Abstract
The majority of patients with head and neck squamous cell carcinoma (HNSCC) present with locally advanced disease, which requires site-specific combinations of surgery, radiation, and chemotherapy. Despite aggressive therapy, survival outcomes remain poor, and treatment-related morbidity is not negligible. For patients with recurrent or metastatic disease, therapeutic options are further limited and prognosis is dismal. With this in mind, molecularly targeted therapy provides a promising approach to optimizing treatment efficacy while minimizing associated toxicity. The ErbB family of receptors (ie, epidermal growth factor receptor [EGFR], ErbB2/human epidermal growth factor receptor [HER]-2, ErbB3/HER3, and ErbB4/HER4) is known to contribute to oncogenic processes, such as cellular proliferation and survival. EGFR, specifically, is upregulated in more than 90% of HNSCC, has been implicated in radiation resistance, and correlates with poorer clinical outcomes. The central role of EGFR in the pathogenesis of HNSCC suggests that inhibition of this pathway represents an attractive treatment strategy. As a result, EGFR inhibition has been extensively studied, with the emergence of two classes of drug therapy: monoclonal antibodies and tyrosine kinase inhibitors. While the monoclonal antibody cetuximab is currently the only US Food and Drug Administration–approved EGFR inhibitor for the treatment of HNSCC, numerous investigational drugs are being evaluated in clinical trials. This paper will review the role of the ErbB family in the pathogenesis of HNSCC, as well as the evidence-based data for the use of ErbB family inhibition in clinical practice.
Introduction
Head and neck cancer is the seventh most common cancer worldwide.Citation1 In the United States, head and neck squamous cell carcinoma (HNSCC) accounts for 3% of cancers diagnosed annually and 2% of cancer-related deaths.Citation2 The 2014 estimates for the number of new cases of HNSCC and anticipated deaths from HNSCC in the United States are approximately 55,000 and 12,000, respectively.Citation2 Tobacco and alcohol use remain the strongest risk factors for HNSCC, act synergistically, and are implicated in the majority of diagnoses.Citation3,Citation4 Viral etiologies have also been implicated; specifically, Epstein–Barr virus is present in a significant proportion of nasopharyngeal cancers, whereas high-risk human papillomavirus (HPV) is now the primary cause of oropharyngeal cancers (OPCs).Citation5–Citation7
More than half the patients with HNSCC present with potentially curable, locally advanced (LA) disease, or disease that has spread to nearby tissue or lymph nodes, but has not metastasized.Citation8 Historically, surgery was the mainstay of treatment for HNSCC; however, the advent of functional organ preservation in the last few decades has shifted the treatment paradigm to include definitive chemoradiation (CRT). While early-stage disease is routinely treated with surgery or radiation (RT) alone, LA disease typically requires site-specific multimodal therapy.Citation9 Although survival rates improved over the last few decades, 30%–60% of patients still develop local recurrences, and approximately 20% develop distant metastases.Citation8 For patients with recurrent or metastatic (R/M) HNSCC, therapeutic options remain limited, and prognosis is dismal. The most active cytotoxic regimens are platinum-based and are associated with response rates (RRs) of up to 30% and median overall survival (OS) of 6–9 months.Citation10,Citation11
Unfavorable survival outcomes coupled with the toxicity of current treatments underscore the importance of incorporating targeted therapies within the treatment paradigm. Epidermal growth factor receptor (EGFR) is the most well-studied member of the ErbB family and is overexpressed in more than 90% of HNSCC.Citation12–Citation15 Furthermore, there is a correlation between increased EGFR levels and higher stage disease, increased lymph node metastasis, shorter relapse-free survival, and decreased OS.Citation12,Citation14–Citation18 Not surprisingly, targeting the ErbB family is an area of avid research. This paper focuses on the role of the ErbB family in the pathogenesis of HNSCC, and the clinical data evaluating ErbB family inhibition for the management of HNSCC.
Methods
To identify relevant clinical trials of ErbB family inhibitors in HNSCC, PubMed and ClinicalTrials.gov databases were searched using the key search terms or aliases “ErbB” and “HNSCC”. In addition, abstracts presented at the European Cancer Congress, European Society of Medical Oncology, and American Society of Clinical Oncology meetings were evaluated.
The ErbB family in HNSCC
The ErbB family consists of four transmembrane receptors, EGFR/ErbB1/human epidermal growth factor receptor (HER)-1, ErbB2/HER2/neu, ErbB3/HER3, and ErbB4/HER4.Citation19,Citation20 ErbB signaling activation begins with binding of natural ligands (typically epidermal growth factor [EGF] and transforming growth factor [TGF]-α) to EGFR, ErbB3, or ErbB4. ErbB2 has no known soluble ligands, but is the preferred heterodimerization partner for EGFR. Ligand binding leads to receptor homo- or heterodimerization with other ErbB family receptors (eg, ErbB2).Citation19,Citation20 Upon dimerization, intracellular tyrosine residues undergo autophosphorylation, triggering a cascade of downstream effects. Four primary signaling pathways have been implicated in downstream EGFR signaling: 1) phosphatidylinositol-3-kinase (PI3K)/v-akt murine thymoma viral oncogene homologue (Akt), 2) Ras/Raf/mitogen-activated protein kinase (MAPK), 3) phospholipase-C (PLC)-γ/protein kinase C (PKC), and 4) signal transducers and activators of transcription (STAT) pathways.Citation21 These pathways culminate in the transcription of genes involved in cellular proliferation, invasion, metastasis, cell survival, and angiogenesis ().Citation19–Citation22
Figure 1 ErbB family of receptors and their associated signaling pathways and downstream effects.
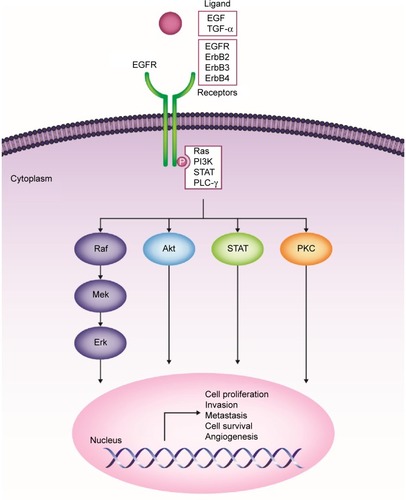
In HNSCC, increased ErbB expression has been linked to poor outcomes, including decreased OS, locoregional relapse, and treatment failure.Citation16,Citation23,Citation24 Biomarker analysis from a prospective Phase III trial demonstrated that high EGFR expression was associated with significantly shorter OS (P=0.0006) and disease-free survival (DFS; P=0.0016), and higher locoregional relapse rates (P=0.0031).Citation16
ErbB2 gene expression and ErbB3 protein expression have been linked to reduced treatment response and poor outcomes in laryngopharyngeal cancer.Citation23,Citation24 In a study that investigated molecular correlates of locoregional failure following CRT, overexpression of ErbB2 or MDM2 proto-oncogene, E3 ubiquitin protein ligase (MDM2) was identified as an independent predictor of decreased locoregional DFS.Citation23 Microarray analysis of samples from primary, metastatic, and recurrent HNSCC demonstrated that ErbB3 overexpression was more common in metastases than in primary lesions (P=0.003), was associated with shorter survival compared with negative ErbB3 levels (median survival, 22 vs 40 months; P=0.027), and was an independent prognostic predictor of OS (hazard ratio [HR], 1.51; 95% confidence interval [CI], 1.01–2.23; P=0.040]).Citation24 In patients with oral squamous cell carcinoma (SCC), combined expression of EGFR, ErbB2, and ErbB3 was more predictive of reduced survival, with ErbB2 demonstrating the strongest correlation.Citation17
ErbB family signaling and RT sensitization
ErbB signaling may modulate response to RT.Citation25,Citation26 EGFR overexpression has been linked to poor RT responses in glioblastoma multiformeCitation25 and SCC cell lines,Citation27,Citation28 and ErbB2 and ErbB3 expression have been associated with gefitinib resistance in HNSCC cell lines.Citation26
Several mechanisms may underlie the association between ErbB family members and responses to RT.Citation27–Citation29 In human SCC cell lines, ionizing RT stimulates kinase activity via ErbB receptors, resulting in downstream activation of intracellular proliferative pathways.Citation27–Citation29 In addition, cytoprotective pathways triggered via EGFR may increase cell survival in response to RT.Citation30 In human SCC cell lines, ionizing RT triggers ligand-independent caveolin-driven nuclear translocation of EGFR and formation of a complex with DNA-dependent protein kinase, thereby preventing DNA repair after RT exposure.Citation31 In addition, RT may allow tumor cells to circumvent EGF-mediated growth inhibition. RT exposure promotes entry of SCC cells into S- and G2/M phases after stimulation with EGF and ionizing RT, significantly increasing SCC proliferation in an EGFR-dependent manner;Citation27 this suggests that EGFR may play a role in post-RT tumor repopulation.Citation27,Citation32 Finally, EGFR overexpression has been implicated in fostering cancer stem cell survival, including expression of certain cancer stem cell genes and tumorsphere formation in HNSCC cell lines.Citation33
Clinical data on ErbB family inhibitors in HNSCC
There are two classes of available agents with anti-EGFR activity: monoclonal antibodies (mAbs) and tyrosine kinase inhibitors (TKIs). mAbs act at the receptor’s extracellular domain, whereas TKIs act on the cytosolic adenosine triphosphate–binding domain of EGFR to inhibit autophosphorylation.Citation34 Cetuximab is the first and only targeted therapy approved by the US Food and Drug Administration (FDA) for the treatment of HNSCC.Citation35 This agent has the most robust clinical data among ErbB family inhibitors and is routinely used in clinical practice. Other targeted agents are currently being investigated in HNSCC. Herein, we discuss Phase II and III data available for ErbB family inhibitors, including completed (–) and ongoing trials ().
Table 1 Selected Phase II and III trials of cetuximab in LA HNSCC
Table 3 Selected Phase II and III data on ErbB family inhibitors in LA and R/M HNSCC
Table 4 Selected ongoing clinical trials of ErbB family inhibitors in Phase II or III development for HNSCC
Approved and investigational anti-EGFR mAbs
Cetuximab
Cetuximab is an IgG1 chimeric (human–murine) mAb that competitively binds with high affinity to EGFR. Cetuximab has two FDA-approved indications: treatment of LA HNSCC (combined with RT) and R/M HNSCC (combined with platinum/5-fluorouracil or as monotherapy for platinum-refractory disease).Citation35–Citation37
Cetuximab for LA HNSCC
Bonner et alCitation36,Citation37 conducted a pivotal multinational, randomized, Phase III trial evaluating the addition of cetuximab to definitive RT for patients with stage III–IV, nonmetastatic HNSCC. Approximately 60% of patients had oropharyngeal primaries. Overall, cetuximab-RT had an acceptable toxicity profile, with the exception of acneiform rash and infusion-related events, which were more common with cetuximab. Median duration of locoregional control (primary endpoint) was better with cetuximab-RT vs RT alone (24.4 vs 14.9 months; P=0.005). Median OS was also improved with cetuximab-RT vs RT alone (49.0 vs 29.3 months; P=0.03).Citation36 After 5 years of follow-up, OS rate was 45.6% for cetuximab-RT vs 36.4% with RT alone (P=0.018). With cetuximab-RT, OS was significantly improved in patients who experienced grade ≥2 acneiform rash compared with patients who had no or grade 1 rash (HR, 0.49; 95% CI, 0.34–0.72; P=0.002).Citation37 Subset analyses demonstrated that the benefit of cetuximab was restricted to patients aged <65 years, or those with a good Karnofsky performance status (ie, 90–100).Citation37 Importantly, no head-to-head comparisons have evaluated cetuximab vs a platinum-based regimen concurrent with RT. The Radiation Therapy Oncology Group (RTOG) recently completed accrual of a randomized Phase III trial to address this comparison in HPV-positive oropharyngeal SCC (RTOG 1016; ). Therefore, cisplatin-based CRT is still widely accepted as standard treatment for patients with LA HNSCC, based on a meta-analysis of 17,346 patients that demonstrated an absolute 5-year survival benefit of 6.5% with concomitant CRT compared with RT alone.Citation38
The addition of cetuximab to definitive cisplatin-based CRT does not further improve survival. RTOG 0522 evaluated 891 patients with stage III–IV nonmetastatic HNSCC who were randomized to receive CRT (cisplatin 100 mg/m2 on days 1 and 22; RT 70–72 Gy) or the same regimen with weekly cetuximab.Citation39 At the third interim analysis, the conditional power was <10%, which triggered early reporting. The addition of cetuximab resulted in more frequent RT interruptions (26.9% vs 15.1% for CRT alone); mean cisplatin delivery was similar. Patients with p16-positive OPC had better 3-year probability of progression-free survival (PFS; 72.8% vs 49.2%; P<0.001) and OS (85.6% vs 60.1%; P<0.001) than patients with p16-negative OPC; EGFR expression did not distinguish outcome. Cetuximab had significantly higher rates of acute grade ≥3 mucositis, skin reactions, fatigue, anorexia, and hypokalemia; after 90 days, adverse event (AE) rates were similar.
Integration of cetuximab into a larynx preservation paradigm was evaluated in TREMPLIN, a randomized Phase II trial of 116 patients with stage III–IV laryngeal or hypopharyngeal SCC suitable for total laryngectomy.Citation40 After three cycles of induction chemotherapy (cisplatin/docetaxel/5-fluorouracil), further treatment was based on response to chemotherapy. Patients with <50% response received salvage surgery; patients with ≥50% response were randomized to definitive RT (70 Gy) with either high-dose cisplatin or concurrent cetuximab (400 mg/m2 loading dose, then 250 mg/m2 weekly). Treatment compliance was higher with cetuximab (71% completed all weekly doses) vs cisplatin (42% received all three doses). There was no difference in acute grade ≥3 mucositis (43% in each arm), but grade ≥3 in-field dermatitis was more common with cetuximab (57% vs 26%). In an intent-to-treat analysis, there was no difference in larynx preservation at 3 months (primary endpoint; 95% with cisplatin vs 93% with cetuximab), larynx function preservation (87% vs 82%), and OS at 18 months (92% vs 89%). Locoregional failure rate (median follow-up, 36 months) was 13.3% with cisplatin and 21.4% with cetuximab. However, due to the increased locoregional failure rate with cetuximab, more salvage laryngectomies were performed in the cetuximab arm, ultimately resulting in similar locoregional failure rates (13.3% vs 10.7%). There was no difference in 2-year laryngoesophageal dysfunction–free survival rate, a composite endpoint included after the study was designed (79% vs 72%).Citation40
Additional studies of cetuximab integrated into standard platinum-based CRT or with RT alone in the induction or adjuvant settings are summarized in ; ongoing trials with RT are listed in .
Cetuximab for R/M HNSCC
The proof-of-principle trial of cetuximab as first-line treatment for R/M HNSCC was published in 2005.Citation41 This Eastern Cooperative Oncology Group randomized, multi-institutional, placebo-controlled, Phase III trial of 117 evaluable patients evaluated cisplatin (100 mg/m2 every 4 weeks) with cetuximab (400 mg/m2 loading dose, then 250 mg/m2 weekly) or placebo. Significant improvement in RR was observed with cetuximab (26% vs 10%; P=0.03). While cetuximab had better median PFS (4.2 vs 2.7 months) and OS (9.2 vs 8.0 months), these findings were not statistically significant. The trial, however, was not adequately powered for survival.
Based on these findings, the EXTREME trial confirmed the benefit of adding cetuximab to chemotherapy as first-line treatment for R/M HNSCC.Citation42 Four hundred forty-two patients were randomized to cisplatin (100 mg/m2) or carboplatin (5 mg/mL/min) on day 1, followed by 5-fluorouracil 1,000 mg/m2 daily for 4 days, every 3 weeks for a maximum of six cycles, or the same chemotherapy plus cetuximab (400 mg/m2 loading dose, then 250 mg/m2 weekly). Patients in the cetuximab arm with response or stable disease received maintenance cetuximab until disease progression or unacceptable toxicity. Crossover was not allowed. Median OS was 7.4 months with chemotherapy alone vs 10.1 months with cetuximab (P=0.04). Addition of cetuximab also prolonged median PFS (from 3.3 to 5.6 months; P<0.001) and RR (from 20% to 36%; P<0.001). These clinical benefits were not associated with adverse quality of life. Of 219 patients receiving cetuximab, 9% had grade 3 skin reactions and 3% had grade ≥3 infusion reactions; there were no cetuximab-related deaths. A subset analysis suggested greater benefit for patients aged <65 years and those who had better performance status or received cisplatin. Additional trials have evaluated cetuximab in the first-line setting and for platinum-refractory R/M HNSCC ().
Table 2 Selected Phase II and III trials of cetuximab in R/M HNSCC
Panitumumab
Panitumumab is a fully human IgG2 mAb with high affinity for EGFR.Citation43 Unlike cetuximab, panitumumab’s human structure results in minimal infusion-related reactions. Results of the SPECTRUM trial were recently published.Citation44 This was a randomized, multinational, Phase III trial of 657 patients with R/M HNSCC who received cisplatin (100 mg/m2 on day 1) and 5-fluorouracil (1,000 mg/m2 daily on days 1–4) every 3 weeks with or without panitumumab (9 mg/kg on day 1) until disease progression or for a maximum of six cycles. Patients without disease progression could continue receiving panitumumab maintenance after the initial six cycles of chemotherapy. Crossover was not allowed. There was no significant difference in median OS (primary endpoint; 11.1 vs 9.0 months; P=0.14). Panitumumab did prolong median PFS by 1.2 months (5.8 vs 4.6 months; P=0.004). Several grade ≥3 toxicities were more frequent with panitumumab, including skin or eye toxicity, diarrhea, hypomagnesemia, and cardiac arrhythmias. There were also more treatment-related deaths with panitumumab (14 [4%] patients) vs chemotherapy (8 [2%] patients). A predefined subanalysis evaluating the prognostic implication of HPV status was performed; however, direct comparisons with other trials may be confounded by the low p16 cutoff threshold (10%) that was utilized. Furthermore, approximately half of p16-positive tumors involved nonoropharyngeal primaries, for which the relative importance of HPV status remains to be defined.Citation45 The randomized Phase II PARTNER trial preliminarily demonstrated improved PFS and RR with docetaxel/cisplatin plus panitumumab vs docetaxel/cisplatin alone as first-line treatment for R/M HNSCC, but with an increased frequency of grade 3/4 AEs (73% vs 56%).Citation46 For LA HNSCC, the randomized Phase II CONCERT-2 trial of 151 patients receiving panitumumab/RT vs CRT demonstrated trends favoring CRT for 2-year locoregional control (primary endpoint; 51% with panitumumab/RT vs 61% with CRT), PFS (P=0.03), and OS (P=0.10); rates of grade ≥3 AEs were similar (85% vs 81%).Citation47 More recently, results from the National Cancer Institute of Canada Clinical Trials Group HN.6 Phase III trial of panitumumab/RT (accelerated fractionation) vs cisplatin/RT (standard fractionation) in LA HNSCC were presented, which failed to establish noninferiority for the primary endpoint of 2-year PFS (76% vs 73%; HR, 0.95; 95% CI, 0.6–1.5; P=0.83) and showed a similar grade >3 nonhematologic AE rate (91% vs 88%; P=0.25).Citation48 In a separately presented quality of life analysis, significant differences favoring the panitumumab arm were seen during the last week of RT; however, there was no durable quality of life benefit relative to cisplatin/RT.Citation49 A Phase II study of panitumumab/chemotherapy vs chemotherapy alone in R/M HNSCC (NCT00756444) was recently completed and data are forthcoming. Several trials of panitumumab for R/M and LA HNSCC are ongoing ().
Zalutumumab
Zalutumumab is a fully human IgG1 mAb that targets EGFR domain III and inhibits binding of EGF and TGF-α to EGFR.Citation50 Zalutumumab also prevents conformational changes in EGFR that are necessary for its activation.Citation50 An open-label, randomized, Phase III trial investigated zalutumumab plus best supportive care (BSC) vs BSC alone in 286 patients with R/M HNSCC after failure of platinum-based chemotherapy.Citation51 Zalutumumab prolonged median PFS compared with BSC alone (9.9 vs 8.4 weeks; P=0.0012). However, the trial failed to meet its primary endpoint of improved median OS (6.7 vs 5.2 months; P=0.06). The most frequent grade ≥3 AEs with zalutumumab were rash, anemia, and pneumonia.Citation51 Although Genmab (Princeton, NJ, USA) suspended clinical development of zalutumumab in 2011,Citation50 there is an ongoing Phase III trial evaluating zalutumumab combined with definitive CRT for pharyngeal and laryngeal primaries (NCT00496652 [DAHANCA 19]). Preliminary results reported no increase in locoregional control, disease-specific survival, or OS with the addition of zalutumumab to CRT.Citation52
Nimotuzumab
Nimotuzumab is a fully humanized IgG1 mAb that binds domain III of EGFR.Citation53 Unlike zalutumumab, nimotuzumab prevents ligand binding, but not conformational receptor changes.Citation54 A Phase II, randomized, placebo-controlled, double-blinded trial compared nimotuzumab-RT with placebo-RT in 106 patients with LA HNSCC who were medically unfit for standard CRT.Citation55 The primary endpoint of complete RR was met (59.5% for nimotuzumab-RT vs 34.2% for RT; P=0.04). The intent-to-treat analysis demonstrated a nonsignificant trend toward improved median OS with nimotuzumab-RT (12.5 vs 9.47 months). However, in a research site–specific subanalysis of 88 patients, nimotuzumab-RT was associated with significant OS benefit (median 14.0 vs 8.83 months; P=0.02). Finally, an analysis of median OS by EGFR status showed that it was significantly longer for patients with EGFR-positive tumors who were receiving nimotuzumab vs placebo (16.5 vs 7.2 months; P=0.004). There was no survival advantage for patients with EGFR-negative tumors. No grade ≥3 AEs or skin toxicity were observed.Citation55 Another study linking nimotuzumab-elicited outcomes with EGFR expression was a randomized, multicenter, Phase IIb study that divided 92 patients with LA HNSCC into two treatment groups (CRT vs RT for those with poor performance status), further stratified by whether they received nimotuzumab or placebo.Citation56 Patients receiving nimotuzumab-CRT had a significantly higher median OS than those receiving placebo-CRT (>30 months vs 22 months; P<0.003). There was a significant correlation between EGFR expression and improved OS in the nimotuzumab-CRT arm (P=0.02), which remained significant at 24 months (P=0.01).Citation56 Recently, preliminary results of a Phase II trial of 56 patients with LA HNSCC who were randomized to CRT with or without nimotuzumab demonstrated significantly higher RR with nimotuzumab vs CRT alone (96% vs 72%; P=0.02).Citation57 Furthermore, there was no potentiation of treatment-related toxicity, suggesting nimotuzumab could be safely added to standard CRT.
A Phase II study of nimotuzumab/chemotherapy vs chemotherapy alone in LA HNSCC (NCT01425736) was recently completed and data are forthcoming. Several ongoing Phase II and III trials evaluating nimotuzumab for treatment of LA HNSCC are summarized in .
MEHD7945A and Sym004
MEHD7945A, a first-in-class human IgG1 mAb targeting EGFR and ErbB3/HER3,Citation58–Citation60 will be evaluated vs cetuximab in a Phase II trial in R/M HNSCC (NCT01577173). Sym004, a novel anti-EGFR therapy containing 2 mAbs targeting nonoverlapping epitopes in domain III,Citation61 was evaluated in a Phase II study of 26 heavily pretreated patients with R/M HNSCC who developed resistance to anti-EGFR mAb-based therapy.Citation62 Preliminary findings revealed tumor shrinkage in 8 patients, while 14 had stable disease; median PFS was 82 days. Skin rash was reported by 96% of patients, including 42% with grade ≥3.
Investigational ErbB family TKIs
While several oral, small-molecule, ErbB family TKIs are being evaluated, none have been approved for HNSCC at the time of publication.
Gefitinib
Gefitinib is a reversible EGFR TKI.Citation63 Based on results from Phase III trials demonstrating that gefitinib has limited activity compared with chemotherapy for R/M HNSCC,Citation64,Citation65 there are no known plans for further development of gefitinib in HNSCC.
Erlotinib
Erlotinib is another reversible EGFR TKI.Citation66,Citation67 In LA HNSCC, erlotinib has demonstrated modest activity as neoadjuvant monotherapy,Citation68 combined with definitive CRT,Citation69 and with definitive bevacizumab-CRT.Citation70 Another Phase II trial, however, demonstrated no improvement in complete RR or PFS when adding erlotinib to definitive CRT for LA HNSCC.Citation71
For R/M HNSCC, Phase II data with erlotinib suggest antitumor activity with acceptable tolerability. Erlotinib monotherapy in 115 patients with R/M HNSCC, regardless of HER1/EGFR status, demonstrated an RR of 4.3% (all partial responses).Citation72 There were no differences in PFS or OS in subgroup analyses; however, patients with grade ≥2 rash had significantly higher OS (P=0.045). Skin rash and diarrhea were the most frequently reported drug-related toxicities. A Phase I/II trial of 45 patients receiving cisplatin and erlotinib for R/M HNSCC demonstrated an RR of 21%, median PFS of 3.3 months, and median OS of 7.9 months.Citation73 There was minimal grade ≥3 toxicity. A Phase II study of 50 patients receiving erlotinib in combination with cisplatin/docetaxel for R/M HNSCC demonstrated an RR of 67% and disease control rate (DCR) of 95%.Citation74 Median OS and PFS at 19 months of follow-up were 11 and 6 months, respectively. Ongoing Phase II trials evaluating erlotinib with CRT for LA HNSCC and with chemotherapy followed by maintenance in R/M HNSCC are summarized in .
Lapatinib
Lapatinib is a reversible EGFR and ErbB2/HER2 TKI.Citation75,Citation76 A randomized, placebo-controlled, Phase II trial of lapatinib monotherapy followed by definitive CRT demonstrated clinical activity (RR, 17% vs 0% with placebo) in 107 patients with treatment-naïve LA HNSCC.Citation77 In the R/M HNSCC setting, however, a Phase II trial of 45 patients receiving lapatinib monotherapy demonstrated good tolerability but no responses.Citation78 Evaluation of lapatinib in Phase II trials with induction chemotherapy was discouraged after Phase I results demonstrated unacceptable toxicities (predominantly renal failure) when combined with standard induction regimens for LA laryngeal and hypopharyngeal SCC.Citation79 A recently completed placebo-controlled Phase III trial of adjuvant lapatinib plus CRT followed by 1 year of lapatinib maintenance in patients with resected, high-risk HNSCC did not improve DFS.Citation80 Ongoing Phase II trials are evaluating lapatinib with definitive CRT followed by 1 year of lapatinib maintenance for LA HNSCC (NCT00387127) and definitive RT for LA HNSCC in patients who cannot tolerate concurrent CRT (NCT00490061).
Afatinib
Afatinib is an irreversible ErbB family inhibitor (targets include EGFR, ErbB2/HER2, and ErbB4/HER4).Citation81,Citation82 Five Phase III studies are evaluating afatinib for LA HNSCC as adjuvant therapy and for R/M HNSCC as monotherapy or in combination with chemotherapy ( and ). In the LUX-Head & Neck 1 trial of afatinib vs methotrexate in R/M HNSCC after failure of platinum-based therapy, afatinib was associated with a significant improvement in the primary endpoint of PFS compared with methotrexate (2.6 vs 1.7 months; P=0.030); OS was not improved (P=0.70).Citation83 The objective RR was 10% with afatinib (vs 6% with methotrexate), and DCR was 49% (vs 39%). PFS benefit was associated with positive patient-reported outcomes, with afatinib-treated patients reporting less pain, improved swallowing, and delayed deterioration of global health status. In subgroup analyses, patients with p16-negative non-OPC and local recurrence (rather than metastasis) without prior EGFR-targeted mAb therapy seemed to derive the most benefit from afatinib. The most common grade 3/4 treatment-related AEs were rash/acne (10%) and diarrhea (9%). A more recently presented biomarker analysis found a propensity for greater PFS benefit with afatinib vs methotrexate in the settings of p16-negative (2.7 vs 1.6 months; HR, 0.70; P=0.029), PTEN-high (2.9 vs 1.4 months; HR, 0.36; P=0.014), HER3-low (2.9 vs 2.0 months; HR, 0.47; P=0.014), and EGFR-amplified (2.8 vs 2.2 months; HR, 0.64; P=0.162) disease.Citation84 Final results of a randomized, open-label, Phase II study that compared afatinib to cetuximab in 124 patients with platinum-refractory R/M HNSCC were recently published.Citation85 In stage I, patients were randomized to daily afatinib or weekly cetuximab until disease progression or unacceptable toxicity, at which time crossover was permitted (stage II). Stage I results demonstrated comparable antitumor activity (tumor shrinkage, RR) and median PFS (13.0 weeks with afatinib vs 15.0 weeks with cetuximab; P=0.71). Approximately half (56%) the patients crossed over to the other treatment arm (stage II); disease progression was the primary reason. DCR by independent central review was 33% for afatinib (vs 19% with cetuximab), and median PFS was 9.3 weeks (vs 5.7 weeks) during stage II. Grade ≥3 toxicities were more frequent in patients treated with afatinib (47% vs 16%). The authors concluded that patients may benefit from sequential therapy, especially treatment with afatinib after cetuximab failure.Citation85 Other Phase II trials of afatinib include one in a neoadjuvant setting (NCT01538381 [EORTC NOCI-HNCG]), another to evaluate potential biomarkers (NCT01415674 [PREDICTOR]), and another in HPV-negative LA HNSCC as a component of induction chemotherapy (NCT01732640).
Dacomitinib
Dacomitinib is an irreversible TKI that targets EGFR, ErbB2/HER2, and ErbB4/HER4.Citation86 A Phase II study of dacomitinib monotherapy in 69 patients with R/M HNSCC demonstrated an RR of 12.7%; median PFS and OS were 12.1 and 34.6 weeks, respectively.Citation87 Diarrhea, acneiform dermatitis, and fatigue were the most frequent grade ≥3 AEs. An ongoing placebo-controlled, Phase I/II study seeks to identify biomarker modulations associated with dacomitinib treatment when given preoperatively for resectable oral cavity HNSCC (NCT01116843).
Vandetanib
Vandetanib is a multitargeted TKI, including EGFR and vascular endothelial growth factor receptor 2.Citation88,Citation89 Preliminary results of vandetanib plus docetaxel (n=15) vs docetaxel alone (n=14) for R/M HNSCC demonstrated a partial RR of 13% with vandetanib plus docetaxel vs 7% with docetaxel alone, and a median PFS of 9 vs 3.2 weeks; serious AEs were comparable between arms.Citation90 A Phase II trial of vandetanib with adjuvant CRT in high-risk, stage III–IV HNSCC was terminated early due to withdrawal of study drug; as only 34 of 170 planned patients were accrued, no analysis was performed (NCT00720083).
Perspectives
EGFR overexpression and its key role in HNSCC carcinogenesis make EGFR inhibition a promising molecular treatment strategy. Two classes of ErbB inhibitors are available: mAbs and small-molecule TKIs. To date, cetuximab remains the only FDA-approved ErbB family inhibitor for HNSCC. For LA disease, cetuximab is approved in combination with definitive RT; however, studies are ongoing to provide direct comparisons with platinum-based regimens. In R/M disease, cetuximab is approved both in combination with platinum-containing regimens and as monotherapy for platinum-refractory disease. The limited effect of other EGFR inhibitors in HNSCC could be explained by the different mechanisms of action of mAbs and TKIs. Notably, cetuximab has been shown to elicit an antibody-dependent cellular cytotoxicity response that is dependent on EGFR expression levels in HNSCC.Citation91,Citation92 Overexpression of EGFR and other ErbB family receptors, ErbB ligands, and downstream pathway components in HNSCC may promote positive feedback of the pathway.Citation93 In cell lines, kinase-inactive EGFR can dimerize with ErbB2 and activate signaling downstream of EGFR, suggesting that the presence of EGFR is important for promoting cell survival, even in the absence of EGFR kinase activity.Citation94 Kinase-inactive EGFR has also been shown to physically interact with several cancer-related proteins, including Axl and ephrin type-A receptor 2.Citation95 Furthermore, EGFR has been shown to have kinase-independent roles in maintaining intracellular glucose levels and initiating autophagy, both of which contribute to increased cell survival.Citation96,Citation97 This evidence for functions of EGFR beyond its tyrosine kinase role may partially explain the lack of substantial efficacy of EGFR TKIs in EGFR-overexpressing cancers like HNSCC.
Because EGFR mutations are rarely detected in HNSCC,Citation93 there is also a need to identify biomarkers to predict those patients most likely to benefit from EGFR-targeted agents, and lack of patient selection may partially explain the minimal responses observed thus far with the majority of EGFR inhibitors tested in HNSCC. Rash has been suggested to be a biomarker for EGFR inhibitor response and has been associated with improved outcomes in several tumor types, including HNSCC.Citation98 In two HNSCC trials, statistically significant improvements in OS have been observed in patients who developed grade ≥2 skin rash following either erlotinib or cetuximab treatment compared with patients who developed no or grade 1 skin rash.Citation37,Citation72 Similarly, in a trial evaluating gefitinib in patients with R/M HNSCC, grade of skin toxicity positively correlated with DCR, PFS, and OS.Citation99 Although the mechanism by which EGFR inhibitors cause dermatological toxicity is not fully understood, there is evidence to suggest that immune cell infiltration and inhibition of EGFR homodimer signaling may be associated with these skin toxicities.Citation100,Citation101
Conclusion
Although ErbB family members represent valid therapeutic targets in HNSCC, the modest RR seen with ErbB family inhibitors illustrates the need for continued research to identify potential resistance mechanisms and biomarkers for response. A detailed understanding of the role this family plays in the pathogenesis of HNSCC is critical so that we may further exploit this promising treatment strategy in our effort to maximize patient survival.
Author contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in either drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Acknowledgments
The authors received no direct compensation related to the development of the manuscript. Writing, editorial support, and formatting assistance were provided by Melissa Brunckhorst, PhD, of MedErgy, which was contracted and funded by Boehringer Ingelheim Pharmaceuticals, Inc. (BIPI). BIPI was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations.
Disclosure
The authors report no conflicts of interest in this work.
References
- FerlayJSoerjomataramIDikshitRCancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012Int J Cancer20151365E359E38625220842
- American Cancer SocietyCancer Facts & Figures, 2014Atlanta, GAAmerican Cancer Society2014
- ArgirisAKaramouzisMVRabenDFerrisRLHead and neck cancerLancet200837196251695170918486742
- BlotWJMcLaughlinJKWinnDMSmoking and drinking in relation to oral and pharyngeal cancerCancer Res19884811328232873365707
- GillisonMLChaturvediAKLowyDRHPV prophylactic vaccines and the potential prevention of noncervical cancers in both men and womenCancer200811310 Suppl3036304618980286
- SturgisEMCinciripiniPMTrends in head and neck cancer incidence in relation to smoking prevalence: an emerging epidemic of human papillomavirus-associated cancers?Cancer200711071429143517724670
- KleinGThe relationship of the virus to nasopharyngeal carcinomaEpsteinMAchongBThe Epstein-Barr VirusBerlin, GermanySpringer-Verlag1979339350
- SeiwertTYCohenEEState-of-the-art management of locally advanced head and neck cancerBr J Cancer20059281341134815846296
- National Comprehensive Cancer NetworkNCCN Clinical Practice Guidelines in Oncology™Head and Neck Cancers. Version 22014 Available from: http://www.nccn.org/professionals/physician_gls/PDF/head-and-neck.pdfAccessed September 22, 2014
- GibsonMKLiYMurphyBRandomized phase III evaluation of cisplatin plus fluorouracil versus cisplatin plus paclitaxel in advanced head and neck cancer (E1395): an intergroup trial of the Eastern Cooperative Oncology GroupJ Clin Oncol200523153562356715908667
- ColevasADChemotherapy options for patients with metastatic or recurrent squamous cell carcinoma of the head and neckJ Clin Oncol200624172644265216763278
- DassonvilleOFormentoJLFrancoualMExpression of epidermal growth factor receptor and survival in upper aerodigestive tract cancerJ Clin Oncol19931110187318788410112
- Rubin GrandisJMelhemMFBarnesELTweardyDJQuantitative immunohistochemical analysis of transforming growth factor-alpha and epidermal growth factor receptor in patients with squamous cell carcinoma of the head and neckCancer1996786128412928826952
- SantiniJFormentoJLFrancoualMCharacterization, quantification, and potential clinical value of the epidermal growth factor receptor in head and neck squamous cell carcinomasHead Neck19911321321392022478
- Rubin GrandisJMelhemMFGoodingWELevels of TGF-alpha and EGFR protein in head and neck squamous cell carcinoma and patient survivalJ Natl Cancer Inst199890118248329625170
- AngKKBerkeyBATuXImpact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinomaCancer Res200262247350735612499279
- XiaWLauYKZhangHZCombination of EGFR, HER-2/neu, and HER-3 is a stronger predictor for the outcome of oral squamous cell carcinoma than any individual family membersClin Cancer Res19995124164417410632356
- O-charoenratPRhys-EvansPHArcherDJEcclesSAC-erbB receptors in squamous cell carcinomas of the head and neck: clinical significance and correlation with matrix metalloproteinases and vascular endothelial growth factorsOral Oncol2002381738011755824
- BazleyLAGullickWJThe epidermal growth factor receptor familyEndocr Relat Cancer200512Suppl 1S17S2716113093
- HynesNELaneHAERBB receptors and cancer: the complexity of targeted inhibitorsNat Rev Cancer20055534135415864276
- EgloffAMGrandisJRTargeting epidermal growth factor receptor and SRC pathways in head and neck cancerSemin Oncol200835328629718544443
- BaselgaJArteagaCLCritical update and emerging trends in epidermal growth factor receptor targeting in cancerJ Clin Oncol200523112445245915753456
- GanlyITalbotSCarlsonDIdentification of angiogenesis/metastases genes predicting chemoradiotherapy response in patients with laryngopharyngeal carcinomaJ Clin Oncol200725111369137617416856
- TakikitaMXieRChungJYMembranous expression of Her3 is associated with a decreased survival in head and neck squamous cell carcinomaJ Transl Med2011912621801427
- BarkerFGSimmonsMLChangSMEGFR overexpression and radiation response in glioblastoma multiformeInt J Radiat Oncol Biol Phys200151241041811567815
- ErjalaKSundvallMJunttilaTTSignaling via ErbB2 and ErbB3 associates with resistance and epidermal growth factor receptor (EGFR) amplification with sensitivity to EGFR inhibitor gefitinib in head and neck squamous cell carcinoma cellsClin Cancer Res200612134103411116818711
- Schmidt-UllrichRKMikkelsenRBDentPRadiation-induced proliferation of the human A431 squamous carcinoma cells is dependent on EGFR tyrosine phosphorylationOncogene19971510119111979294612
- BowersGReardonDHewittTThe relative role of ErbB1-4 receptor tyrosine kinases in radiation signal transduction responses of human carcinoma cellsOncogene200120111388139711313882
- ContessaJNHamptonJLammeringGIonizing radiation activates Erb-B receptor dependent Akt and p70 S6 kinase signaling in carcinoma cellsOncogene200221254032404112037685
- DittmannKMayerCFehrenbacherBRadiation-induced epidermal growth factor receptor nuclear import is linked to activation of DNA-dependent protein kinaseJ Biol Chem200528035311823118916000298
- DittmannKMayerCKehlbachRRodemannHPRadiation-induced caveolin-1 associated EGFR internalization is linked with nuclear EGFR transport and activation of DNA-PKMol Cancer200876918789131
- KavanaghBDLinPSChenPSchmidt-UllrichRKRadiation-induced enhanced proliferation of human squamous cancer cells in vitro: a release from inhibition by epidermal growth factorClin Cancer Res1995112155715629815956
- AbholdELKiangARahimyEEGFR kinase promotes acquisition of stem cell-like properties: a potential therapeutic target in head and neck squamous cell carcinoma stem cellsPLoS One201272e3245922384257
- BozecAPeyradeFFischelJLMilanoGEmerging molecular targeted therapies in the treatment of head and neck cancerExpert Opin Emerg Drugs200914229931019519286
- ERBITUX® (cetuximab) injection, for intravenous infusion [package insert]Branchburg, NJImClone LLC2013
- BonnerJAHarariPMGiraltJRadiotherapy plus cetuximab for squamous-cell carcinoma of the head and neckN Engl J Med2006354656757816467544
- BonnerJAHarariPMGiraltJRadiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survivalLancet Oncol2010111212819897418
- PignonJPle MaîtreAMaillardEBourhisJMeta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patientsRadiother Oncol200992141419446902
- AngKKZhangQRosenthalDIRandomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522J Clin Oncol201432272940295025154822
- LefebvreJLPointreauYRollandFInduction chemotherapy followed by either chemoradiotherapy or bioradiotherapy for larynx preservation: the TREMPLIN randomized phase II studyJ Clin Oncol201331785385923341517
- BurtnessBGoldwasserMAFloodWMattarBForastiereAAPhase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer: an Eastern Cooperative Oncology Group studyJ Clin Oncol200523348646865416314626
- VermorkenJBMesiaRRiveraFPlatinum-based chemotherapy plus cetuximab in head and neck cancerN Engl J Med2008359111116112718784101
- YangXDJiaXCCorvalanJRWangPDavisCGDevelopment of ABX-EGF, a fully human anti-EGF receptor monoclonal antibody, for cancer therapyCrit Rev Oncol Hematol2001381172311255078
- VermorkenJBStohlmacher-WilliamsJDavidenkoICisplatin and fluorouracil with or without panitumumab in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck (SPECTRUM): an open-label phase 3 randomised trialLancet Oncol201314869771023746666
- ChungCIsaranuwatchaiWDi MaioMEconomic analysis of TORCH: erlotinib versus cisplatin and gemcitabine as first-line therapy for advanced non-small cell lung cancer (NSCLC)J Clin Oncol201331Suppl Abstract e19031
- WirthLJDakhilSRKornekGPARTNER: a randomized phase II study of docetaxel/cisplatin (doc/cis) chemotherapy with or without panitumumab (pmab) as first-line treatment (tx) for recurrent or metastatic squamous cell carcinoma of the head and neck (R/M SCCHN)J Clin Oncol201331Suppl Abstract 6029
- GiraltJTrigoJMNuytsSPhase 2, randomized trial (CONCERT-2) of panitumumab (PMAB) plus radiotherapy (PRT) compared with chemoradiotherapy (CRT) in patients (PTS) with unresected, locally advanced squamous cell carcinoma of the head and neck (LASCCHN) [Abstract 1016O]Ann Oncol201223Suppl 9ix334
- SiuLLWaldronJNChenBEPhase III randomized trial of standard fractionation radiotherapy (SFX) with concurrent cisplatin (CIS) versus accelerated fractionation radiotherapy (AFX) with panitumumab (PMab) in patients (pts) with locoregionally advanced squamous cell carcinoma of the head and neck (LA-SCCHN): NCIC Clinical Trials Group HN.6 trialJ Clin Oncol201533Suppl Abstract 6000
- RingashJWaldronJNSiuLLQuality of life (QOL) in a phase III randomized trial of standard fractionation radiotherapy (SFX) with concurrent cisplatin (CIS) versus accelerated fractionation radiotherapy (AFX) with panitumumab (PMab) in patients (pts) with locoregionally advanced squamous cell carcinoma of the head and neck (LA-SCCHN): NCIC Clinical Trials Group HN.6 (NCT00820248)J Clin Oncol201533Suppl Abstract 6053
- SchickUGujralDMRichardsTMHarringtonKJNuttingCMZalutumumab in head and neck cancerExpert Opin Biol Ther201212111912522171666
- MachielsJPSubramanianSRuzsaAZalutumumab plus best supportive care versus best supportive care alone in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck after failure of platinum-based chemotherapy: an open-label, randomised phase 3 trialLancet Oncol201112433334321377930
- EriksenJGMaareCJohansenJDAHANCA 19: first results of a randomized phase III study of the importance of the EGFR-inhibitor zalutumumab for the outcome of primary curative radiotherapy for squamous cell carcinoma of the head and neckLate breaking abstract presented at: The European Cancer CongressSeptember 27–October 1, 2013Amsterdam, Netherlands Abstract 12
- BolandWKBebbGNimotuzumab: a novel anti-EGFR monoclonal antibody that retains anti-EGFR activity while minimizing skin toxicityExpert Opin Biol Ther2009991199120619624281
- TalaveraAFriemannRGomez-PuertaSNimotuzumab, an antitumor antibody that targets the epidermal growth factor receptor, blocks ligand binding while permitting the active receptor conformationCancer Res200969145851585919584289
- RodriguezMORiveroTCdel CastilloBRNimotuzumab plus radiotherapy for unresectable squamous-cell carcinoma of the head and neckCancer Biol Ther20109534334920448462
- BasavarajCSierraPShivuJMelarkodeRMonteroENairPNimotuzumab with chemoradiation confers a survival advantage in treatment-naive head and neck tumors over expressing EGFRCancer Biol Ther201010767368120647773
- BhatnagarARSinghDPSharmaRA comparative study of monoclonal antibody against EGFR (nimotuzumab) used in combination with chemoradiation versus chemoradiation alone in the treatment of locally advanced inoperable squamous cell carcinoma of the head and neckJ Clin Oncol201230Suppl Abstract e16012
- KamathAVLuDGuptaPPreclinical pharmacokinetics of MEHD7945A, a novel EGFR/HER3 dual-action antibody, and prediction of its human pharmacokinetics and efficacious clinical doseCancer Chemother Pharmacol20126941063106922203367
- SchaeferGHaberLCrockerLMA two-in-one antibody against HER3 and EGFR has superior inhibitory activity compared with mono-specific antibodiesCancer Cell201120447248622014573
- HuangSLiCArmstrongEADual targeting of EGFR and HER3 with MEHD7945A overcomes acquired resistance to EGFR inhibitors and radiationCancer Res201373282483323172311
- PedersenMWJacobsenHJKoefoedKSym004: a novel synergistic anti-epidermal growth factor receptor antibody mixture with superior anticancer efficacyCancer Res201070258859720068188
- MachielsJ-PHSpecenierPMKraussJSym004, a novel strategy to target EGFR with an antibody mixture, in patients with advanced SCCHN progressing after anti-EGFR monoclonal antibody: a proof of concept studyJ Clin Oncol201331Suppl Abstract 6002
- AdamoVFranchinaTAdamoBGefitinib in lung cancer therapy: Clinical results, predictive markers of response and future perspectivesCancer Biol Ther20098320621219182534
- StewartJSCohenEELicitraLPhase III study of gefitinib compared with intravenous methotrexate for recurrent squamous cell carcinoma of the head and neckJ Clin Oncol200927111864187119289630
- ArgirisAGhebremichaelMGilbertJPhase III randomized, placebo-controlled trial of docetaxel with or without gefitinib in recurrent or metastatic head and neck cancer: an Eastern Cooperative Oncology Group trialJ Clin Oncol201331111405141423460714
- CohenMHJohnsonJRChenYFSridharaRPazdurRFDA drug approval summary: erlotinib (Tarceva) tabletsOncologist200510746146616079312
- Tarceva® (erlotinib tablets) [package insert]South San Francisco, CAGenentech, Inc2013
- ThomasFRochaixPBenlyazidAPilot study of neoadjuvant treatment with erlotinib in nonmetastatic head and neck squamous cell carcinomaClin Cancer Res200713237086709218056187
- HerchenhornDDiasFLViegasCMPhase I/II study of erlotinib combined with cisplatin and radiotherapy in patients with locally advanced squamous cell carcinoma of the head and neckInt J Radiat Oncol Biol Phys201078369670220421154
- HainsworthJDSpigelDRGrecoFACombined modality treatment with chemotherapy, radiation therapy, bevacizumab, and erlotinib in patients with locally advanced squamous carcinoma of the head and neck: a phase II trial of the Sarah Cannon oncology research consortiumCancer J201117526727221952273
- MartinsRGParvathaneniUBaumanJECisplatin and radiotherapy with or without erlotinib in locally advanced squamous cell carcinoma of the head and neck: a randomized phase II trialJ Clin Oncol201331111415142123460709
- SoulieresDSenzerNNVokesEEHidalgoMAgarwalaSSSiuLLMulticenter phase II study of erlotinib, an oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with recurrent or metastatic squamous cell cancer of the head and neckJ Clin Oncol2004221778514701768
- SiuLLSoulieresDChenEXPhase I/II trial of erlotinib and cisplatin in patients with recurrent or metastatic squamous cell carcinoma of the head and neck: a Princess Margaret Hospital phase II consortium and National Cancer Institute of Canada Clinical Trials Group StudyJ Clin Oncol200725162178218317538162
- KimESKiesMSGlissonBSFinal results of a phase II study of erlotinib, docetaxel and cisplatin in patients with recurrent/metastatic head and neck cancerJ Clin Oncol20072518S Abstract 6013
- KondoNTsukudaMIshiguroYAntitumor effects of lapatinib (GW572016), a dual inhibitor of EGFR and HER-2, in combination with cisplatin or paclitaxel on head and neck squamous cell carcinomaOncol Rep201023495796320204279
- TYKERB® (lapatinib) tablets [package insert]Research Triangle Park, NCGlaxoSmithKline2012
- Del CampoJMHittRSebastianPEffects of lapatinib monotherapy: results of a randomised phase II study in therapy-naive patients with locally advanced squamous cell carcinoma of the head and neckBr J Cancer2011105561862721829197
- de SouzaJADavisDWZhangYA phase II study of lapatinib in recurrent/metastatic squamous cell carcinoma of the head and neckClin Cancer Res20121882336234322371453
- LalamiYSpecenierPMAwadaAEORTC 24051: unexpected side effects in a phase I study of TPF induction chemotherapy followed by chemoradiation with lapatinib, a dual EGFR/ErbB2 inhibitor, in patients with locally advanced resectable larynx and hypopharynx squamous cell carcinomaRadiother Oncol2012105223824022989664
- HarringtonKJTemamSD’CruzAFinal analysis: a randomized, blinded, placebo (P)-controlled phase III study of adjuvant postoperative lapatinib (L) with concurrent chemotherapy and radiation therapy (CH-RT) in high-risk patients with squamous cell carcinoma of the head and neck (SCCHN)J Clin Oncol2014325s Abstract 6005
- LiDAmbrogioLShimamuraTBIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer modelsOncogene200827344702471118408761
- SolcaFDahlGZoephelATarget binding properties and cellular activity of afatinib (BIBW 2992), an irreversible ErbB family blockerJ Pharmacol Exp Ther2012343234235022888144
- MachielsJPHaddadRIFayetteJAfatinib versus methotrexate as second-line treatment in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck progressing on or after platinum-based therapy (LUX-Head & Neck 1): an open-label, randomised phase 3 trialLancet Oncol201516558359425892145
- CohenEEWFayetteLJGaulerTCBiomarker analysis in recurrent and/or metastatic head and neck squamous cell carcinoma (R/M HNSCC) patients (pts) treated with second-line afatinib versus methotrexate (MTX): LUX-Head & Neck 1 (LUX-H&N1)J Clin Oncol201533Suppl Abstract 6023
- SeiwertTYFayetteJCupissolDA randomized, phase II study of afatinib versus cetuximab in metastatic or recurrent squamous cell carcinoma of the head and neckAnn Oncol20142591813182024928832
- GonzalesAJHookKEAlthausIWAntitumor activity and pharmacokinetic properties of PF-00299804, a second-generation irreversible pan-erbB receptor tyrosine kinase inhibitorMol Cancer Ther2008771880188918606718
- Abdul RazakARSoulieresDLaurieSAA phase II trial of dacomitinib, an oral pan-human EGF receptor (HER) inhibitor, as first-line treatment in recurrent and/or metastatic squamous-cell carcinoma of the head and neckAnn Oncol201324376176923108949
- WedgeSROgilvieDJDukesMZD6474 inhibits vascular endothelial growth factor signaling, angiogenesis, and tumor growth following oral administrationCancer Res200262164645465512183421
- CAPRELSA® (vandetanib) tablets [prescribing information]Wilmington, DEAstraZeneca Pharmaceuticals LP2011
- LimayeSRileySZhaoSA randomized phase II study of docetaxel with or without vandetanib in recurrent or metastatic squamous cell carcinoma of head and neck (SCCHN)Oral Oncol201349883584123727257
- HansenARSiuLLEpidermal growth factor receptor targeting in head and neck cancer: have we been just skimming the surface?J Clin Oncol201331111381138323460713
- KimuraHSakaiKAraoTShimoyamaTTamuraTNishioKAntibody-dependent cellular cytotoxicity of cetuximab against tumor cells with wild-type or mutant epidermal growth factor receptorCancer Sci20079881275128017498200
- KalyankrishnaSGrandisJREpidermal growth factor receptor biology in head and neck cancerJ Clin Oncol200624172666267216763281
- DebTBSuLWongLEpidermal growth factor (EGF) receptor kinase-independent signaling by EGFJ Biol Chem200127618155541556011279155
- GusenbauerSVlaicuPUllrichAHGF induces novel EGFR functions involved in resistance formation to tyrosine kinase inhibitorsOncogene201332333846385623045285
- WeihuaZTsanRHuangWCSurvival of cancer cells is maintained by EGFR independent of its kinase activityCancer Cell200813538539318455122
- TanXThapaNSunYAndersonRAA kinase-independent role for EGF receptor in autophagy initiationCell20151601–214516025594178
- DienstmannRBranaIRodonJTaberneroJToxicity as a biomarker of efficacy of molecular targeted therapies: focus on EGFR and VEGF inhibiting anticancer drugsOncologist201116121729174022135123
- CohenEEKaneMAListMAPhase II trial of gefitinib 250 mg daily in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neckClin Cancer Res200511238418842416322304
- SolomonBMJatoiARash from EGFR inhibitors: opportunities and challenges for palliationCurr Oncol Rep200810430430818778556
- LauxIJainASinghSAgusDBEpidermal growth factor receptor dimerization status determines skin toxicity to HER-kinase targeted therapiesBr J Cancer2006941859216306877
- SalamaJKHaddadRIKiesMSClinical practice guidance for radiotherapy planning after induction chemotherapy in locoregionally advanced head-and-neck cancerInt J Radiat Oncol Biol Phys200975372573319362781
- MesiaRRuedaAVeraRAdjuvant therapy with cetuximab for locally advanced squamous cell carcinoma of the oropharynx: results from a randomized, phase II prospective trialAnn Oncol201324244845323041591
- MarurSLeeJ-WCmelakAECOG 1308: a phase II trial of induction chemotherapy followed by cetuximab with low dose versus standard dose IMRT in patients with HPV-associated resectable squamous cell carcinoma of the oropharynx (OP)J Clin Oncol201230Suppl Abstract 5566
- MarurSLiSCmelakAE 1308: a phase II trial of induction chemotherapy (IC) followed by cetuximab with low dose versus standard dose IMRT in patients with human papilloma virus (HPV)-associated resectable squamous cell carcinoma of the oropharynx (OPSCC)J Clin Oncol201331Suppl Abstract 6005
- MassarelliEHaddadRILeeJJWeekly paclitaxel, carboplatin, cetuximab (PCC), and cetuximab, docetaxel, cisplatin, and fluorouracil (C-TPF), followed by risk-based local therapy in previously untreated, locally advanced head and neck squamous cell carcinoma (LAHN-SCC)J Clin Oncol201533Suppl Abstract 6001
- GuigayJFayetteJDilliesAFCetuximab, docetaxel, and cisplatin (TPEx) as first-line treatment in patients with recurrent or metastatic (R/M) squamous cell carcinoma of the head and neck (SCCHN): final results of phase II trial GORTEC 2008-03J Clin Oncol201230Suppl Abstract 5505
- HittRIrigoyenACortes-FunesHGrauJJGarcia-SaenzJACruz-HernandezJJPhase II study of the combination of cetuximab and weekly paclitaxel in the first-line treatment of patients with recurrent and/or metastatic squamous cell carcinoma of head and neckAnn Oncol20122341016102221865152
- BaselgaJTrigoJMBourhisJPhase II multicenter study of the antiepidermal growth factor receptor monoclonal antibody cetuximab in combination with platinum-based chemotherapy in patients with platinum-refractory metastatic and/or recurrent squamous cell carcinoma of the head and neckJ Clin Oncol200523245568557716009950
- KnoedlerMDietzAGaulerTCCetuximab, fluorouracil (5-FU), cisplatin, and docetaxel as first-line treatment in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck (R/M SCCHN): interim results of a randomized phase II clinical trial (CeFCiD)J Clin Oncol201331Suppl Abstract e17021
- VermorkenJBLicitraLStohlmacher-WilliamsJPhase II study of pemetrexed in combination with cisplatin and cetuximab in recurrent or metastatic squamous cell carcinoma of the head and neckEur J Cancer201349132877288323726971
- HerbstRSArquetteMShinDMPhase II multicenter study of the epidermal growth factor receptor antibody cetuximab and cisplatin for recurrent and refractory squamous cell carcinoma of the head and neckJ Clin Oncol200523245578558716009949
- FuryMGShermanELisaDA randomized phase II study of cetuximab every 2 weeks at either 500 or 750 mg/m2 for patients with recurrent or metastatic head and neck squamous cell cancerJ Natl Compr Canc Netw201210111391139823138167
- VermorkenJBTrigoJHittROpen-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapyJ Clin Oncol200725162171217717538161
- CrombetTOsorioMCruzTUse of the humanized anti-epidermal growth factor receptor monoclonal antibody h-R3 in combination with radiotherapy in the treatment of locally advanced head and neck cancer patientsJ Clin Oncol20042291646165415117987
- HarringtonKBerrierARobinsonMRandomised phase II study of oral lapatinib combined with chemoradiotherapy in patients with advanced squamous cell carcinoma of the head and neck: rationale for future randomised trials in human papilloma virus-negative diseaseEur J Cancer20134971609161823265705
- WaldronJNParulekarWO’SullivanBA phase III study of standard fractionation radiotherapy with concurrent high-dose cisplatin versus accelerated fractionation radiotherapy (RT) with panitumumab in patients with locally advanced stage III and IV squamous cell carcinoma of the head and neck (SCCHN) (NCIC Clinical Trials Group HN.6)J Clin Oncol201230Suppl Abstract TPS5600
