Abstract
Endometrial carcinoma with a germ cell tumor component is a rare event. Here we report a uterine neoplasm with a unique combination of endometrioid adenocarcinoma and mixed germ cell malignant elements. A 28-year-old woman with abnormal vaginal bleeding, an abdominal mass, and elevated alfa-fetoprotein and beta-human chorionic gonadotropin (β-hCG) levels had a history of biopsy of an omental mass and chemotherapy in another hospital one month before her referral to our department. Histologic examination of the mass removed from the omentum revealed an endometrioid adenocarcinoma with yolk sac tumor-like differentiation. Total abdominal hysterectomy, bilateral salpingo-oophorectomy, infracolic omentectomy, and removal of metastatic disease were then undertaken at our hospital. Postoperative chemotherapy was given. Eight months postoperatively, serum alfa-fetoprotein and β-hCG rose again. Cases with primary yolk sac tumors of the endometrium or endometrial carcinoma with trophoblastic differentiation in the literature were reviewed.
Introduction
Yolk sac tumors, also known as endodermal sinus tumors, and trophoblastic neoplasms are both malignant germ cell tumors. Producing alfa-fetoprotein and human chorionic gonadotropin (hCG), respectively, yolk sac tumors and trophoblastic neoplasms are strongly suggested by elevated serum alfa-fetoprotein and hCG levels.
Primary yolk sac tumors of the endometrium are extremely rare; to our knowledge, only nine cases have been reported in the literature,Citation1–Citation9 among which only two cases are in coexistence with endometrial carcinomas. Endometrial carcinomas with trophoblastic differentiation are also very rare, with only 17 cases in the literature.Citation10–Citation24 Despite being uncommon, such cases have a distinct prognosis from pure endometrial adenocarcinoma. These two groups of cases do not share an intersection set. However, we report a case of endometrial carcinoma with yolk sac tumor-like differentiation, as well as elevated serum level of the beta-subunit of hCG (β-hCG), which suggests trophoblastic differentiation although there is a lack of histologic evidence. A dramatic postoperative reduction of β-hCG further supports the existence of a trophoblastic component.
Case report
A 28-year-old, nulligravid, married Chinese woman presented in December 2010 with a 14-month history of abnormal vaginal bleeding. The patient had been on barrier contraception, and to her knowledge had never been pregnant. Past medical history included epilepsy and hypothyroidism due to thyroidectomy. In October 2010, a diagnostic curettage specimen revealed endometrial adenocarcinoma with glandular squamous metaplasia, and an exploratory laparotomy was performed in a local hospital. A large omental mass and diffuse miliary nodules in the pelvic peritoneum made exposure of the surgical field difficult, so biopsy of the mass and peritoneum only was performed and histologic examination showed endometrial adenocarcinoma with yolk sac tumor-like differentiation. Chemotherapy, including intravenous paclitaxel 120 mg, adriamycin 60 mg, and intraperitoneal cisplatin 150 mg for one course, was administered at the local hospital. The patient was then referred to our hospital, where laboratory tests showed serum alfa-fetoprotein level of 1,522 ng/mL (normal value ≤20 ng/mL), β-hCG 518.9 mIU/mL (normal value ≤5 mIU/mL, chemiluminescent technology, Advia Centaur™ XP immunoassay system, Siemens, Erlangen, Germany), and CA 125 129 U/mL (normal value ≤35 U/mL). A chest computed tomography scan was negative.
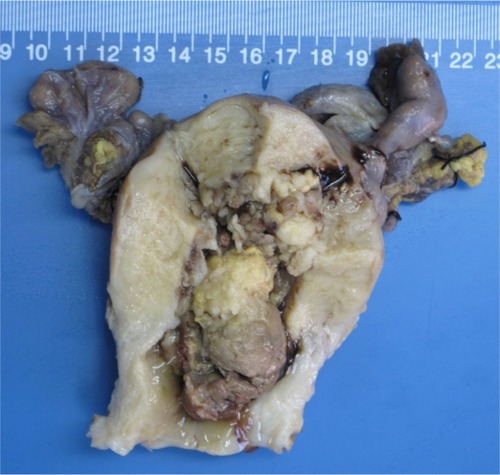
The patient underwent cytoreductive surgery, including total abdominal hysterectomy, bilateral salpingo-oophorectomy with pelvic lymphadenectomy, omentectomy, appendectomy, partial sigmoidectomy with anastomosis, and resection of abdominal and pelvic metastases, without residual visible metastases. The uterus measured 10 × 8 × 5 cm and the cavity was filled with a cauliflower-like tumor measuring 6 × 3 × 2.5 cm and containing areas of ulceration (see ). This solid tumor had a grayish-white cut surface and moderate texture. Histopathologic examination of the uterine tumor revealed well to moderately differentiated endometrial adenocarcinoma with yolk sac tumor-like differentiation intraendometrially, with a close transition between the two components (). No trophoblastic component was found. The metastases were identical to the primary lesion on histology. The myometrium, cervix, appendix, bilateral adnexa, and iliac lymph nodes were negative for tumor. Periodic-acid Schiff stain was positive in yolk sac tumor cells. Immunohistochemical analysis revealed that both endometrial adenocarcinoma and yolk sac tumor components were positive for AE1/AE3. The yolk sac tumor component was strongly positive for alfa-fetoprotein, while the endometrial adenocarcinoma was relatively negative (). The endometrial adenocarcinoma component was positive for EMA, CA125, and CK7, suggesting epithelial neoplasms. There was focal positive staining for p53. Estrogen receptor and progesterone receptor status were both negative, as were hCG and β-hCG.
Figure 2 Mixed components: a close transition from the endometrial adenocarcinoma to the yolk sac tumor areas (hematoxylin and eosin, 100×).
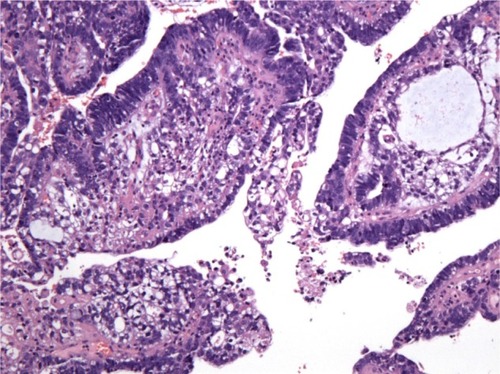
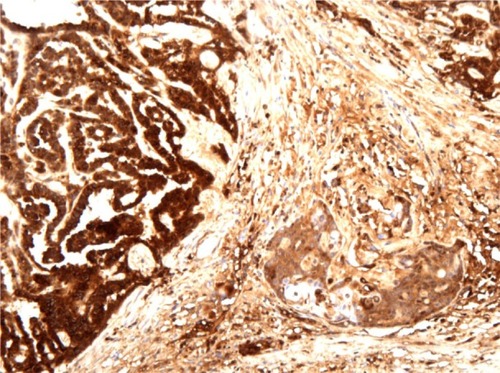
Figure 3 Immunohistochemical staining results for alfa-fetoprotein confirm the existence of two components of endometrial adenocarcinoma and yolk sac tumor.
Two days after surgery, serum alfa-fetoprotein and β-hCG levels decreased dramatically to 166.4 ng/mL and 13.7 mIU/mL, respectively, and the CA125 level dropped into the normal range. Six courses of intravenous chemotherapy with paclitaxel 175 mg/m2 per day followed by carboplatin for an area under the concentration-time curve of 5 mg/mL per minute were administered every 21 days. Serum alfa-fetoprotein dropped to the normal range after three courses, while β-hCG fluctuated in the range of 5–20 mIU/mL despite two additional courses of chemotherapy with etoposide, methotrexate, actinomycin D, etoposide, and cisplatinCitation25 followed by two courses with bleomycin, etoposide, and platinum.Citation26 The persistent slightly elevated β-hCG was considered due to abnormal pituitary feedback, so chemotherapy was stopped. Unfortunately, β-hCG and alfa-fetoprotein rose again 2–3 months later. Despite salvage chemotherapy with two cycles of floxuridine, dactinomycin, etoposide, and vincristine,Citation27 serum alfa-fetoprotein reached 311.1 ng/mL and β-hCG reached 2,716.5 mIU/mL. After the final course with oxaliplatin 200 mg and cyclophosphamide 800 mg, the patient abandoned further treatment and was lost to follow-up.
Discussion
Here we report a case of endometrial carcinoma with yolk sac tumor-like differentiation as well as elevated serum β-hCG level suggesting trophoblastic differentiation although there was a lack of histologic evidence. Yolk sac tumors and trophoblastic neoplasms are both malignant germ cell tumors, and when found simultaneously, mixed germ cell tumor is diagnosed, and usually within the gonads.Citation28 Primary extragonadal concurrent yolk sac tumors and trophoblastic neoplasms are extremely rare, but have been known to occur in the thyroid,Citation29 Barrett’s esophagus,Citation30 and in gastricCitation31 and colon carcinoma.Citation32 The histogenetic mechanism for primary extragonadal germ cell tumors remains controversial.Citation7 The close transition from the endometrioid adenocarcinoma to the yolk sac tumor areas () in the present case supports an origin involving aberrant differentiation of somatic cells. Mixed tumors with a germ cell tumor component usually manifest as tumors in corresponding organs; for instance the present case had a medical history of abnormal vaginal bleeding of 14 months’ duration, which unfortunately was not paid enough attention. Unlike typical endometrial carcinoma, she was young and presented with peritoneal metastasis without myometrial infiltration or lymphadenopathy. Hence, histopathologic examination of specimens from diagnostic curettage and exploratory laparotomy are of great clinical importance in such circumstances.
Primary yolk sac tumors of the endometrium are extremely rare. To our knowledge, only nine cases have been reported in the literature (),Citation1–Citation9 among which seven cases are pure yolk sac tumors and only two casesCitation6,Citation7 are in coexistence with endometrial carcinoma. All the patients presented with a medical history of abnormal vaginal bleeding and elevated serum alfa-fetoprotein levels before or immediately after surgery. Extragonadal germ cell tumors are diagnosed histopathologically. Yolk sac tumor presents variously under the microscope, and occasionally there is confusion in differentiating a microcystic or endodermal sinus-like structure from a clear cell uterine carcinoma and a papillary structure from uterine serous papillary carcinoma. In addition to morphologic differences, immunohistochemical staining is helpful. Yolk sac tumors are strongly positive for alfa-fetoprotein. In addition, serum alfa-fetoprotein determinations are important in the diagnosis of yolk sac tumors and monitoring metastasis or recurrence after therapy. Most of the reviewed cases experienced a reduction in serum alfa-fetoprotein after surgery and adjuvant therapy, as did our patient.
Table 1 Cases of primary yolk sac tumors of the endometrium
Yolk sac tumors usually occur in the gonads of young people.Citation1 In the previously published literature, cases with pure yolk sac tumors are younger (age range 27–49 years, mean 32.7 years) than those with mixed tumors (age range 59–65 years, mean 62 years). In the seven patients with pure yolk sac tumors, fiveCitation3–Citation5,Citation8,Citation9 presented no metastasis (or not described) and no evidence of disease at last follow-up more than one year (6 year at most) after diagnosis. Among these five, two casesCitation8,Citation9 had a unilateral ovary or bilateral ovaries retained after surgery because they were young women, indicating a more favorable prognosis. In contrast with these cases, those with endometrial neoplasms with yolk sac tumor-like differentiation were all postmenopausal women, and presented with early metastasis to the liver, diaphragm, or abdominal lymph nodes. This second group of cases tends to have a worse prognosis. The case reported by PatsnerCitation6 used a potential carcinogen (tamoxifen for prior breast cancer) and the tumor metastasized 19 months after diagnosis despite two surgeries and administration of combined chemotherapy and radiotherapy. The different components of the tumor have a transition zone, so erroneous differentiation of somatic cells was proposed as the histogenetic mechanism of the tumor cells, ie, the yolk sac tumor cells were derived from dedifferentiation or retrodifferentiation of somatic endometrial (tumor) cells. The present case had concurrent endometrioid adenocarcinoma and yolk sac tumor components in both primary and metastatic tumors, and metastasis occurred early. Despite multiple courses and regimens of chemotherapy, alfa-fetoprotein rose again 8 months postoperatively. These clinical and pathologic features strongly resemble the cases in the latter group mentioned above, except for the very young age of 28 years.
The present self-reported nulligravid case had elevated serum β-hCG level during her entire medical course, but no trophoblastic differentiation was observed under the microscope. Grenache et alCitation33 reported a similar case of endometrial adenocarcinoma without trophoblastic differentiation and with an elevated serum free β-hCG and no evidence of pregnancy. In their case, endometrial adenocarcinoma cells showed hCG immunoreactivity and were believed to produce free β-hCG. In the present case, however, the decrease in β-hCG following surgery and chemotherapy excluded the possibility of phantom β-hCG or β-hCG elevation associated with the testing method used. Given that the tumor cells seen were negative for hCG and β-hCG by immunohistochemical analysis, the authors believe that a trophoblastic component did exist, and the negative histology findings might be due to the chemotherapy before admission, and failure to get the positive section in pathological slice-making.
Endometrial neoplasms with trophoblastic differentiation are also very rare, with only 17 cases reported in the literature ().Citation10–Citation24 These 17 patients are relatively older (age range 34–88 years, mean 65.4 years). All cases, including the present one, presented with abnormal genital bleeding except for one without description, and elevated serum or urinary β-hCG before or shortly after therapy, with a median serum β-hCG level of 3,050 mIU/mL, except one case with normal serum β-hCG first measured after histologic diagnosis of surgical specimens.Citation21 Most of the cases had been pregnant, so gestational trophoblastic neoplasms could not be excluded completely. However, Olson et alCitation23 reported a case with clonal evolution from endometrioid carcinoma to trophoblastic tumor proven by morphologic and molecular genetic analysis, suggesting great utility of this method. Endometrioid adenocarcinoma is the most frequently reported histologic type in the predominant component of the concurrent tumor, occurring in 12 of the reported cases, with serous papillary carcinoma in two cases and clear cell carcinoma in one case. Khuu et alCitation17 and Nguyen et alCitation18 reported two cases of malignant Müllerian mixed tumor containing an endometrioid adenocarcinomatous component with trophoblastic differentiation as well as a sarcomatous component. The median follow-up duration was 11 months, and at last follow-up, seven cases had died (follow-up 1.5–24 months), two were alive with disease (1 and 5 months), and five with relatively low initial serum β-hCG (median 283 mIU/mL) were alive without evidence of disease (6–50 months).
Table 2 Cases of endometrial neoplasm with trophoblastic differentiation
Horn et alCitation20 proposed two prognostically relevant types of endometrial carcinoma containing trophoblastic differentiation. One type presents with only a few syncytiotrophoblastic-like giant cells and the other with a notable extension of trophoblastic differentiation, resembling a choriocarcinoma. The latter type is associated with strongly elevated β-hCG, early metastasis, and often a fatal course. The present case presented with no observable trophoblastic cells and a moderately elevated β-hCG at admission, suggesting a more favorable prognosis according to Horn’s proposition. However, despite the rapid decrease in β-hCG after hysterectomy, it remained slightly and persistently elevated and finally rose again to 2,716.5 mIU/mL. The patient was lost to follow-up 10 months after diagnosis, implying an unfavorable outcome.
Conclusion
To the author’s knowledge, endometrial adenocarcinoma associated with both yolk sac tumor-like differentiation and an elevated serum β-hCG level has never been reported in the literature. We present such a case and share our experiences in treatment. Unlike the reviewed cases of endometrial adenocarcinoma with a single germ cell tumor component, our patient was very young and presented with peritoneal metastasis early but without myometrial infiltration or lymphadenopathy. The histology of the metastases was identical to that of the primary tumor, with mixed components. The cytoreductive surgery and chemotherapy tend to show an effective immediate efficacy. The choice of subsequent chemotherapies was based on the major tumor component at each follow-up. However, the disease progressed rapidly and was resistant to salvage chemotherapy. Because medical history, gynecologic examination, and imaging results contribute little to early recognition of extragonadal germ cell tumors, histopathologic examination of specimens from diagnostic curettage and exploratory laparotomy are of great clinical significance in such conditions. Once diagnosed, serum alfa-fetoprotein and β-hCG determinations are important in monitoring metastasis or recurrence. The histogenetic mechanism is unclear, and further investigations with molecular genetic analysis are required.
Disclosure
The authors report no conflicts of interest in this work.
References
- PileriSMartinelliGSerraLBazzocchiFEndodermal sinus tumor arising in the endometriumObstet Gynecol19805633913966158720
- ClementPBYoungRHScullyREExtraovarian pelvic yolk sac tumorsCancer19886236206263292037
- OhtaMSakakibaraKMizunoKSuccessful treatment of primary endodermal sinus tumor of the endometriumGynecol Oncol19883123573643169623
- JosephMGFellowsFGHearnSAPrimary endodermal sinus tumor of the endometrium. A clinicopathologic, immunocytochemical, and ultrastructural studyCancer19906522973021688508
- SpatzABouronDPautierPCastaigneDDuvillardPPrimary yolk sac tumor of the endometrium: a case report and review of the literatureGynecol Oncol19987022852889740707
- PatsnerBPrimary endodermal sinus tumor of the endometrium presenting as “recurrent” endometrial adenocarcinomaGynecol Oncol2001801939511136577
- OguriHSumitomoRMaedaNFukayaTMorikiTPrimary yolk sac tumor concomitant with carcinosarcoma originating from the endometrium: case reportGynecol Oncol2006103136837116814851
- RossiRStacchiottiDBernardiniMGCalvieriGLo VoiRPrimary yolk sac tumor of the endometrium: a case report and review of the literatureAm J Obstet Gynecol20112044e3e421345404
- WangCLiGXiLGuMMaDPrimary yolk sac tumor of the endometriumInt J Gynaecol Obstet2011114329129321696729
- CivantosFRywlinAMCarcinomas with trophoblastic differentiation and secretion of chorionic gonadotrophinsCancer19722937897984334242
- SavageJSubbyWOkagakiTAdenocarcinoma of the endometrium with trophoblastic differentiation and metastases as choriocarcinoma: a case reportGynecol Oncol19872622572623026934
- PesceCMerinoMJChambersJTNogalesFEndometrial carcinoma with trophoblastic differentiation. An aggressive form of uterine cancerCancer1991688179918021717127
- KalirTSeijoLDeligdischLCohenCEndometrial adenocarcinoma with choriocarcinomatous differentiation in an elderly virginal womanInt J Gynecol Pathol19951432662698600080
- BlackKSykesPOstorAGTrophoblastic differentiation in an endometrial carcinomaAust N Z J Obstet Gynaecol19983844724739890238
- BradleyCSBenjaminIWheelerJERubinSCEndometrial adenocarcinoma with trophoblastic differentiationGynecol Oncol199869174779571002
- TuncMSimsekTTrakBUnerMEndometrium adenocarcinoma with choriocarcinomatous differentiation: a case reportEur J Gynaecol Oncol19981954894919863921
- KhuuHMCriscoCPKilgoreLRodgersWHConnerMGCarcinosarcoma of the uterus associated with a nongestational choriocarcinomaSouth Med J200093222622810701796
- NguyenCPLeviAWMontzFJBristowRECoexistent choriocarcinoma and malignant mixed mesodermal tumor of the uterusGynecol Oncol200079349950311104628
- Le BretTTranbalocPBenbunanJLSalet-LizeeDVilletREndometrial choriocarcinoma in peri-menopausal womenJ Gynecol Obstet Biol Reprod (Paris)2005341 Pt 18589 French15767921
- HornLCHanelCBartholdtEDietelJSerous carcinoma of the endometrium with choriocarcinomatous differentiation: a case report and review of the literature indicate the existence of 2 prognostically relevant tumor typesInt J Gynecol Pathol200625324725116810062
- AkbulutMTosunHSoysalMEOztekinOEndometrioid carcinoma of the endometrium with choriocarcinomatous differentiation: a case report and review of the literatureArch Gynecol Obstet20082781798418066564
- YamadaTMoriHKanemuraMOhmichiMShibayamaYEndometrial carcinoma with choriocarcinomatous differentiation: a case report and review of the literatureGynecol Oncol2009113229129419232701
- OlsonMTGockeCDGiuntoliRL2ndShih IeMEvolution of a trophoblastic tumor from an endometrioid carcinoma – a morphological and molecular analysisInt J Gynecol Pathol201130211712021293290
- WakahashiSSudoTNakagawaEEndometrioid adenocarcinoma with high-grade transformation with serous and choriocarcinomatous differentiation – a case reportJ Cancer20123141822211141
- BehtashNKarimi ZarchiMPlacental site trophoblastic tumorJ Cancer Res Clin Oncol200813411617701427
- AlazzamMTidyJOsborneRColemanRHancockBWLawrieTAChemotherapy for resistant or recurrent gestational trophoblastic neoplasiaCochrane Database Syst Rev201212CD00889123235667
- FengFXiangYWanXGengSWangTSalvage combination chemotherapy with floxuridine, dactinomycin, etoposide, and vincristine (FAEV) for patients with relapsed/chemoresistant gestational trophoblastic neoplasiaAnn Oncol20112271588159421239399
- ZhaoSKatoNEndohYJinZAjiokaYMotoyamaTOvarian gonadoblastoma with mixed germ cell tumor in a woman with 46, XX karyotype and successful pregnanciesPathol Int200050433233510849320
- Wierzbicka-ChmielJChroszczMSlomianGMixed germ cells tumour primarily located in the thyroid – a case reportEndokrynol Pol2012635388390 Russian23115073
- WasanHSSchofieldJBKrauszTSikoraKWaxmanJCombined choriocarcinoma and yolk sac tumor arising in Barrett’s esophagusCancer19947335145177507793
- SatakeNChikakiyoMYagiTSuzukiYHiroseTGastric cancer with choriocarcinoma and yolk sac tumor components: case reportPathol Int201161315616021355958
- KawaharaMTakadaATachibanaAGerm cell tumor of the colon with an adenocarcinomatous componentInt J Clin Oncol200914653754019967492
- GrenacheDGMollerKAGrobenPMEndometrial adenocarcinoma associated with elevated serum concentrations of the free beta subunit of human chorionic gonadotropinAm J Clin Pathol2004121574875315151215