clear cytoplasm. These nests of trophoblastic cells are surrounded by extensive necrosis and a hyaline-like matrix, giving ETT its characteristic map-like appearance. This allows it to be distinguished histologically from PSTT. Immunohistochemical staining of ETT often shows AE1/AE3, hCG, hPL, P63, and Ki-67 indexes to be lower than those found in choriocarcinoma. Positive p63 and hCG expression can help to distinguish ETT from PSTT and cervical squamous cell carcinoma, respectively. In 1998, Shih and KurmanCitation1 reported a mean Ki-67 labeling index of 17.7% (range, 10%–25%) for ETT. Both CC and cervical SCC have much higher labeling indices. The patient was considered for ETT, squamous cell carcinoma and malignant melanoma based on HE staining in the Beijing Obstetrics and Gynecology Hospital. Negative expression of vimentin and melan-A can be used to distinguish ETT from malignant melanoma. The immunohistochemical staining of the patient in the present study was consistent with ETT.
Currently, surgical resection remains the primary treatment for ETT. Palmer et alCitation11 have summarized treatment strategies performed in 52 cases of ETT diagnosed from 1989 to 2007. Of these patients, 39% were treated with surgery alone (31%, total abdominal hysterectomy; 4%, dilation and curettage; 4%, lung resection). Hysterectomy and localized tumor resection have been used successfully in the treatment of ETT. Feng and Xiang have proposed that the best time to operate upon refractory patients with gestational trophoblastic diseases was when serum β-hCG levels decreased to normal levels, or below 10 mIU/mL.Citation12 For patients with strong fertility requirements, hysterectomy should be performed only if serum β-hCG levels do not rapidly decreased to normal. If β-hCG levels do decrease to normal, close follow-up is necessary. Excision is also the main surgical treatment for extra-uterine lesions.
Like PSTT, ETT is relatively resistant to the chemotherapeutic drugs used in the treatment of other types of gestational trophoblastic diseases. There is no standard and effective chemotherapeutic regimen because of the small number of cases. Scott et alCitation2 reported that 29% of ETT patients underwent preoperative chemotherapy and 48% had chemotherapy postoperatively, though the specific regimens varied considerably. At present, various combinations of chemotherapeutic agents are in use, including etoposide, methotrexate, and dactinomycin alternating with cyclophos-phamide and vincristine (EMA/CO) and platinum-etoposide combinations with methotrexate and actinomycin D (EMA/EP). In general, the patient should undergo one to two courses after β-hCG levels decrease to within the normal range. The patient discussed herein initially underwent vaginal lesion resection in another hospital and was followed up for 5 months until β-hCG levels increased. Local recurrence of vaginal wall lesions was observed by physical examination. Given one course of consolidated chemotherapy after vaginal lesion resection and curettage, the serum hCG titer remained undetectable during 8 months of outpatient followup. Therefore, we recommend that chemotherapy should be conducted as far as possible after surgery to reduce the recurrence of ETT.
Conclusion
Epithelioid trophoblastic tumor is a rare gestational tropho-blastic tumor. The present case presented with a single vaginal lesion. This is the first reported case of isolated vaginal ETT. Postoperative chemotherapy may be beneficial to some patients. However, because isolated vaginal ETT is so rare, more data must be accumulated before this conclusion can be verified.
Disclosure
The authors declare no conflicts of interest in this work.
References
- ShihIMKurmanRJEpithelioid trophoblastic tumor: a neoplasm distinct from choriocarcinoma and placental site trophoblastic tumor simulating carcinomaAm J Surg Pathol19982211139314039808132
- ScottEMSmithALDesoukiMMOlawaiyeABEpithelioid tropho-blastic tumor: a case report and review of the literatureCase Rep Obstet Gynecol2012201286247223243530
- NaritaFTakeuchiKHamanaSOhbayashiCAyataMMaruoTEpithelioid trophoblastic tumor (ETT) initially interpreted as cervical cancerInt J Gynecol Cancer20031355155412911738
- CoulsonLEKongCSZaloudekCEpithelioid trophoblastic tumor of the uterus in a postmenopausal woman: a case report and review of the literatureAm J Surg Pathol200024111558156211075860
- KuoKTChenMJLinMCEpithelioid trophoblastic tumor of the broad ligament: a case report and review of the literatureAm J Surg Pathol200428340540915104307
- UrabeSFujiwaraHMiyoshiHEpithelioid trophoblastic tumor of the lungJ Obstet Gynaecol Res200733339740117578376
- ParkerALeeVDalrympleCValmadreSRussellPEpithelioid trophoblastic tumour: report of a case in the fallopian tubePathology200335213614012745461
- KhunamornpongSSettakornJSukpanKSuprasertPSiriaunkgulSOvarian involvement of epithelioid trophoblastic tumor: a case reportInt J Gynecol Pathol201130216717221293282
- OhiraSYamazakiTHatanoHHaradaOTokiTKonishiIEpithelioid trophoblastic tumor metastatic to the vagina: an immunohistochemical and ultrastructural studyInt J Gynecol Pathol200019438138611109170
- ShenXXiangYGuoLAnalysis of clinicopathologic prognostic factors in 9 patients with epithelioid trophoblastic tumorInt J Gynecol Cancer20112161124113021738043
- PalmerJEMacdonaldMWellsMHancockBWTidyJAEpithelioid trophoblastic tumor: a review of the literatureJ Reprod Med200853746547518720920
- FengFXiangYSurgical management of chemotherapy-resistant gestational trophoblastic neoplasiaExpert Rev Anticancer Ther2010101718020014887