Abstract
Background
Chemotherapy and epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors are controversial in the treatment of patients with brain metastases from non-small-cell lung cancer (NSCLC).
Methods
We retrospectively studied the effects of solely localized treatment or localized treatment in combination with chemotherapy and/or EGFR tyrosine kinase inhibitors on outcomes in 210 NSCLC patients with brain metastases. The effects of treatment modality, Karnofsky performance status, age, primary tumor histology, number of brain metastases, and other factors on survival time were analyzed, and the robustness of two prognostic indices, ie, recursive partitioning analysis and graded prognostic assessment, was evaluated.
Results
The median survival time in patients with systemic medication and localized treatments was higher than in those with localized treatments alone (11 versus 3 months, P=0.000). Within the systemic medication group, median survival time was significantly longer for EGFR tyrosine kinase inhibitors than for other types of chemotherapy (12 versus 9 months, P=0.002). In the EGFR tyrosine kinase inhibitor group, median survival time for patients with EGFR gene mutation was 20 months versus 8 months for those with the wild-type EGFR gene. The median survival time with pemetrexed was significantly higher than with other chemotherapies (13 versus 7 months, P=0.006). In multivariate analysis, the prognosis was significantly correlated with treatment modality (P=0.000), Karnofsky performance status (P=0.000), number of brain metastases (P=0.001), and histologic tumor type (P=0.007). In the graded prognostic assessment model, survival curves for the subgroups showed clear separations.
Conclusion
NSCLC patients with brain metastasis benefited from pemetrexed and/or tyrosine kinase inhibitors along with localized treatments, and the graded prognostic assessment index is a robust model for prognostic evaluation.
Introduction
Brain metastases are a common complication in non-small-cell lung cancer (NSCLC) patients, have incidence rates as high as 25%–40%,Citation1–Citation3 and severely impair patient survival outcome and quality of life. Current treatment options include surgical resection, whole brain radiation therapy, stereotactic radiosurgery, and combined modality approaches. Whole brain radiation therapy is the standard treatment approach for multiple brain metastases, with a response rate of 60%.Citation4,Citation5 However, the median survival time following whole brain radiation therapy alone is rather short, in the range of 2.4–4.8 months,Citation2,Citation5,Citation6 which might be due to the fact that most NSCLC patients with brain metastasis also develop extracranial metastases and thus require systemic medication in addition to local treatment of their brain tumors.
Previously published studies of whole brain radiation therapy used in combination with traditional chemotherapeutic agents, such as platinum, nitrosourea, paclitaxel, and temozolomide, have failed to show a significant improvement in overall survival compared with whole brain radiation therapy alone,Citation7–Citation12 thus creating an urgent need to find more effective systemic therapies. In recent years, pemetrexed (a new multitarget antimetabolite) and epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors have become regular medications for the treatment of advanced NSCLC and had good results in treating patients with brain metastases. One study showed that pemetrexed as sole medication for NSCLC brain metastases achieved a partial response in 38.4% of cases, with an overall clinical benefit obtained in 69% of patients and a median survival time of 10 months.Citation13
Tyrosine kinase inhibitors used as single medication showed response rates ranging from 10% to 86%, progression-free survival of 3–10 months, and a median survival time of 8.3–18.8 months.Citation14–Citation18 In order to evaluate the effectiveness of these new agents in enhancing survival rates in NSCLC patients with brain metastases, we retrospectively investigated 210 such patients for factors such as treatment modality, Karnofsky performance status, patient age, histologic type, smoking status, and number of brain metastases as well as medication regimens.
Patients and methods
Patients
We enrolled 210 NSCLC patients first diagnosed with brain metastases at Zhejiang Cancer Hospital between July 2006 and July 2010. All the patients had a Karnofsky performance status score ≥50, were aged 18–75 years, had histologically or cytologically confirmed NSCLC, and had received whole brain radiation therapy previously. All patients had complete clinical and follow-up records. The research was approved by the ethical committee of Zhejiang Cancer Hospital and informed consent was obtained from all participants.
Study design
Using patient hospital records, follow-up registration records, and follow-up phone records, we collected clinical and survival information about the patients. The following data were collected and recorded: age when first diagnosed with brain metastasis, sex, Karnofsky performance status when first diagnosed with brain metastasis, number of brain metastases, primary tumor histologic type, whether the brain metastasis was synchronous or asynchronous, presence or absence of extracranial metastases, and systemic therapy regimens. Patients were categorized by age (<65 years, ≥65 years), sex (male, female), Karnofsky performance status (<70, ≥70), smoking status (never, former, current), number of brain metastases (one tumor, two to three tumors, more than three tumors), whether the brain metastasis was synchronous or asynchronous, presence or absence of extracranial metastases, primary tumor histology (adenocarcinoma, squamous cell carcinoma, other), and local treatment regimen (whole brain radiation therapy only; whole brain radiation therapy plus surgical resection or stereotactic radiosurgery).
Based on local treatment regimens, patients were divided into two groups, ie, whole brain radiation therapy only (n=179) and whole brain radiation therapy plus surgical resection or stereotactic radiosurgery (n=31). Patients were also divided into two groups according to whether they received systemic therapy, ie, 24 patients were treated with localized treatment only and 186 patients received both local and systemic therapy. Of the 186 patients who received systemic medication, 111 received chemotherapy and 75 were treated with EGFR tyrosine kinase inhibitors (46 patients with gefitinib and 29 with erlotinib). Of these patients, 29 were genotyped using the amplification refractory mutation system and 58.6% (17/29) had a mutated EGFR gene in which exon 19 contained a mutation in nine cases and exon 21 contained a mutation in eight cases, whereas 12 patients had a wild-type EGFR gene.Citation19 Within the chemotherapy group, 21 patients with adenocarcinoma were treated with pemetrexed and 90 were treated with other chemotherapeutic agents, mainly gemcitabine or docetaxel in combination with platinum (Supplementary ). Two prognostic models were established separately using the recursive partitioning analysis (RPA) and revised graded prognostic assessment (GPA) models proposed by the Radiation Therapy Oncology Group.Citation20–Citation22 RPA has three prognostic classes based on age at diagnosis, presence or absence of extracranial metastases, Karnofsky performance status, and primary tumor status. A higher RPA class represents a worse prognosis. GPA, on the other hand, uses four criteria (age at diagnosis, Karnofsky performance status, presence or absence of extracranial metastases, and number of brain metastases) to produce a score from 0 to 4, with a higher score corresponding to a better prognosis (). A major focus of the study was overall survival, defined as the duration of time from the start of therapy for brain metastases until death or the most recent follow-up.
Table 1 Patient characteristics at baseline
Statistical analysis
All statistical analyses were performed using Statistical Package for the Social Sciences version 19 software (SPSS Inc, Chicago, IL, USA). Chi-square tests and nonparametric tests (Mann–Whitney) were used to compare differences in patient characteristics. Kaplan–Meier survival curves were used to compare survival differences between subgroups (sex, age, and other factors) by using a single variant survival analysis. Log-rank tests were used to determine statistically significant differences between the survival curves for each group. Finally, the Cox proportional hazard regression model (forward Wald method) was used in multivariate analysis of groups to study the effect of prognostic factors from the Kaplan–Meier single variant test (GPA, number of metastatic sites, treatment modality, RPA classes, Karnofsky performance status, histology, smoking history, sex) and clinical factors (age, treatment, Karnofsky performance status score), and to evaluate which factors were associated with patient survival as well as to analyze differences in the survival curves for each subgroup.
Results
Patients
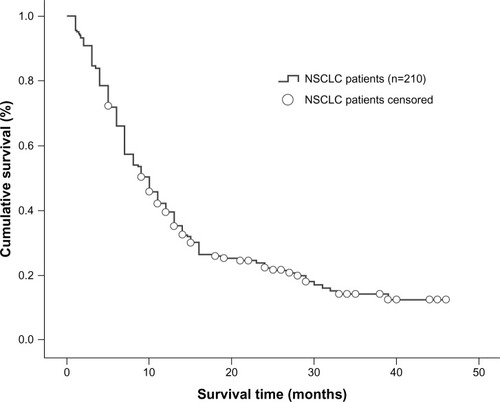
A total of 210 NSCLC patients diagnosed with brain metastases were enrolled in the study and 142 (67.6%) were male. The median age was 55 (range 23–73) years and 146 patients (69.5%) had a Karnofsky performance score ≥70. In 171 patients (81.4%), the tumor type was not squamous cell carcinoma. A total of 125 patients (59.5%) were smokers or had a smoking history. Synchronous brain metastasis occurred in 114 patients (54.3%), 147 patients (70.0%) developed more than one metastatic brain tumor, and 129 patients (61.4%) had extracranial metastases. In total, 179 patients (85.2%) received whole brain radiation therapy only while 14.8% underwent whole brain radiation therapy plus surgical resection or stereotactic radiosurgery. The overall median clinical follow-up duration was 12.5 (range 1–49) months. At the time of the analysis, 164 patients had died (78.1%) and 46 (21.9%) were still alive. Based on the RPA index, 46.7% of the patients were in class II, and based on the GPA index, 62.9% were in the 1.5–2.5 range (). Overall median survival time was determined to be 10 months (95% confidence interval [CI] 8.226–11.774, ).
Correlation between treatment modality and patient prognosis and survival
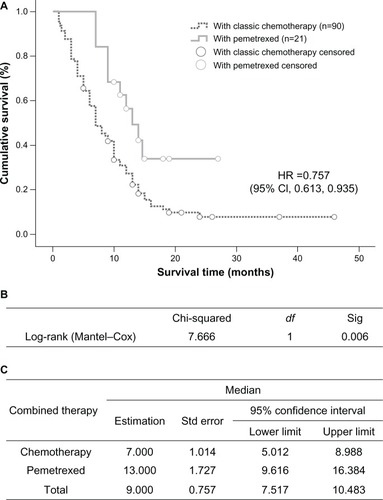
The group that received localized treatment had a median survival time of 3 months (95% CI 1.628–4.372), which is significantly shorter than the 11 months (95% CI 9.205–12.795) in the group receiving both localized treatment and systemic medication (P=0.000, ). Within the medication groups, treatments with EGFR tyrosine kinase inhibitors led to an overall median survival time of 12 months (95% CI 6.660–17.340), which was significantly longer than the 9-month median survival time (95% CI 7.517–10.483) in the chemotherapy group (P=0.002, ).
Figure 2 Comparison of median survival time in patients who received only localized treatment or localized treatment plus systemic medication. (A) Kaplan–Meier survival curve, (B) Mantel–Cox test, and (C) median values with 95% CI.
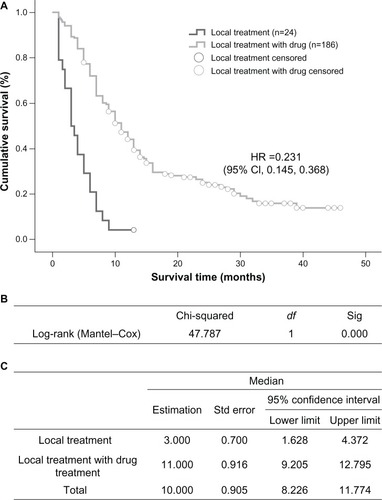
Figure 3 Comparison of median survival time in patients who received EGFR tyrosine kinase inhibitors or chemotherapy (*pemetrexed included). (A) Kaplan–Meier survival curve, (B) Mantel–Cox test, and (C) median values with 95% CI.
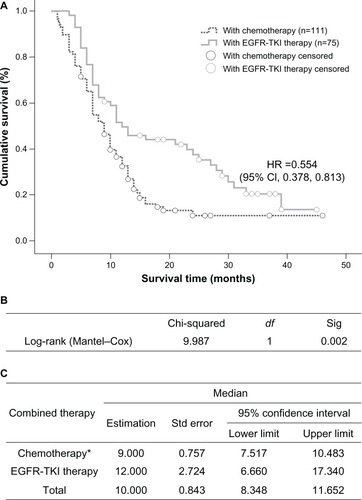
In the group treated with EGFR tyrosine kinase inhibitors, median survival time in the 17 patients with an EGFR mutation was 20 months (95% CI 16.155–23.845), which was longer than that in wild-type EGFR patients (12 cases), whose median survival time was 8 months (95% CI 4.653–11.347, P=0.000, ). In the chemotherapy-treated patients, those receiving pemetrexed (n=21) had a median survival time of 13 months (95% CI 9.616–16.384), which was significantly longer than the 7 months (95% CI 5.012–8.988) recorded in the group treated with gemcitabine or docetaxel combined with platinum (n=90, P=0.006, ).
Figure 4 (A) Kaplan–Meier curve illustrating overall survival based on EGFR status (EGFR mutation and EGFR wild-type) and (B) median values with 95% CI.
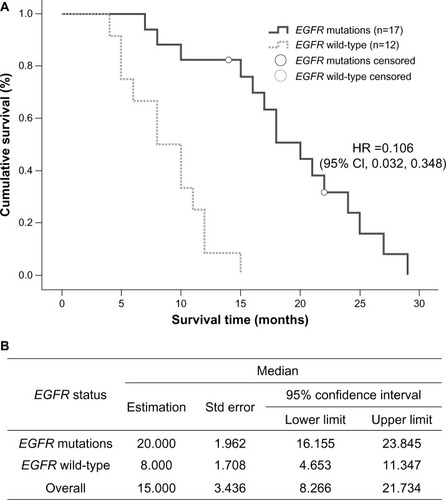
Figure 5 Comparison of median survival time in patients who received pemetrexed or other chemotherapies. (A) Kaplan–Meier survival curve, (B) Mantel–Cox test, and (C) median values with 95% CI.
Correlation between prognostic factors and patient survival
Univariate analysis of the entire sample indicated that, of the 10 prognostic factors taken into account, only treatment modality, number of brain metastases, Karnofsky performance score, tumor histologic type, smoking status, and sex were related to overall survival, while age, presence or absence of extracranial metastases, whether the brain metastasis was synchronous or asynchronous, and local treatment regimens did not correlate with overall survival (). On the other hand, multivariate analysis of the entire sample indicated that four factors were significantly correlated with patient outcome, ie, treatment modality (P=0.000, relative risk 0.527), Karnofsky performance status (P=0.000, relative risk 1.974), number of brain metastases (P=0.001, relative risk 1.412), and tumor histology (P=0.007, relative risk 1.220, ).
Table 2 Univariate analysis of factors affecting overall survival
Table 3 Multivariate analysis of factors affecting overall survival in patients without RPA and GPA in the Cox regression model
Correlation between prognostic model and survival
Univariate analysis showed that the RPA and GPA prognostic index models were both correlated with overall survival (P=0.001 and P=0.000, respectively). According to the RPA prognostic index model, median survival time in patients categorized into classes I, II, and III was 11, 11, and 6 months, respectively, and a statistically significant difference was observed between class III patients and class I/II patients (). Using the GPA prognostic index model, 66.5% of patients with a GPA score of 3.5–4.0 were alive at the end of the study; for patients with a GPA score of 3.0, median survival time was 13 months, while it was 10 months for patients with GPA scores of 1.5–2.5 and 5 months for patients with a GPA score of 0–1. Using the GPA index, statistically significant differences in median survival time could be detected between all subgroups (P=0.000), with clear separations of the survival curves (). However, when the RPA and GPA models were included in multivariate analysis, only treatment modality (P=0.000, relative risk 0.529) and GPA index (P=0.000, relative risk 0.474) continued to show a correlation with treatment outcomes ().
Figure 6 Kaplan–Meier curve illustrating overall survival based on RPA.
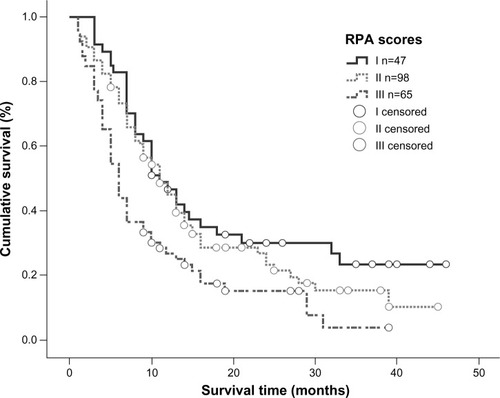
Figure 7 Kaplan–Meier survival curve illustrating overall survival based on GPA.
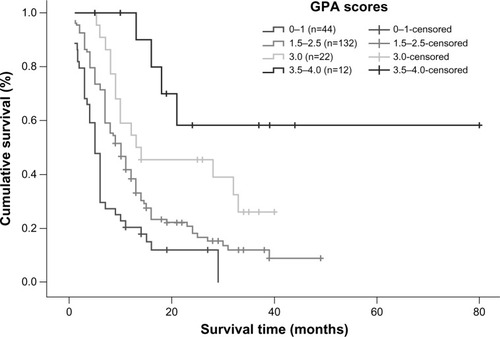
Table 4 Multivariate analysis of factors affecting overall survival in patients with RPA and GPA included in the Cox regression model
These results suggest that when treating NSCLC brain metastases, a combined modality of localized therapy plus systemic medication should be the first choice. Within the medication options, EGFR tyrosine kinase inhibitors should be given preference, and within the chemotherapeutic agents, pemetrexed showed better efficacy than the others. Treatment modality, Karnofsky performance status, number of brain metastases, and tumor histology are four factors that were significantly correlated with patient outcome. The GPA index model showed superior correlations for prognostic evaluation.
Discussion
Patients with brain metastases from NSCLC usually have a poor prognosis, with a median survival time of about one month without treatment,Citation23 2 months with glucocorticoid therapy, and 2.4–4.8 months with whole brain radiation therapy.Citation2,Citation5,Citation6,Citation24 The majority also develop extracranial and multiple brain metastases. Even though there are localized treatment methods available for managing intracranial lesions, their effectiveness is limited and additional medication is required for these patients. There is currently a lack of randomized, controlled studies with large sample sizes that address this issue; further, several smaller Phase II/III clinical studies and some retrospective analyses disagree about the efficacy of traditional chemotherapeutic agents for the treatment of brain metastases.Citation7–Citation12,Citation25,Citation26 Some of the studies showed that whole brain radiation therapy combined with traditional chemotherapy failed to enhance patient survival,Citation7–Citation12 which is thought to be largely due to the inability of drugs to cross the blood–brain barrier or because they possess low activity in NSCLC. However, other studies have shown that chemotherapy enhances survival time to a certain extent,Citation25,Citation26 especially since the introduction of EGFR tyrosine kinase inhibitors and pemetrexed.
The results of our study indicate that localized treatment combined with medication increases the overall survival rate more than localized treatment alone. Further, median survival time in our EGFR tyrosine kinase inhibitor group was significantly longer than in our chemotherapy group. Within the chemotherapies, median survival time was significantly longer in patients on pemetrexed than in those receiving other medications. In a study by Bearz et al,Citation13 of 39 patients with NSCLC brain metastases treated with pemetrexed alone, 38.4% showed a partial response, 69% showed an overall clinical benefit, 82% showed radiologic cerebral benefit, and overall median survival was 10 months. Barlesi et alCitation27 reported that when pemetrexed was used in combination with platinum as the primary treatment for NSCLC patients with asymptomatic and inoperable brain metastases, the cerebral response rate was 41.9% and the extracerebral response rate was 34.9%, with a median survival time of 7.4 months and a time to progression of 4.0 months. Studies have shown that the EGFR mutation rate in patients with brain metastases is about 50% in Western populations and as high as 63% in Asian populations.Citation28,Citation29 Because EGFR mutation status significantly affects the efficacy of EGFR tyrosine kinase inhibitors,Citation30 the fact that patients treated with these agents in this study showed an enhanced survival rate may be related to the fact that NSCLC patients with brain metastases have a higher than normal EGFR mutation rate. In our study, mutations were detected in 58.6% of the 29 patients analyzed for EGFR mutation, and median survival time was longer in these patients than in those with wild-type EGFR.
Several Phase II clinical trials and retrospective studies in unselected NSCLC patients with brain metastases have shown that treatment with tyrosine kinase inhibitors alone achieved response rates of 10%–60%, with progression-free survival of 3–9 months and overall survival of 8.3–15 months.Citation14,Citation16,Citation17 The survival benefit using tyrosine kinase inhibitors in patients with adenocarcinoma or EGFR mutations was even greater, with objective response rates reaching 56.0%–82.0%, progression-free survival times of 6.6–10.1 months, and overall survival times of 12.9–19.8 months.Citation15,Citation18,Citation31,Citation32 Studies have shown that concomitant treatment using tyrosine kinase inhibitors and whole brain radiation therapy in NSCLC patients with brain metastases produced response rates as high as 81%–86%, with overall survival times of 11.8–13 months.Citation28,Citation33 Dai et al believe that the effectiveness of pemetrexed and tyrosine kinase inhibitors in the treatment of brain metastases is related to their ability to penetrate the blood–brain barrier to a certain extent. The small molecule tyrosine kinase inhibitors reach cerebrospinal fluid concentrations that are 1.5%–7% of their plasma concentration,Citation34,Citation35 and pemetrexed has a greater ability to penetrate the blood–brain barrier than methotrexate, another antimetabolite.Citation36
The results of our retrospective analysis also indicate that NSCLC patients with brain metastases could benefit from combinations of localized treatment with chemotherapy and/or tyrosine kinase inhibitors. However, one shortcoming of this study was the lack of EGFR mutation data for the majority of patients; because of this, the overall effect of EGFR mutation rates could not be determined with certainty. We also analyzed prognostic factors for patients with brain metastases. In both univariate and multivariate analyses, we found that Karnofsky performance score, number of brain metastases, and histologic type were all significant predictors of patient prognosis. However, if the RPA and GPA prognostic index models were included in multivariate analysis, only treatment modality and the GPA model remained correlated with prognosis.
The correlation between Karnofsky performance status and prognosis in univariate analysis has been supported in the majority of studies. Sanghavi et alCitation37 retrospectively analyzed 502 patients with brain metastases, who had received stereotactic radiosurgery ± whole brain radiation therapy, and their results showed that patients with a higher Karnofsky performance score, a controlled primary tumor, no extracranial metastases, and a lower RPA class had a better prognosis. Frazier et alCitation38 retrospectively analyzed 273 patients with brain metastases who had undergone stereotactic radiosurgery ± whole brain radiation therapy, and showed that patients under 65 years of age with a Karnofsky performance score >70 had a longer median survival time.
The negative correlation between number of brain metastases and patient outcome is in agreement with several other studies,Citation39,Citation40 indicating that number of brain metastases significantly affects patient outcome. A correlation analysis between histologic tumor type and prognosis indicated that patients with adenocarcinoma had a better prognosis. This is possibly due to the fact that 96 patients in the study (45.7%) received tyrosine kinase inhibitors or pemetrexed, and these two drugs are both more effective against adenocarcinoma. Our study found no correlation between presence or absence of extracranial metastases and patient prognosis; whether or not this is related to the fact that 88.6% of the patients received systemic treatment is a question that warrants further research.
NSCLC patients with brain metastases are a very heterogenous group, with a wide variation in terms of prognosis. Any prediction of outcome must be done based on an overall evaluation of factors affecting survival. In this study, when patients were divided into three classes according to the RPA prognostic model, only median survival time in class III was significantly different from that in the other two classes, while the survival curves of classes I and II showed no significant differences. In comparison, the GPA model provided a more valuable prognostic classification; it was significantly correlated with patient prognosis in both univariate and multivariate analyses and all GPA subgroups had significantly different survival curves. A recent study by Sperduto et al provides further evidence of the prognostic value of the GPA model in NSCLC patients with brain metastases.Citation40 Thus, it is likely that the GPA prognostic model will be more widely utilized in predicting survival for NSCLC patients.
In summary, treatment modality, Karnofsky performance score, number of brain metastases, and histologic type were significantly correlated with patient prognosis. Our results suggest that patients who receive chemotherapy, especially pemetrexed and/or tyrosine kinase inhibitors concomitantly with localized treatments, are likely to have a greater survival benefit, and further clinical studies focusing on tumor histology and molecular characteristics may be warranted. We also found that the GPA index is a robust model for prognostic evaluation.
Acknowledgments
The authors thank Tao Lei and Xiangtao Luo for acquisition of data.
Supplementary table
Table S1 The clinical characteristics of patients in different treatment groups
Disclosure
The authors report no conflicts of interest in this work.
References
- DelattreJYKrolGThalerHTPosnerJBDistribution of brain metastasesArch Neurol19884577417443390029
- LangerCJMehtaMPCurrent management of brain metastases, with a focus on systemic optionsJ Clin Oncol200523256207621916135488
- PatchellRABrain metastasesNeurol Clin1991948178241758427
- GasparLEMehtaMPPatchellRAThe role of whole brain radiation therapy in the management of newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guidelineJ Neurooncol2010961173219960231
- KhuntiaDBrownPLiJMehtaMPWhole-brain radiotherapy in the management of brain metastasisJ Clin Oncol20062481295130416525185
- EichlerAFLoefflerJSMultidisciplinary management of brain metastasesOncologist200712788489817673619
- AntonadouDParaskevaidisMSarrisGPhase II randomized trial of temozolomide and concurrent radiotherapy in patients with brain metastasesJ Clin Oncol200220173644365012202665
- ChuaDKrzakowskiMChouaidCWhole-brain radiation therapy plus concomitant temozolomide for the treatment of brain metastases from non-small-cell lung cancer: a randomized, open-label phase II studyClin Lung Cancer201011317618120439193
- CortesJRodriguezJAramendiaJMFront-line paclitaxel/cisplatin-based chemotherapy in brain metastases from non-small-cell lung cancerOncology2003641283512457029
- GuerrieriMWongKRyanGA randomised phase III study of palliative radiation with concomitant carboplatin for brain metastases from non-small cell carcinoma of the lungLung Cancer200446110711115364138
- UshioYAritaNHayakawaTChemotherapy of brain metastases from lung carcinoma: a controlled randomized studyNeurosurgery19912822012051997887
- VergerEGilMYayaRTemozolomide and concomitant whole brain radiotherapy in patients with brain metastases: a phase II randomized trialInt J Radiat Oncol Biol Phys200561118519115629610
- BearzAGarassinoITiseoMActivity of pemetrexed on brain metastases from non-small cell lung cancerLung Cancer201068226426819632738
- CeresoliGLCappuzzoFGregorcVGefitinib in patients with brain metastases from non-small-cell lung cancer: a prospective trialAnn Oncol20041571042104715205197
- KimJELeeDHChoiYEpidermal growth factor receptor tyrosine kinase inhibitors as a first-line therapy for never-smokers with adenocarcinoma of the lung having asymptomatic synchronous brain metastasisLung Cancer200965335135419157632
- NambaYKijimaTYokotaSGefitinib in patients with brain metastases from non-small-cell lung cancer: review of 15 clinical casesClin Lung Cancer20046212312815476598
- WuCLiYLWangZMGefitinib as palliative therapy for lung adenocarcinoma metastatic to the brainLung Cancer200757335936417434236
- WuYLZhouCChengYErlotinib as second-line treatment in patients with advanced non-small-cell lung cancer and asymptomatic brain metastases: a phase II study (CTONG-0803)Ann Oncol201324499399923129122
- NewtonCRGrahamAHeptinstallLEAnalysis of any point mutation in DNA. The amplification refractory mutation system (ARMS)Nucleic Acids Res1989177250325162785681
- GasparLScottCRotmanMRecursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trialsInt J Radiat Oncol Biol Phys19973747457519128946
- GasparLEScottCMurrayKCurranWValidation of the RTOG recursive partitioning analysis (RPA) classification for brain metastasesInt J Radiat Oncol Biol Phys20004741001100610863071
- SperdutoPWBerkeyBGasparLEMehtaMCurranWA new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG databaseInt J Radiat Oncol Biol Phys200870251051417931798
- ZimmSWamplerGLStableinDHazraTYoungHFIntracerebral metastases in solid-tumor patients: natural history and results of treatmentCancer19814823843947237407
- RudermanNBHallTCUse of glucocorticoids in the palliative treatment of metastaic brain tumorsCancer1965829830614264031
- MoscettiLNelliFFeliciAUp-front chemotherapy and radiation treatment in newly diagnosed nonsmall cell lung cancer with brain metastases: survey by Outcome Research Network for Evaluation of Treatment Results in OncologyCancer2007109227428117154161
- RobinetGThomasPBretonJLResults of a phase III study of early versus delayed whole brain radiotherapy with concurrent cisplatin and vinorelbine combination in inoperable brain metastasis of non-small-cell lung cancer: Groupe Francais de Pneu-mo-Cancerologie (GFPC) Protocol 95-91Ann Oncol2001121596711249050
- BarlesiFGervaisRLenaHPemetrexed and cisplatin as first-line chemotherapy for advanced non-small-cell lung cancer (NSCLC) with asymptomatic inoperable brain metastases: a multicenter phase II trial (GFPC 07-01)Ann Oncol201122112466247021321089
- WelshJWKomakiRAminiAPhase II trial of erlotinib plus concurrent whole-brain radiation therapy for patients with brain metastases from non-small-cell lung cancerJ Clin Oncol201331789590223341526
- MatsumotoSTakahashiKIwakawaRFrequent EGFR mutations in brain metastases of lung adenocarcinomaInt J Cancer200611961491149416642476
- MokTSWuYLThongprasertSGefitinib or carboplatin-paclitaxel in pulmonary adenocarcinomaN Engl J Med20093611094795719692680
- PortaRSanchez-TorresJMPaz-AresLBrain metastases from lung cancer responding to erlotinib: the importance of EGFR mutationEur Respir J201137362463120595147
- ParkSJKimHTLeeDHEfficacy of epidermal growth factor receptor tyrosine kinase inhibitors for brain metastasis in non-small cell lung cancer patients harboring either exon 19 or 21 mutationLung Cancer201277355656022677429
- MaSXuYDengQYuXTreatment of brain metastasis from non-small cell lung cancer with whole brain radiotherapy and gefitinib in a Chinese populationLung Cancer200965219820319091441
- BroniscerAPanettaJCO’ShaughnessyMPlasma and cerebrospinal fluid pharmacokinetics of erlotinib and its active metabolite OSI-420Clin Cancer Res20071351511151517332296
- ZhaoJChenMZhongWCerebrospinal fluid concentrations of gefitinib in patients with lung adenocarcinomaClin Lung Cancer201314218819322846582
- DaiHChenYElmquistWFDistribution of the novel antifolate pemetrexed to the brainJ Pharmacol Exp Ther2005315122222915987831
- SanghaviSNMiranpuriSSChappellRRadiosurgery for patients with brain metastases: a multi-institutional analysis, stratified by the RTOG recursive partitioning analysis methodInt J Radiat Oncol Biol Phys200151242643411567817
- FrazierJLBatraSKaporSStereotactic radiosurgery in the management of brain metastases: an institutional retrospective analysis of survivalInt J Radiat Oncol Biol Phys20107651486149219619958
- SperdutoPWChaoSTSneedPKDiagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patientsInt J Radiat Oncol Biol Phys201077365566119942357
- SperdutoPWKasedNRobergeDSummary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastasesJ Clin Oncol201230441942522203767