Abstract
Bevacizumab when combined with chemotherapy exerts significant activity against many solid tumors through tumor angiogenesis inhibition; however, it can induce severe side effects. We report the rare case of a 27-year-old premenopausal woman with locally advanced breast cancer that was marked by rapid tumor necrosis followed by massive hemorrhage shortly after bevacizumab and paclitaxel administration. On the basis of histopathological examination of a biopsy specimen and computed tomography findings, she was diagnosed with stage IV estrogen and progesterone receptor-negative and human epidermal growth factor receptor type 2-positive breast cancer with multiple organ metastases when she had entered gestational week 24. Cyclophosphamide, Adriamycin®, fluorouracil therapy was initiated, but multiple liver metastases continued to progress. A healthy fetus was delivered by induced delivery and trastuzumab-based treatment was initiated. Although the multiple liver metastases were controlled successfully by trastuzumab combined with paclitaxel, the primary tumor continued to expand even after subsequent administration of three other treatment regimens including anti-human epidermal growth factor receptor type 2 agents and cytotoxic drugs. To inhibit primary tumor growth, a combination therapy with paclitaxel and bevacizumab was subsequently initiated. Following therapy initiation, however, the large tumor occupying the patient’s entire left breast became necrotic and ulcerated rapidly. Furthermore, massive hemorrhage from the tumor occurred 5 weeks after bevacizumab-based therapy initiation. Although hemostasis was achieved by manual compression, the patient required blood transfusion for the massive blood loss. She eventually succumbed to respiratory failure. This case report demonstrates that primary breast cancer lesions with skin involvement have the potential to cause massive hemorrhage after bevacizumab-based treatment.
Introduction
Bevacizumab is a humanized monoclonal antibody that targets vascular endothelial growth factor and has significant activity against many solid tumors when combined with chemotherapy.Citation1–Citation3 Because bevacizumab inhibits tumor angiogenesis, which is a vital process in tumor growth and development, it induces distinguishing side effects, including hypertension, proteinuria, thromboembolism, delayed wound healing, gastrointestinal hemorrhage, bowel perforation, hemoptysis, and nasal septum perforation.Citation1,Citation4–Citation7 Furthermore, primary tumor ulceration induced by combined therapy with bevacizumab and chemotherapy has been reported in cases of locally advanced breast cancer.Citation8 However, massive hemorrhage subsequent to rapid tumor necrosis following treatment with bevacizumab combined with paclitaxel in a primary breast cancer has not been reported to the best of our knowledge. Herein we report a case of locally advanced breast cancer that showed a rapid necrosis of the tumor and serious hemorrhage after treatment with bevacizumab combined with paclitaxel.
Case report
A 27-year-old premenopausal woman with no family history of breast cancer noticed a 1-cm lump in her left breast in January 2010. Thereafter, she became pregnant, and the lump gradually increased in size to occupy her entire left breast by July. She first visited a breast clinic that referred her to the Shinshu University Hospital with suspected breast cancer. Physical examination at her initial visit revealed a hard, swollen, and erythematous left breast, with many palpable and enlarged lymph nodes in the bilateral axillae and left supraclavicular to lateral cervical region. Histopathological examination of a core needle biopsy specimen revealed invasive ductal carcinoma (scirrhous carcinoma) that was histological grade 3, estrogen receptor-negative, progesterone receptor-negative, and human epidermal growth factor receptor type 2 (HER2)-positive. A relatively high Ki-67 index (30%–40%) was also observed. Computed tomography revealed multiple metastases to the liver, mediastinal lymph nodes, and supine and pelvic bones. A final diagnosis of stage IV breast cancer was made when she had entered gestational week 24.
The patient was treated with three triweekly cycles of cyclophosphamide (500 mg/m2), Adriamycin® (Kyowa Hakko Kirin Co., Ltd., Tokyo, Japan) (60 mg/m2), and fluorouracil (500 mg/m2) therapy (). A slight decrease in size of the primary tumor in the left breast was induced by this therapy; however, the multiple liver metastases enlarged. Therefore, induced delivery was planned to enable the administration of trastuzumab-based therapy. A healthy fetus was delivered through the vagina without blood transfusion at gestational week 35 in February 2011, and weekly paclitaxel (80 mg/m2) with trastuzumab (2 mg/kg) treatment was initiated thereafter. A partial response was observed for the liver metastases; however, the primary lesion gradually increased in size and the inflammation became exacerbated despite the new treatment. In July 2011, her treatment was changed to lapatinib and capecitabine as a third-line therapy. The size of the primary tumor decreased slightly and its progression was temporarily inhibited. Although the multiple liver metastases were successfully controlled by these therapies, the primary tumor began to enlarge again in December 2011. Thereafter, treatment was changed to weekly trastuzumab (2 mg/kg on days 1, 8, and 15) and vinorelbine (25 mg/m2 on days 1 and 8) as a fourth-line therapy; however, almost no clinical response was observed. Therefore, in January 2012, a fifth-line combination therapy was initiated with docetaxel (75 mg/m2 on day 1) and carboplatin (6 area under the curve [AUC] on day 1) plus trastuzumab (2 mg/kg on days 1, 8, and 15). This treatment also had to be discontinued after one cycle because of marked anorexia. In the meantime, the primary tumor grew rapidly and extended beyond the skin in April 2012. To inhibit this growth, eribulin (1.4 mg/m2) was initiated as a sixth-line therapy; however, she was readmitted with dyspnea due to massive left pleural effusion after one cycle. By that time, the size of the primary tumor had increased to 15 × 12 × 9 cm, and its surface had become nodular and partially erosive.
Figure 1 The patient’s clinical course before administration of paclitaxel and bevacizumab.
Abbreviations: Ca15-3, cancer antigen 15-3; CAF, cyclophosphamide, adriamycin, and 5-fluorouracil therapy; CEA, carcinoembryonic antigen; E, eribulin; TCH, docetaxel, carboplatin, and trastuzumab; tras, trastuzumab; Vino, vinorelbine.
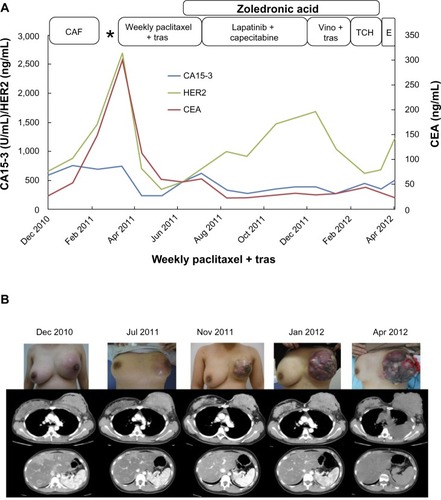
As it was considered that the primary tumor became resistant to anti-HER2-based therapy, combination therapy with paclitaxel (90 mg/m2 on days 1, 8, and 15) and bevacizumab (10 mg/m2 on days 1 and 15) was initiated as a seventh-line therapy after removal of the pleural effusion by thoracocentesis (). Three days after the first infusion of paclitaxel and bevacizumab, the volume of the primary tumor occupying her entire left breast began to decrease and its color turned to dusky red, followed by spread of skin erosion. The entire mass flattened drastically because of rapid tumor necrosis. Next, the erosion developed deep ulceration and necrosis, with loss of all skin layers over an extensive area. This led to exposure of the underlying pectoralis major muscles by the end of the first cycle of treatment. Gradually, most of the tumor became necrotic and a large area of the pectoralis major muscle was exposed, with the remaining tumor at the rim of the ulcerated lesion 4 weeks after bevacizumab initiation.
Figure 2 The patient’s clinical course after administration of paclitaxel and bevacizumab combination therapy.
Abbreviations: B, bevacizumab; p, paclitaxel.
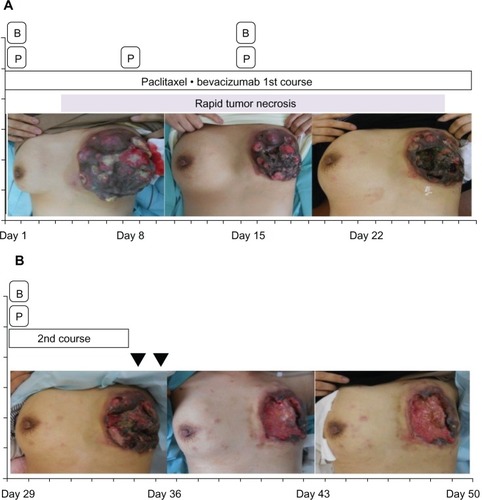
Six days after initiation of the second treatment cycle, she was readmitted to our hospital because of massive hemorrhage from the surface of the necrotic residual tumor in her left breast. Hemostasis was achieved by applying pressure with gauze and oxidized cellulose. To prevent further hemorrhage, the surface of the residual tumor was covered and compressed firmly with thick gauze; however, massive hemorrhage occurred again on the following day, necessitating several hours of manual compression. The total blood loss during these 2 days was approximately 1,800 mL; therefore, she required 560 mL of leukocyte-reduced red cell concentrate transfusion on the first day and an additional 280 mL on the following day. The bleeding from the ulcerated tumor finally ceased. The combination therapy of paclitaxel and bevacizumab was discontinued and local treatment of the ulcerated lesion was continued. Because the ulcerated lesion caused severe pain, both opioid and nonopioid analgesics were administered. The patient desired to be with her family; therefore, she was discharged after 2 weeks of hospitalization. Thereafter, she visited our hospital daily for local treatment. Because of the lack of healing in the large ulcerated lesion as well as the patient’s request for temporary cessation of anticancer therapy, only local treatment was administered. Twelve days after discharge, however, her respiratory status suddenly deteriorated and she experienced cardiopulmonary arrest in the ambulance en route to the hospital. Cardiopulmonary resuscitation was unsuccessful and the patient eventually succumbed. Although an autopsy was not performed, we considered that she died from respiratory failure caused by carcinomatous lymphangitis as determined by a chest X-ray.
Discussion
Several reports have demonstrated the adverse event of hemorrhage in patients with breast cancer treated with bevacizumab;Citation8–Citation10 however, to the best of our knowledge, none have described rapid primary tumor necrosis accompanied by massive hemorrhage that required blood transfusion in response to this drug. Therefore, this is the first report to describe massive hemorrhage caused by bevacizumab-based treatment for a primary breast tumor.
Adverse events of bleeding, including major pulmonary hemorrhage associated with squamous cell histology, cavitated or necrotic tumors, and tumors located close to major blood vessels, have been noted in cases of lung cancer. Furthermore, because tumor-associated hemorrhage may become fatal, squamous cell lung cancer or a centrally located lung tumor is contraindicated for bevacizumab-based treatment.Citation11,Citation12 With regard to breast cancer, fatal massive hemorrhage 3 days after the administration of bevacizumab and paclitaxel was reported in a patient with multiple lung metastases localized close to a blood vessel.Citation9 As evidenced in the present and referenced cases, bevacizumab should be administrated very cautiously in patients with breast cancer accompanied by centrally located lung metastases.
Cottu et al reported that six of 12 consecutive advanced breast cancer patients with extensive skin involvement who were treated with bevacizumab-based therapy showed a transformation from skin erosion to deep ulceration and necrosis, with loss of all skin layers over an extensive area and exposure of underlying fat and muscle tissues.Citation8 However, severe hemorrhage from the ulcerated tumor was not observed in their case series. Our patient experienced a similar transformation from skin erosion to deep ulceration and necrosis followed by massive hemorrhage. Although fatal hemorrhage from a primary tumor is recognized as a critical complication in patients with colon and lung cancer, possible massive hemorrhage from a primary breast cancer lesion should not be overlooked. Our case demonstrated that a primary breast cancer lesion with skin involvement also has the potential of fatal hemorrhage following administration of bevacizumab-based treatment.
In our patient, the large ulcerated lesion showed no healing tendency. Cottu et al reported a long-term delay in wound healing in several cases once necrosis occurred after bevacizumab discontinuation.Citation8 Furthermore, healing of the flap was substantially delayed in patients who underwent surgery aimed at complete resection of skin lesions after the washout of bevacizumab for at least 4 weeks, while complete healing of the flap was not achieved in two patients. An adverse event, such as that in the present case, considerably impairs the patient’s quality of life; therefore, careful assessment of the skin lesion is recommended before initiated bevacizumab-based treatment. On the other hand, Lazzati et al reported impaired wound healing and flap necrosis after mastectomy in a case of advanced breast cancer treated with neoadjuvant fluorouracil, epirubicin, and cyclophosphamide chemotherapy followed by weekly paclitaxel combined with bevacizumab.Citation13 In that case, although surgery was performed 6 weeks after the last administration of bevacizumab, skin necrosis occurred within the center of the wound 2 days after surgery. Moreover, histopathological examination revealed vascular thrombosis with dermal and subdermal infarctions in the excised skin, indicating that bevacizumab inhibits angiogenesis required for wound healing long after its discontinuation. Therefore, the indications of bevacizumab should be carefully considered before initiating the treatment of patients with locally advanced breast cancer scheduled for surgery.
In 2011, the US Food and Drug Administration excluded metastatic breast cancer as an indication of bevacizumab because it did not improve overall patient survival. However, a meta-regression analysis of five clinical trials showed that bevacizumab combined with chemotherapy for advanced breast cancer significantly improved progression-free survival when used as a first-line therapy.Citation14 With regard to the effect of bevacizumab for HER2-positive breast cancer, the results of BEVERLY- 2 trial have recently been published. In this Phase II trial, the efficacy and safety of neoadjuvant bevacizumab combined with trastuzumab and chemotherapy in patients with primary HER2-positive inflammatory breast cancer were assessed, and 63.5% of patients showed a pathological complete response with the combination therapy without serious adverse events.Citation15 As for the treatment of HER2-positive metastatic breast cancer, a combination of bevacizumab with trastuzumab and docetaxel as the first-line therapy showed a longer progression-free survival in the bevacizumab arm than that in the non-bevacizumab arm, although the benefit was not statistically significant. Thus, bevacizumab-containing treatments have demonstrated promising results in HER2-positive breast cancer.Citation16 In addition, as shown in the present case report, bevacizumab combined with chemotherapy can induce shrinkage of locally advanced breast cancer followed by drastic necrosis in a manner different from that when conventional cytotoxic agents are used. Therefore, bevacizumab exerts obvious potent and targeted activity against a subgroup of breast cancers. Nonetheless, further investigations are required to develop biomarkers that can predict tumor response to bevacizumab. Before administering bevacizumab in the treatment of locally advanced breast cancer, physicians should be aware of this drug’s potential to severely impair quality of life by causing deep ulcerations of the skin and soft tissues as well as massive tumor hemorrhage.
Disclosure
The authors declare no conflicts of interest in this work.
References
- HurwitzHFehrenbacherLNovotnyWBevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancerN Engl J Med2004350232335234215175435
- JohnsonDHFehrenbacherLNovotnyWFRandomized Phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancerJ Clin Oncol200422112184219115169807
- YangJCHaworthLSherryRMA randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancerN Engl J Med2003349542743412890841
- ChenHXMooneyMBoronMPhase II multicenter trial of bevacizumab plus fluorouracil and leucovorin in patients with advanced refractory colorectal cancer: an NCI Treatment Referral Center Trial TRC-0301J Clin Oncol200624213354336016849749
- KabbinavarFHurwitzHIFehrenbacherLPhase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancerJ Clin Oncol2003211606512506171
- ScappaticciFASkillingsJRHoldenSNArterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumabJ Natl Cancer Inst200799161232123917686822
- MailliezABaldiniCVanJTServentVMalletYBonneterreJNasal septum perforation: a side effect of bevacizumab chemotherapy in breast cancer patientsBr J Cancer2010103677277520736943
- CottuPHFourchotteVVincent-SalomonAKriegelIFromantinINecrosis in breast cancer patients with skin metastases receiving bevacizumab-based therapyJ Wound Care2011209403404406408 passim22068139
- Philippin-LauridantGThureauSOuvrierMJBlotEFatal hemoptysis in a patient with breast cancer treated with bevacizumab and paclitaxelAnn Oncol200819111977197818801882
- TrainaTANortonLDruckerKSinghBNasal septum perforation in a bevacizumab-treated patient with metastatic breast cancerOncologist200611101070107117110625
- CabebeEWakeleeHRole of anti-angiogenesis agents in treating NSCLC: focus on bevacizumab and VEGFR tyrosine kinase inhibitorsCurr Treat Options Oncol200781152717634832
- GiacconeGThe potential of antiangiogenic therapy in non-small cell lung cancerClin Cancer Res20071371961197017404076
- LazzatiVZygónJLohsiriwatVVeronesiPPetitJYImpaired wound healing and bilateral mastectomy flap necrosis in a patient with locally advanced breast cancer after neoadjuvant Paclitaxel with bevacizumabAesthetic Plast Surg201034679679720567970
- CupponeFBriaEVaccaroVMagnitude of risks and benefits of the addition of bevacizumab to chemotherapy for advanced breast cancer patients: meta-regression analysis of randomized trialsJ Exp Clin Cancer Res2011305421569417
- PiergaJYPetitTDelozierTNeoadjuvant bevacizumab, trastuzumab, and chemotherapy for primary inflammatory HER2-positive breast cancer (BEVERLY-2): an open-label, single-arm phase 2 studyLancet Oncol201213437538422377126
- GianniLRomieuGHLichinitserMAVEREL: a randomized phase III trial evaluating bevacizumab in combination with docetaxel and trastuzumab as first-line therapy for HER2-positive locally recurrent/metastatic breast cancerJ Clin Oncol201331141719172523569311