Abstract
Abstract: Breast cancer is the fifth cause of cancer death among women worldwide and represents a global health concern due to the lack of effective therapeutic regimens that could be applied to all disease groups. Nowadays, strategies based on pharmacogenomics constitute novel approaches that minimize toxicity while maximizing drug efficacy; this being of high importance in the oncology setting. Besides, genetic profiling of malignant tumors can lead to the development of targeted therapies to be included in effective drug regimens. Advances in molecular diagnostics have revealed that breast cancer is a multifaceted disease, characterized by inter-tumoral and intra-tumoral heterogeneity and, unlike the past, molecular classifications based on the expression of individual biomarkers have led to devising novel therapeutic strategies that improve patient survival. In this review, we report and discuss the molecular classification of breast cancer subtypes, the heterogeneity resource, and the advantages and disadvantages of current drug regimens with consideration of pharmacogenomics in response and resistance to treatment.
Introduction
Providing the most effective treatment is a task of paramount importance in personalized medicine for breast cancer.Citation1 This approach enables categorizing cancer subtypes and, ultimately, assigning the best treatment regimen based on patient characteristics, medical history and response to therapy.Citation2 The combination of pharmacological approaches (eg, pharmacogenetics, and pharmacodynamics) aids personalized medicine therapeutic decisions. Furthermore, pharmacogenetics and pharmacogenomics enable clinicians selecting appropriate drugs and doses to decrease adverse effects and increase efficacy. The systematic study of drug absorption, distribution, and metabolism is crucial for efficacy, and this may depend on genetic and epigenetic variations.Citation3 In addition to the role of genetic polymorphisms, epigenetics, microbiome alterations as well as demographic characteristics are involved in the occurrence of multi-drug resistance.Citation4–Citation6 Historically, pharmacogenetics and pharmacogenomics refer to the effect of genetic variations on drug metabolism, and the influence of the whole genome might have in response to drugs.Citation7 These approaches are frequently deployed in the setting of oncology as new strategies to minimize toxicity while maximizing the efficacy of target therapy and chemotherapy. They are also applied to develop new drugs based on genetic profiling and gene expression, this process being facilitated by new innovative techniques and profiling instruments. Here we overview the current molecular classification of breast cancer, the source of tumor heterogeneity and pharmacogenetics approaches in the targeted therapy and chemotherapy of the main subtypes of breast cancer.
Molecular classification
Based on molecular stratification, breast cancer is categorized into five distinct molecular classesCitation8 1, and 2 hormone receptor positive (luminal A and luminal B), 3 human epidermal growth factor receptor-2 (HER2-positive), 4 basal-like and normal-like based on microarray and gene expression profiling described by Perou and Sorlie,Citation9,Citation10 and the 5 claudin-low,Citation11 which is triple-negative breast cancer (TNBC), classified by medullary and metaplastic differentiation.Citation12,Citation13 The patterns of each molecular subtype differ in response to treatment.Citation9,Citation10 The management of the most common class – luminal A (predominantly ER+/PR+ and HER2-negative) – which accounts for 40%-60% of the diseaseCitation8,Citation14 has low pathological grade and proliferationCitation15 and shows low response to chemotherapyCitation16 while being sensitive to endocrine therapy,Citation17 is focused on anti-endocrine strategiesCitation8 In luminal B (ER+/PR+ and HER2-positive), which accounts for 15% of the disease,Citation14 and compared with luminal A,Citation15 has higher pathological grade and proliferation, chemotherapy rather than anti-estrogen drugs is beneficial.Citation8,Citation18 10% of breast cancers fall into the HER2 positive categoryCitation14 that displays high pathological grade.Citation15 Although this group of tumors is chemosensitive,Citation19 the disease prognosis has been dismal up until the introduction of targeted therapeutics.Citation20,Citation21 Basal-like tumors that represent 10–25% of the disease,Citation13 and are characterized by ER−/PR− or HER2− overlap with triple-negative (TN) tumors, express either HER2 or ER or both, in 15–45% of the cases.Citation21 Studies showed that basal-like tumors are chemo responsive,Citation19 although they are associated with aggressive behavior and poor prognosisCitation10,Citation14,Citation22–Citation24; 3–10% of the cases classified as normal-like type.Citation12 Those tumors that express gene expression characteristics of adipose tissue show an intermediate prognosis. In some studies, it has been proposed that these cells might represent a technical artifact during the microarrays.Citation25 The type Claudin-low is predominantly present as TNBC that accounts for 7–14% of the tumors and displays low expression of genes claudin and E-cadherin genesCitation26; 15% of these tumors express ER, while HER2 is overexpressed in 15% of the other cases belonging to the claudin-low type.Citation12 Response to chemotherapy in claudin-low tumors is intermediate.Citation12,Citation13 represents subtypes of breast cancer and their corresponding management strategies for cancer therapy. Despite the remarkable improvement achieved upon the use of therapeutic agents, de novo and acquired resistance remains an unsolved issue in breast cancer. The knowledge of the molecular mechanisms involved in the molecular subgroups of breast cancer might guide the development of new therapeutic strategies.Citation27,Citation28 Individual or multiple molecules or mutated genes could be deployed as a target of specific therapeutic strategies.
Table 1 Breast cancer classification based on molecular profiling
Heterogeneity resources
Heterogeneity of a subtype of breast cancer is the most frequent cause of therapy failure and unexpected outcome. In a distinct subgroup of cancer, alterations in genetic and epigenetic features of a clone of cells lead to changes in the prognosis and drug response.Citation5,Citation29 This heterogeneity can be intra-tumoral when involving cells within a tumor in an individual patient, or inter-tumoral when involving cells of the same subgroup of tumors in different patients. Intra-tumor heterogeneity is named “spatial” when involving a certain part of the tumor; or “temporal” when cells within the same tumor undergo changes during the course of the disease, from the primary tumor to metastasis. The heterogeneity associated with the morphological levels takes from comparing variable cell types in histopathological tests and provides grading systems.Citation30 When considering heterogeneity at a genetic level, alterations are related to the copy number variation (CNV), overexpression and down-regulation of a gene; or from missense, nonsense and frameshift mutations. Nowadays, there is a distinct open source database (COSMIC and TCGA) that provides information related to high throughput techniques. There are different heterogeneity sources for breast cancer patients who categorize in defined groups. These include local and systemic sources.Citation5 The COSMIC database contains data from hundreds of breast cancer tumors detected across different platforms and provides information about different analysis, mutation, CNV, methylation, over and underexpression. shows the genetic information of different categories of breast cancer categorized based on cellular and molecular signatures. The extensive variation in top 10 genes translates to the high rate of heterogeneity not only across but also within the groups; it would, therefore, be unlikely to observe a certain molecular pattern in all patients even if they all belong to the same group. This variation may be related to spatial or temporal heterogeneity that results in different tumor responses upon exposure to the therapeutic regimens.
Table 2 Breast cancer subgroups identified by genome profiling using different analysis platforms in COSMIC database
The pharmacogenomics approach enables identifying all the genetic variations in driver genes that account for the selective growth of cells in tumor bulk. As therapeutic targets, these help the clinician to select the appropriate therapeutic regimens to overcome resistance. The use of anticancer against these driver genes requires several considerations such as the choice of drug combinations, or selected target based on the predominant subclonal population that may undergo adaptive responses or toxic effects.Citation3 The detection of a subclonal population by accuracy methods like the ex vivo culture of tumor spheres in the tumor on-chip technique remains a critical issue.Citation31,Citation32 Other somatic mutations, introduced as passenger mutations, are those present in genes that help tumor survival. These mutations become acquired when cells are in the normal state or after they have undergone neoplastic transformation.Citation5 In some cases, genes implicated in cancer development have not been found to be mutated but are frequently found to be inactivated as a result of epigenetic mechanisms.
Hereditary breast cancer is linked to genetic mutations. BRCA1, BRCA2, PALB2, TP53, CDH1, and PTEN are genes that may undergo mutations associated with cumulative breast cancer risk.Citation33 Moreover, epigenetic mechanisms, which are known to play an essential role in the regulation of gene expression, may be involved in a hereditary form of the disease. Previous studies have shown that the tumor suppressor gene – RASSF1A– is often silenced following hypermethylation in breast cancer.Citation34 In familial breast cancer, <5% of mutations affect the BRCA1 and BRCA2 genes; whereas sporadically arising breast cancer can be associated with 10–15% rate of BRCA1 methylation. In most subtypes of breast cancer (), the molecular pathwayCitation35 related to cancer progression include the PI3K/AKT/mTOR and the RAS/RAF/MEK pathways. These are abnormally activated and are associated with resistance. Clinical trials are currently underway to test various PI3K inhibitors, which have been developed to target different components of the pathway. In the PI3K pathway, PTEN inactivation by epigenetic mechanisms is likely to extend the use of drug inhibitors effective in case of PTEN defective cancer cells. Another gene involved in this pathway is PPP2R2B, the negative regulator of AKT, and for which promoter methylation occurs in breast cancer.Citation36
Figure 1 Signaling pathways implicated in different categories of breast cancer. Notes: Adapted with permission from Kanehisa M, Goto S. KEGG: Breast cancer - Reference pathway; 2018. Available at: https://www.genome.jp/kegg-bin/show_pathway?map05224. Accessed May 15, 2019. Copyright Kanehisa Laboratories.Citation154
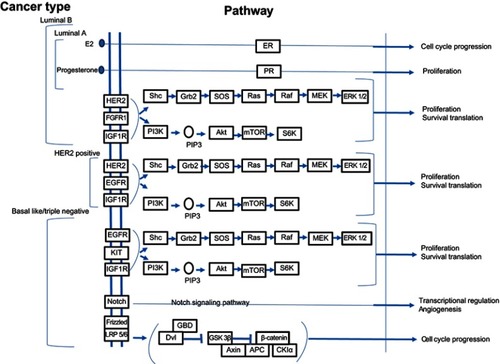
In some gene families, overlaps of mutation and methylation are observed, these leading to variable responses. As an example, when considering the RUNX family, RUNX1 is mutated while RUNX3 is inactivated by epigenetic mechanisms.Citation15,Citation37 DNA methylation can be analyzed in a blood sample using a powerful bisulfite-based PCR technique.Citation15 The tumor microenvironment, which comprises tumor-associated macrophage, fibroblast, bone marrow-derived cell and lymphatic growth factors, chemokine, cytokines, and exosome, is also responsible for tumor heterogeneity and contributes to both growth and metastasisCitation5 It is envisaged that in the near future genetic, genomic and immunologic consultants might help the oncologists to implement different strategies and therefore plan for the most successful therapy regimens. Pharmacogenomics will pave the way for such planning and generation of individualized treatments.
Pharmacogenetics in breast cancer subtypes
Estrogen receptor positive
The predominant type of breast cancer is associated with expression of estrogen receptor, which is used as a predictive marker in the follow up of patients (disease free). These cases benefit from hormonal therapy based on the administration of the artificial estrogen analog tamoxifen (TAM). TAM binds to estrogen receptor and disrupts the activation of the classical pathway that leads to ductal hyperplasia. Subsequent alteration of the tumor microenvironment makes the invasion state.Citation38,Citation39 represents the signaling pathways, which modulate tumor cells and tumor microenvironment components, as well as the effect that certain cancer therapeutic agents exerts on these pathways in patient with ER+. The other signaling pathways related to ER overexpression relate to nonclassical functions that facilitate genomics activity in an independent hormone manner. In this context, ER acts through growth factor signaling (FGFR, IGFR, GPCR) that activate intracellular kinase and phosphatase. Several studies determined the effect of kinases in the phosphorylation of ER protein; this phenomenon is associated with sensitivity or resistance to endocrine therapy. Baron et al provided an extensive review of all phosphorylation sites and mRNA splicing regions in ER and associated drug response. Further, the interaction between ER with other transcription factors like C-Fos/C-Jun (AP-1), Sp1 and NF-KB was found to result in tumor cell division, angiogenesis and progression to metastasisCitation40 In addition to TAM, the ER down-regulator Fulvestrant has been approved for the treatment of ER+ breast cancer. By forming a complex with ER, Fulvestrant inhibits its dimerization, finally leading to its degradation. Moreover, Anastrozole, an aromatase inhibitor that blocks the conversion of adrenal androgen into estrogen, can be administered to a postmenopausal woman who could also benefit from Fulvestrant treatment. TAM is equally valid regardless of menopausal status.Citation39 As indicated in , there are variations in hormone receptor subtypes, which are associated with genetic differences. Progesterone receptor gene expression is dependent on ER expression in epithelial cells so that half of the ER+ tumors are also PR+. Previous work has shown that patients who do not express PR have the worse prognosis since the start of TAM treatment when compared to those who are PR+. This group of patients benefits from the administration of Anastrozole.Citation41 Primary endocrine resistance can occur in half of the cases and finally developed resistance in another half as acquired resistance. Several factors are contributing to resistance, and these include mutation rate, methylation, acetylation and downregulation of ERα, overexpression of ERβ as well as crosstalk between ER and growth factor and signaling pathways.Citation40,Citation42 ARN-810 is a selective ERα antagonist that induces proteasomal-mediated degradation of ERα. This drug is active against tumors with the ESR1 mutation, whereas conventional endocrine therapy is not sufficient.Citation43 Mutations in the ligand binding domain of ERα are associated with ligand-independent transcriptional activity. Hot spots mutations located in the ligand-binding domain include Y537S, Y537N, Y537C, and D538G, these representing >80% of ESR1 mutations associated with acquired resistance to endocrine therapy.Citation44–Citation46 This type of mutations is regarded as the main resistance mechanism that is rare in primary tumors but is reported in >20% of cases of recurrence and metastatic cancer in patients treated with endocrine therapy.Citation47–Citation49 Since the frequency mutation of the ESR1 is higher in metastatic than in primary breast cancer, assessment of this gene mutation in plasma cfDNA could help selecting treatment strategies, eg, administration of Fulvestrant in patients with the ESR1 mutation was found to improve tumor-free survival.Citation48,Citation49 Although the mechanism related to the effect of Fulvestrant alone or in combination with Palbociclib in a patient with ER mutation is not known, patients with metastatic cancer benefit upon exposure to combination therapy.Citation50 The COSMIC database reported that the ESR1 mutation Y537S affects the phosphorylation of IGF1R.Citation51 This is associated with shorter overall survival and contributes to resistance to target therapy.Citation42 K303R is another significant mutation that was found to reduce sensitivity to TAM through phosphorylation of AKT.Citation52 According to the COSMIC database, most of the ER+ cases display PIK3CA mutations. In luminal B subtype with endocrine resistance generated the moderate PTEN reduction leading enhance PI3K signaling. Alone or in combination mTOR, AKT, or MEK inhibitors with Fulvestrant improved endocrine therapy.Citation53 Luminal B are more aggressive and endocrine therapy resistant than luminal A. Moreover they are known with a different rate of PI3K pathway activation.Citation53,Citation54
Figure 2 The interaction between tumor microenvironment components, including stromal cells, and tumor cells leads to enhanced cell growth, proliferation, angiogenesis and invasion. Breast cancer epithelial cells increase tumor cell proliferation and invasion while inhibiting apoptosis through either of these following pathways: (1) classical pathway in which estrogen binds to its receptor, ER, or (2) non-classical pathway that involves post-translational modifications of ER by activation kinases, and transcription factors. Anti-cancer agents including tamoxifen (TAM), whose metabolites, 4-OH TAM and endoxifen, have higher affinity for ER when compared with TAM, exert their effects by modulating signaling pathways that regulate tumor cells.Note: Data adapted from RussellCitation39 and Barone et al.Citation40
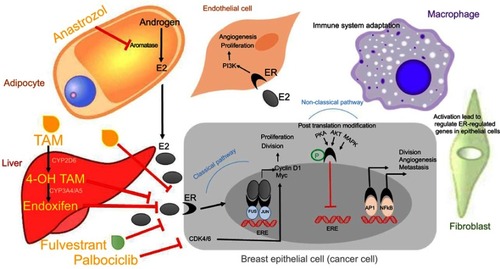
Primary and secondary endocrine resistance can be overcome by additional agents like PI3K and CDK4/6 inhibitors. Genetic alterations were noted in sensitive endocrine patients; however, de novo resistance was related to loss of ER, loss of amplification of co-receptor or co-amplificatory molecules as well as to activation of the cyclin D pathway. In the case of acquired resistance, which develops after an initial response to endocrine therapy, the activation of the PI3Kpathway represents a common resistance mechanism.Citation43 Previous studies have determined the relationship between ESR1 and PIK3CA mutations with clinical features. Thus, mutations of the PI3K/mTOR pathway observed in one-third of ER+ cases associated with good prognosis in early stage and with poor prognosis in the metastatic setting after start of endocrine therapy.Citation43 A recent study that used cfDNA to detect mutations in the ESR1 and PIK3CA genes revealed higher level of heterogeneity in ESR1 than PIK3CA.Citation55 PIK3CA mutation occurs in the early stage of tumor development;Citation56 whereas the ESR1 mutation occurred later during endocrine treatment and was detected in metastatic lesions.Citation57 Therefore, a mutation might result from the pressure endocrine therapy exerts on tumors. Duration of response and development of resistance are related to the rate of ESR1 mutation.Citation57 In ER+ patients resistance to endocrine therapy deriving from aberrant activation of PI3K signaling proved to benefit from the combination of a PI3K inhibitor with the antiestrogen. Combination of Alpelisib and Letrozole were more effective in ER+/HER2 negative metastatic patients with PIK3CA mutation, patients with FGFR1/2 amplification and KRAS and P53 mutations.Citation58 A limited number of clinical trials of luminal B HER2-enriched combination between HER2-targeted therapy (lapatinib or trastuzumab) and aromatase inhibitors provided a clinical benefit.Citation59
Further, combinatorial treatment based on cell cycle arrest agents targeting CDK and endocrine therapy has been proven successful, especially in those cases with primary endocrine resistance. Several studies have investigated the synergistic effect of the CDK4/6 inhibitor Palbociclib and endocrine therapy in both endocrines sensitive and endocrine-resistant cases; the combined action of these drugs leads to improved prognosis. Additional agents could be deployed to target methylation by histone deacetylase; this enabling to reverse resistance.Citation43 Hypermethylation of CPG islands within the promoter of ESR1 gene is associated with lack of response to TAM. Methylation of PITX2 has also been related to TAM-resistance. Methylation analysis of candidate genes could help in sparing patients from ineffective treatment by indicating the administration of demethylation agents such as 5-azacytidine (vidaza) or decitabine (5aza 2-deoxycytidine, dacogen) along with histone deacetylase inhibitors like vorinostat or romidepsin.Citation15 TAM metabolites 4-OH TAM and N-desmethyl TAM (endoxifen) have a higher affinity than TAM for ER. Patients with defective alleles of CYP2D6, an enzyme involved in drug metabolism, are less responsive to TAM. In this regard, the mutation G1934A in the CYP2D6 splicing site resulting in loss of enzyme activity.Citation3 Because the use of TAM, especially in association with chemotherapy, increases the risk of thromboembolic events, breast cancer patients should be screened before prescribing the drug.Citation60,Citation61 Although promising, targeted therapy might not be applicable to all patients; therefore the development of new agents based on pharmacogenomics and pharmacogenetics, resistance profile and maintenance of systemic hemostasis is needed. As highlighted above, drug resistance remains one of the most prominent clinical obstacles in breast cancer treatment. Studies have shown that 40–50% of ER+ breast cancer patients ultimately develop TAM-resistance. Thus, useful biomarkers are needed for the early diagnosis of these TAM-resistant patients.Citation62,Citation63
HER2 positive
HER2 amplification has been identified in >14% of metastatic breast cancer that associated with increased cell proliferation, angiogenesis, invasion and reduced apoptosis.Citation64 It has been found that in HER2- tumors there are compensatory driver genes like BRF2 and DSN1, which undergo amplification or overexpression as oncogenes that bestow a neoplastic advantage.Citation29 HER2+ patients are sensitive to HER2 antibodies and kinase inhibitors lapatinib, pertuzumab, trastuzumab, and ado-trastuzumab and emtansine.Citation65 Trastuzumab (Herceptin™, Genentech/Roche, South San Francisco, CA, USA) was the first humanized monoclonal antibody against the domain IV of human epidermal receptor 2 to be developed.Citation66 This antibody was the first to receive FDA approval as a targeted drug for breast cancer.Citation67 In some clinical studies, a combination of trastuzumab with standard chemotherapy regimens resulted in better response rates than prescription chemotherapy alone.Citation68–Citation70 Subsequent studies, however, showed that some patients tend to develop resistance to therapy.Citation71 The predominant mechanisms of resistance to trastuzumab appear to be related to the HER2 signaling pathwayCitation71,Citation72 and be associated with activating mutations in PIK3CA, RAS, Src, NF-KB and inactivating mutations in PTEN, a negative regulator.Citation73–Citation75
Moreover, truncated isoforms of HER2, which lack trastuzumab target epitope,Citation76 constitute an active source of HER2 generation due to the formation of stable HER2 homodimers.Citation77,Citation78 Overexpression of EGFR and HER-3 – the HER2 co-receptors – and their ligands,Citation73 interaction with adhesion molecules such as MUC1-C or MUC4Citation79,Citation80 and incorporation into heterologous receptors (HER-3) with HER2, are additional mechanisms of resistance to trastuzumab. Targeted strategies to overcome resistance to this drug include: 1) use of pan PI3K inhibitor, specific PIK3CA inhibitors, AKT inhibitors, and mTOR inhibitors when resistance results from PIK3CA alterations; 2) Lapatinib chemotherapy to overcome the high level of p95HER2; 3) Tyrosine kinase inhibitors or IGF1R monoclonal antibodies to overcome activation of IGF1R tyrosine kinase receptor; 4) MET inhibitors to overcome MET alterations (mutation and amplification); and 5) immune checkpoints inhibitors to overcome low immune response.Citation81 Heterogeneity in HER2+ breast cancers was associated with failure of target therapy, for example, like in the case of HER2+ and luminal molecular subtype that also expresses ER. In this regard, clinical trials highlighted the variability in the efficacy of regimens based on the combination of trastuzumab and endocrine therapy.Citation82 As depicted in , HER2+ patients display various molecular alterations and gene expression mutations that may impact prognosis and response to treatment.
Trastuzumab-DM1 (T-DM1: Genentech/Roche, South San Francisco, CA, USA) a novel monoclonal antibody that is conjugated with maytansine - a fungal toxin,Citation80 requires a high level of HER2 expression on the targeted cells in order to be effective. Trastuzumab and its metabolites require accumulating in the cytosol of the target cancer cell to reach an optimum concentration and therefore induce cell death.Citation83 In this context, it appears that low intra-tumor levels of HER2 and poor internalization of the HER2-drug, which is associated with insufficient intracellular expression of DM1, represent the primary mechanisms to drug resistance.Citation84 CYD985 is another antibody-drug conjugate administered to and well tolerated in TDM1 pretreated patients.Citation85 Lapatinib (Tykerb™, GlaxoSmithKline, Research Triangle Park, NC, USA) is a tyrosine kinase receptor inhibitor that inhibits autophosphorylation of both EGFR and HER2.Citation86,Citation87 Administration of lapatinib in combination with trastuzumab represents a strategy to target both intracellular and extracellular domains of HER2.Citation88 However, the main issue related to lapatinib is the acquired resistance due to the Presence of HER2 overexpression in tumor cells which are primarily sensitive to lapatinib apoptotic effects.Citation89 Activation of AXL, a membrane-bound RTK, constitutes another mechanism of resistance to lapatinib.Citation90 Pertuzumab (Omnitarg™, Genentech/Roche, South San Francisco, CA, USA), a humanized monoclonal antibody that binds to an extracellular domain of HER2, different from that bound by trastuzumab, inhibits hetero and homo-dimerization of HER2Citation91 and could partially reverse resistance to trastuzumab in metastatic breast cancer patients.Citation92 Administration of pertuzumab in combination with lapatinib could be a promising strategy to overcome lapatinib resistance, as demonstrated in an animal model. Pertuzumab inhibited NRG1-induced signaling that is activated 24 hrs following lapatinib administration.Citation93 Ertumaxomab (Rexoman™, Fresenius Biotech, Hamburg, Germany) is an antibody that activates and aggregates T-cells, NK-cells, dendritic cells, and macrophages through the formation of the HER2-drug-CD3 complex.Citation93 Although the drug is still being evaluated in clinical trials, it might represent a promising targeted therapy. The presence of PIK3CA mutation might account for resistance to anti-HER2 therapy in HER2+ tumors. Neratinib has received increasing interest in the treatment of recurrent and metastatic tumors characterized by non-amplifying HER2 alterations.Citation94 Neratinib, a reversible tyrosine kinase inhibitor, can inhibit HER-1, HER2, and HER-4.Citation95 In a study including 1,420 women with breast cancer, neratinib significantly improved disease-free survival by 12 months.Citation96 In the presence of HER2 mutations, afatinib, another kinase inhibitor has also been used. Afatinib efficacy has been evaluated both in vivo and in vitro.Citation97 The duration of afatinib effects is longer compared with other reversible EGFR inhibitors.Citation98 In a recent study, administration of afatinib with letrozole to metastatic breast cancer patients resulted in stabilization of the disease in 54% of cases, who previously progressed while they were on letrozole therapy.Citation99
Triple negative
Triple-negative cancers include three subtypes: normal like, basal-like and non-basal like; 75% of basal-like cancers are TN, and 80% of these TN breast cancer display P53 mutations (nonsense and frameshift).Citation100 Basal and non-basal TN also present a different type of mutation, ie, the homologous recombination deficiency (HRD) and homologous recombination repair (HRR). Homologous recombination is the repair mechanism of double-strand DNA breaks (DSB). Several clinical trials on patients with TN cancers highlighted the importance of HRD genomic signature in the prediction of therapeutic response.Citation100–Citation102
The COSMIC database had identified the genes mainly involved in TN variations. These genes have been extensively studied and include TP53, BRCA1, PIK3CA, RB, and PTEN. In TN tumors, there is no correlation between TP53 and BRCA1 mutations,Citation103 although the methylation rate of BRCA1 was found to correlate with the TP53 mutation.Citation104
Increased copy number and mutations of the PIK3CA gene, loss of PTEN and INPP4B and overexpression of EGFR were found to activate the PI3K pathway. In the basal-like tumor, 72% of cases are RB-/P16+ and display p53 overexpression, which is correlated with high proliferation. Further, mutations in BRCA1, PTEN, ERBB2 genes were correlated with increased risk of metastasis.Citation100 Genetic and epigenetic alterations in the DNA repair system may act as initial causes of cell transformation. Drugs targeting cells with a deficit in the DSB repair are highly relevant as anti-cancer agents. These are platinum-based compounds that lead to DSB.Citation105 The PARP inhibitor is effective against cancer cells with a deficit in DNA repair of DSBs. This inhibitor blocks the activity of the PARP enzyme, of which PARP1 is an important target indicating the presence of single-strand breaks. Therefore, the inhibition of PARP1 leads to more “unrepaired” single-strand breaks. These breaks in replication forks lead to the formation of DSB that cannot be repaired in BRCA1 or BRCA2 defective cells, thereby leading to the accumulation of DSB and subsequent cellular death.Citation15 There are similarities between BRCA1 mutated breast cancer and basal-like breast cancer. The TN cancer due to inherited BRCA1 mutation is the same as basal-like breast cancer in the presence of acquired BRCA1 mutations or other mutations in genes within same pathways. This phenotype has been found in the sporadic case of basal-like or TNBC. PARP inhibitors initially gave promising results in TN tumors; however, subsequent trials failed to confirm these findings. Therefore, it remains unclear which subset of TN or inherited BRCA1 or BRCA2 can benefit from treatment with the PARP inhibitors olaparib and iniparib.Citation106,Citation107 Genetic studies of TN and basal-like cancers have reported defects in BRCA1 resulting in half of TN tumors having acquired BRCA1 methylation. Preclinical studies indicate that the use of BRCA1 methylation might present a promising biomarker of response to PARP inhibitor.Citation15 In the case of mutation or methylation impacting BRCA1 and BRCA2, defective cells could benefit from platinum-based agents like cisplatin. By crosslinking to DNA, this drug can target the lesions that are ineffectively repaired because of DSB formation. Mutations in the TP53 gene have been associated with resistance to cisplatin treatment.Citation108 In TN subtype, mutations in the PI3K/AKT/mTOR pathway could be overcome by target therapy in combination with chemotherapy.Citation5
Chemotherapy
One of the cornerstones in the treatment of disease, both when dealing with primary and metastatic breast cancer, is to administer medications systematically.Citation108 Until now numerous classes of drugs used in the treatment of breast cancer have been given systematically, and these include anthracyclines such as doxorubicin, epirubicin, and mitoxantrone that have pleiotropic effectsCitation109 and induce cell death via a number of proposed mechanisms.Citation110–Citation112 Resistance to anthracylines is related to the increase in expression of P-170 glycoprotein that results in increased drug efflux.Citation113 A number of approaches have been applied to overcome tumor resistance to anthracyclines, and these are based on the use of fostriecin, merbarone, aclarubicin and bis (2,6-dioxopiperazine) as novel inhibitors of topoisomerase II,Citation114,Citation115 altered topoisomerase II,Citation116 use of non-cross-resistant drugs after administration of anthracyclinesCitation117,Citation118 and changes in the route and time of drug delivery.Citation119 Another class of drugs, the taxanes, includes paclitaxel, docetaxel, and nab-paclitaxel that bind to microtubules with high-affinity; inhibit mitosis and therefore disrupting cell division.Citation120 Cancer cells manifest resistance to these drugs by changes in the expression levels of beta-tubulin isotopes and by upregulating caveolin-1.Citation121 The effects of cyclosporine A, PC833 and verapamil have been evaluated with the view of overcoming resistance to this class of chemotherapeutic agents.Citation122 New microtubule inhibitors like ixabepilone and eribulin were developed to overcome resistance against anthracyclines and taxanes; these were approved by FDA but not by EMA.Citation123 Recently, irinotecan pegol (NKTR-102), a long-acting topoisomerase I inhibitor, has been launched as a new agent to overcome resistance related to anthracyclines and taxanes.Citation124,Citation125 A study reported that CPG island hypermethylation in the promoter of the GSTP1 gene was associated with a therapeutic response to doxorubicin.Citation15 Antimetabolites such as methotrexate (MTX) and 5-fluorouracil (5-FU) inhibit dihydrofolate reductase (DHFR)Citation126 and thymidylate synthase enzymesCitation127 respectively. Resistance to MTX may be conferred by a decrease in drug uptake,Citation128,Citation129 increase in drug efflux,Citation130,Citation131 reduction in polyglutamation,Citation132 increase in the level of DHFRCitation133 and expression of Bcl2 during apoptosis.Citation134 Resistance to 5-FU results from alterations in enzymes involved in the metabolism of fluoropyrimidine.Citation135 Strategies have been evaluated to overcome resistance to antimetabolite drugs. These include manipulation of drug metabolism, using high-dose medications and the development of novel antimetabolites.Citation136 Alkylating agents and platinum-based drugs alkylate DNA through the formation of reactive intermediates formation.Citation109 Tumors can develop resistance to these drugs through decreased alterations linked to transmembrane cellular drug accumulation,Citation137 increase in drug inactivation in the cytosol, increase in repair of damaged DNACitation138 and anti-apoptotic mechanisms.Citation139 Changes in the regulators of apoptosis have been considered as an approach to overcome resistance observed with the existing drugs.Citation140 Vinca alkaloids that comprise vincristine, vinblastine, vinorelbine, vindesine, vinflunine, and vinpocetine have clinical usesCitation141 and are also susceptible to multidrug resistance.Citation142 The mechanism of drug resistance in this class of drugs results from decreased drug accumulation and retention. In this case, methods to overcome resistance have considered administration of DNA polymerase alpha inhibitors like Gemcitabine.Citation109
New challenges in precision medicine
Despite the progress achieved in targeted therapy, numerous unsolved challenges still exist. The lack of safe and effective drugs is an important issue that might impact the effective control of the disease. Even though the molecular mechanisms underlying the anticancer effects of some common drugs like metformin – an antihyperglycemic drug – remain to be fully understood, growing evidence suggests that diabetic patients who receive metformin exhibit lower rate of cancers, including breast cancer.Citation143 A recent study investigating the effects of metformin on cells overexpressing the tumor suppressor microRNA (miR-200c), showed that the drug inhibits proliferation, migration, and invasion of cancer cells while augmenting the levels of miR-200c.Citation144 The Authors suggested miR-200c as a potential target for the treatment of breast cancer patients. With the view of revealing the molecular mechanisms involved in the anticancer effects of metformin and to introduce novel therapeutic approaches, a recent proteomics analysis proposed developing further generations of metformin.Citation145
On the other hand, some factors such as diet and psycho-social status should be considered in personalized medicine. The steroid hormone – vitamin D – which affects almost every cell in the human body plays a significant role in breast cancer prevention.Citation146
Epidemiological studies have reported the role of vitamin D and vitamin D-binding protein-related genes in the association between vitamin D and the risk of the disease. There is mounting evidence indicating that psycho-social factors such as stress, depression, anxiety, and spiritual status markedly impact the development of breast cancer and response to treatment.Citation147–Citation149 It is plausible that the involved molecular mechanisms are used as a step towards precision medicine. Recent studies have shown the potential role for dopamine and serotonin in this context. Experimental research on Iranian women has shown that spiritual intervention causes a decrease in dopamine receptor gene expression in peripheral blood mononuclear cells (PBMCs) of breast cancer patients compared with a control group who did not receive the intervention.Citation150 Another study on PBMCs of breast cancer patients revealed that serotonin receptor gene expression was increased compared to the healthy group.Citation151 The authors proposed antagonists of serotonin receptors as a new therapeutic agent.
Microbiome profiling is one of the emerging tools in the clarification of the role of environmental factors in the etiology of breast cancer. Recent work indicates there are differences between bacteria present in the normal tumor-adjacent tissue of breast cancer patients and tissue from healthy women.Citation152
The advents of modern diagnostics and therapeutics have provided significant advances compared with traditional medicine. Nowadays, novel biomarkers, therapeutic targets and data mining constitute essential parts of science. Hence, researchers spend a long time analyzing existing databases. Analytic and modeling tools accompanied by experimental modules have been key in providing a comprehensive and meticulous view of the interactions between cellular components in biological processes. This so-called 'systems biology'Citation153 proves pivotal in implementing personalized approaches, especially therapeutics.
Acknowledgments
The studies reported in this publication were supported by Proteomics Research Centre of Shahid Beheshti University of Medical Sciences; there was no conflict of interest in relation to the support.
Disclosure
The authors report no conflicts of interest in this work.
References
- Wang C, Machiraju R, Huang K. Breast cancer patient stratification using a molecular regularized consensus clustering method. Methods. 2014;67(3):304–312. doi:10.1016/j.ymeth.2014.03.00524657666
- Chen X, Shachter RD, Kurian AW, Rubin DL. Dynamic strategy for personalized medicine: an application to metastatic breast cancer. J Biomed Inform. 2017;68:50–57. doi:10.1016/j.jbi.2017.02.01228232241
- Nerenz RD. Pharmacogenomics and personalized medicine in the treatment of human diseases. In: Coleman WB, Tsongalis GJ, editors. Molecular pathology. 2nd ed. Elsevier; New York: Chapel Hill; 2018;731–743.
- Li H, Jia W. Cometabolism of microbes and host: implications for drug metabolism and drug‐induced toxicity. Clin Pharmacol Ther. 2013;94(5):574–581. doi:10.1038/clpt.2013.15723933971
- Nandy A, Gangopadhyay S, Mukhopadhyay A. Individualizing breast cancer treatment—the dawn of personalized medicine. Exp Cell Res. 2014;320(1):1–11. doi:10.1016/j.yexcr.2013.09.00224051330
- Alomar MJ. Factors affecting the development of adverse drug reactions. Saudi Pharm J. 2014;22(2):83–94. doi:10.1016/j.jsps.2013.02.00324648818
- Dickmann LJ, Ware JA. Pharmacogenomics in the age of personalized medicine. Drug Discov Today Technol. 2016;21:11–16. doi:10.1016/j.ddtec.2016.11.00327978982
- Eroles P, Bosch A, Pérez-Fidalgo JA, Lluch A. Molecular biology in breast cancer: intrinsic subtypes and signaling pathways. Cancer Treat Rev. 2012;38(6):698–707. doi:10.1016/j.ctrv.2011.11.00522178455
- Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747. doi:10.1038/3502055710963602
- Sørlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci. 2001;98(19):10869–10874. doi:10.1073/pnas.19136709811553815
- Herschkowitz JI, Simin K, Weigman VJ, et al. Identification of conserved gene expression features between murine mammary carcinoma models and human breast tumors. Genome Biol. 2007;8(5):R76. doi:10.1186/gb-2007-8-5-r8117493263
- Prat A, Perou CM. Deconstructing the molecular portraits of breast cancer. Mol Oncol. 2011;5(1):5–23. doi:10.1016/j.molonc.2010.11.00321147047
- Perou CM. Molecular stratification of triple-negative breast cancers. Oncologist. 2011;16(Supplement 1):61–70. doi:10.1634/theoncologist.2011-S1-61
- Sørlie T, Tibshirani R, Parker J, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc National Acad Sci. 2003;100(14):8418–8423. doi:10.1073/pnas.0932692100
- Stefansson OA, Esteller M. Epigenetic modifications in breast cancer and their role in personalized medicine. Am J Pathol. 2013;183(4):1052–1063. doi:10.1016/j.ajpath.2013.04.03323899662
- Gnant M, Harbeck N, St. Gallen TC. summary of the consensus discussion. Breast Care. 2011;6(2):136–141.21633630
- Del Mastro L, De Placido S, Bruzzi P, et al. Fluorouracil and dose-dense chemotherapy in adjuvant treatment of patients with early-stage breast cancer: an open-label, 2×2 factorial, randomised phase 3 trial. Lancet. 2015;385(9980):1863–1872. doi:10.1016/S0140-6736(14)62048-125740286
- Creighton CJ. The molecular profile of luminal B breast cancer. Biologics. 2012;6:289.22956860
- Rouzier R, Perou CM, Symmans WF, et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res. 2005;11(16):5678–5685. doi:10.1158/1078-0432.CCR-04-242116115903
- Network CGA. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61. doi:10.1038/nature1141223000897
- Colozza M, de Azambuja E, Cardoso F, Bernard C, Piccart MJ. Breast cancer: achievements in adjuvant systemic therapies in the pre-genomic era. Oncologist. 2006;11(2):111–125. doi:10.1634/theoncologist.11-2-11116476832
- Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the carolina breast cancer study. JAMA. 2006;295(21):2492–2502. doi:10.1001/jama.295.21.249216757721
- Rakha EA, El-Rehim DA, Paish C, et al. Basal phenotype identifies a poor prognostic subgroup of breast cancer of clinical importance. Eur J Cancer. 2006;42(18):3149–3156. doi:10.1016/j.ejca.2006.08.01517055256
- Nielsen TO, Hsu FD, Jensen K, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10(16):5367–5374. doi:10.1158/1078-0432.CCR-04-022015328174
- Weigelt B, Mackay A, A‘Hern R, et al. Breast cancer molecular profiling with single sample predictors: a retrospective analysis. Lancet Oncol. 2010;11(4):339–349. doi:10.1016/S1470-2045(10)70008-520181526
- Prat A, Parker JS, Karginova O, et al. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res. 2010;12(5):R68. doi:10.1186/bcr272220813035
- Cadoo KA, Traina TA, King TA. Advances in molecular and clinical subtyping of breast cancer and their implications for therapy. Surg Oncol Clin. 2013;22(4):823–840. doi:10.1016/j.soc.2013.06.006
- Fang L, Barekati Z, Zhang B, Liu Z, Zhong X. Targeted therapy in breast cancer: what’s new. Swiss Med Wkly. 2011;141:w13231.21706452
- Ng CK, Martelotto LG, Gauthier A, et al. Intra-tumor genetic heterogeneity and alternative driver genetic alterations in breast cancers with heterogeneous HER2 gene amplification. Genome Biol. 2015;16(1):107. doi:10.1186/s13059-015-0667-425994018
- Zardavas D, Irrthum A, Swanton C, Piccart M. Clinical management of breast cancer heterogeneity. Nat Rev Clin Oncol. 2015;12(7):381. doi:10.1038/nrclinonc.2015.7325895611
- Tsai H-F, Trubelja A, Shen AQ, Bao G. Tumour-on-a-chip: microfluidic models of tumour morphology, growth and microenvironment. J R Soc Interface. 2017;14(131):20170137. doi:10.1098/rsif.2017.013728637915
- Ahn J, Sei Y, Jeon N, Kim Y. Tumor microenvironment on a chip: the progress and future perspective. Bioengineering. 2017;4(3):64. doi:10.3390/bioengineering4020044
- Peters ML, Garber JE, Tung N. Managing hereditary breast cancer risk in women with and without ovarian cancer. Gynecol Oncol. 2017;146(1):205–214. doi:10.1016/j.ygyno.2017.04.01328454658
- Kristiansen S, Nielsen D, Sölétormos G. Detection and monitoring of hypermethylated RASSF1A in serum from patients with metastatic breast cancer. Clin Epigen. 2016;8(1):35. doi:10.1186/s13148-016-0199-0
- Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28(1):27–30.10592173
- Sangodkar J, Farrington CC, McClinch K, Galsky MD, Kastrinsky DB, Narla G. All roads lead to PP 2A: exploiting the therapeutic potential of this phosphatase. FEBS J. 2016;283(6):1004–1024. doi:10.1111/febs.1357326507691
- Lau QC, Raja E, Salto-Tellez M, et al. RUNX3 is frequently inactivated by dual mechanisms of protein mislocalization and promoter hypermethylation in breast cancer. Cancer Res. 2006;66(13):6512–6520. doi:10.1158/0008-5472.CAN-06-036916818622
- Pietras RJ, Márquez-Garbán DC. Membrane-associated estrogen receptor signaling pathways in human cancers. Clin Cancer Res. 2007;13(16):4672–4676. doi:10.1158/1078-0432.CCR-07-137317699844
- Russell CA. Personalized medicine for breast cancer: it is a new day! Am J Surg. 2014;207(3):321–325. doi:10.1016/j.amjsurg.2013.10.01624581758
- Barone I, Brusco L, Fuqua SA. Estrogen receptor mutations and changes in downstream gene expression and signaling. Clin Cancer Res. 2010;15;16(10):1078–1432. CCR-09-1753.
- De Abreu FB, Wells WA, Tsongalis GJ. The emerging role of the molecular diagnostics laboratory in breast cancer personalized medicine. Am J Pathol. 2013;183(4):1075–1083. doi:10.1016/j.ajpath.2013.07.00223920325
- Yanagawa T, Kagara N, Miyake T, et al. Detection of ESR1 mutations in plasma and tumors from metastatic breast cancer patients using next-generation sequencing. Breast Cancer Res Treat. 2017;163(2):231–240. doi:10.1007/s10549-017-4190-z28283903
- Mayer IA. Advanced hormone-sensitive breast cancer: overcoming resistance. J Natl Compr Canc Netw. 2015;13(5S):655–657.25995422
- Downey C, Simpkins S, White J, et al. The prognostic significance of tumour–stroma ratio in oestrogen receptor-positive breast cancer. Br J Cancer. 2014;110(7):1744. doi:10.1038/bjc.2014.6924548861
- Takeshita T, Yamamoto Y, Yamamoto-Ibusuki M, et al. Clinical significance of monitoring ESR1 mutations in circulating cell-free DNA in estrogen receptor positive breast cancer patients. Oncotarget. 2016;7(22):32504. doi:10.18632/oncotarget.883927102299
- Tabarestani S, Motallebi M, Akbari ME. Are estrogen receptor genomic aberrations predictive of hormone therapy response in breast cancer? Iran J Cancer Prev. 2016;9:4. doi:10.17795/ijcp
- Segal CV, Dowsett M. Estrogen receptor mutations in breast cancer—new focus on an old target. Clin Cancer Res. 2014;20(7):1724–1726. doi:10.1158/1078-0432.CCR-14-006724583794
- Fribbens C, O‘Leary B, Kilburn L, et al. Plasma ESR1 mutations and the treatment of estrogen receptor-positive advanced breast cancer. J Clin Oncol. 2016;34:2961–2968.
- Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17(4):425–439. doi:10.1016/S1470-2045(15)00613-026947331
- Lauring J, Wolff AC. Evolving role of the estrogen receptor as a predictive biomarker: ESR1 mutational status and endocrine resistance in breast cancer. J Clin Oncol. 2016;34(25):2950–2952. doi:10.1200/JCO.2016.68.472027382095
- Gelsomino L, Gu G, Rechoum Y, et al. ESR1 mutations affect anti-proliferative responses to tamoxifen through enhanced cross-talk with IGF signaling. Breast Cancer Res Treat. 2016;157(2):253–265. doi:10.1007/s10549-016-3829-527178332
- Fuqua SA, Gu G, Rechoum Y. Estrogen receptor (ER) α mutations in breast cancer: hidden in plain sight. Breast Cancer Res Treat. 2014;144(1):11–19. doi:10.1007/s10549-014-2847-424487689
- Fu X, Creighton CJ, Biswal NC, et al. Overcoming endocrine resistance due to reduced PTEN levels in estrogen receptor-positive breast cancer by co-targeting mammalian target of rapamycin, protein kinase B, or mitogen-activated protein kinase kinase. Breast Cancer Res. 2014;16(5):430. doi:10.1186/s13058-014-0492-925212826
- Rimawi MF, Wiechmann LS, Wang Y-C, et al. Reduced dose and intermittent treatment with lapatinib and trastuzumab for potent blockade of the HER pathway in HER2/neu-overexpressing breast tumor xenografts. Clin Cancer Res. 2011;17(6):1351–1361. doi:10.1158/1078-0432.CCR-10-190521138857
- Takeshita T, Yamamoto Y, Yamamoto-Ibusuki M, et al. Analysis of ESR1 and PIK3CA mutations in plasma cell-free DNA from ER-positive breast cancer patients. Oncotarget. 2017;8(32):52142. doi:10.18632/oncotarget.1847928881720
- Van Loo P, Wedge D, Nik-Zainal S, Stratton M, Futreal P, Campbell P. 5 proffered paper: the life history of 21 breast cancers. Eur J Cancer. 2012;48:S2. doi:10.1016/S0959-8049(12)70709-8
- Takeshita T, Yamamoto Y, Yamamoto-Ibusuki M, et al. Droplet digital polymerase chain reaction assay for screening of ESR1 mutations in 325 breast cancer specimens. Transl Res. 2015;166(6):540–53. e2. doi:10.1016/j.trsl.2015.09.00326434753
- Perez EA. Treatment strategies for advanced hormone receptor-positive and human epidermal growth factor 2-negative breast cancer: the role of treatment order. Drug Resist Update. 2016;24:13–22. doi:10.1016/j.drup.2015.11.001
- Zanardi E, Bregni G, De Braud F, Di Cosimo S, editors. Better together: targeted combination therapies in breast cancer. Semin Oncol. 2015. Elsevier. doi:10.1053/j.seminoncol.2015.09.029
- Garber JE, Halabi S, Tolaney SM, et al. Factor V Leiden mutation and thromboembolism risk in women receiving adjuvant tamoxifen for breast cancer. J Natl Cancer Inst. 2010;102(13):942–949. doi:10.1093/jnci/djq21120554945
- Onitilo AA, McCarty CA, Wilke RA, et al. Estrogen receptor genotype is associated with risk of venous thromboembolism during tamoxifen therapy. Breast Cancer Res Treat. 2009;115(3):643–650. doi:10.1007/s10549-008-0264-219082882
- Conway K, Parrish E, Edmiston SN, et al. The estrogen receptor-α A908G (K303R) mutation occurs at a low frequency in invasive breast tumors: results from a population-based study. Breast Cancer Res. 2005;7(6):R871. doi:10.1186/bcr94916280033
- Roodi N, Bailey LR, Kao W-Y, et al. Estrogen receptor gene analysis in estrogen receptor-positive and receptor-negative primary breast cancer. Jnci. 1995;87(6):446–451. doi:10.1093/jnci/87.6.4467861463
- Weinreb I, Piscuoglio S, Martelotto LG, et al. Hotspot activating PRKD1 somatic mutations in polymorphous low-grade adenocarcinomas of the salivary glands. Nat Genet. 2014;46(11):1166. doi:10.1038/ng.289525240283
- Ross JS, Gay LM, Wang K, et al. Nonamplification ERBB2 genomic alterations in 5605 cases of recurrent and metastatic breast cancer: an emerging opportunity for anti‐HER2 targeted therapies. Cancer. 2016;122(17):2654–2662. doi:10.1002/cncr.3010227284958
- Carter P, Presta L, Gorman CM, et al. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc National Acad Sci. 1992;89(10):4285–4289. doi:10.1073/pnas.89.10.4285
- Gajria D, Chandarlapaty S. HER2-amplified breast cancer: mechanisms of trastuzumab resistance and novel targeted therapies. Expert Rev Anticancer Ther. 2011;11(2):263–275. doi:10.1586/era.10.22621342044
- Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–792. doi:10.1056/NEJM20010315344110111248153
- Vogel CL, Cobleigh MA, Tripathy D, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20(3):719–726. doi:10.1200/JCO.2002.20.3.71911821453
- Jahanzeb M. Adjuvant trastuzumab therapy for HER2-positive breast cancer. Clin Breast Cancer. 2008;8(4):324–333. doi:10.3816/CBC.2008.n.03718757259
- Nahta R, Esteva F. Trastuzumab: triumphs and tribulations. Oncogene. 2007;26(25):3637. doi:10.1038/sj.onc.121037917530017
- Bedard PL, de Azambuja E, Cardoso F. Beyond trastuzumab: overcoming resistance to targeted HER-2 therapy in breast cancer. Curr Cancer Drug Targets. 2009;9(2):148–162.19275756
- Rimawi MF, Schiff R, Osborne CK. Targeting HER2 for the treatment of breast cancer. Annu Rev Med. 2015;66:111–128.
- Arteaga CL, Sliwkowski MX, Osborne CK, Perez EA, Puglisi F, Gianni L. Treatment of HER2-positive breast cancer: current status and future perspectives. Nat Rev Clin Oncol. 2012;9(1):16. doi:10.1038/nrclinonc.2012.154
- Yamaguchi H, Chang S, Hsu J, Hung M. Signaling cross-talk in the resistance to HER family receptor targeted therapy. Oncogene. 2014;33(9):1073. doi:10.1038/onc.2013.7423542173
- Arribas J, Baselga J, Pedersen K, Parra-Palau JL. p95HER2 and breast cancer. Cancer Res. 2011;71(5):1515–1519. doi:10.1158/0008-5472.CAN-10-379521343397
- Castiglioni F, Tagliabue E, Campiglio M, Pupa S, Balsari A, Menard S. Role of exon-16-deleted HER2 in breast carcinomas. Endocr Relat Cancer. 2006;13(1):221–232. doi:10.1677/erc.1.0104716601290
- Mitra D, Brumlik MJ, Okamgba SU, et al. An oncogenic isoform of HER2 associated with locally disseminated breast cancer and trastuzumab resistance. Mol Cancer Ther. 2009;8(8):2152–2162. MCT-09-0295.
- Funes M, Miller JK, Lai C, Carraway KL, Sweeney C. The mucin Muc4 potentiates neuregulin signaling by increasing the cell-surface populations of ErbB2 and ErbB3. J Biol Chem. 2006;281(28):19310–19319. doi:10.1074/jbc.M60322520016690615
- Price‐Schiavi SA, Jepson S, Li P, et al. Rat Muc4 (sialomucin complex) reduces binding of anti‐ErbB2 antibodies to tumor cell surfaces, a potential mechanism for herceptin resistance. Int J Cancer. 2002;99(6):783–791. doi:10.1002/ijc.1041012115478
- de Melo Gagliato D, Jardim DLF, Marchesi MSP, Hortobagyi GN. Mechanisms of resistance and sensitivity to anti-HER2 therapies in HER2+ breast cancer. Oncotarget. 2016;7(39):64431.26824988
- Ferrari A, Vincent-Salomon A, Pivot X, et al. A whole-genome sequence and transcriptome perspective on HER2-positive breast cancers. Nat Commun. 2016;7:12222. doi:10.1038/ncomms1222227406316
- Kovtun YV, Goldmacher VS. Cell killing by antibody–drug conjugates. Cancer Lett. 2007;255(2):232–240. doi:10.1016/j.canlet.2007.04.01017553616
- Barok M, Joensuu H, Isola J. Trastuzumab emtansine: mechanisms of action and drug resistance. Breast Cancer Res. 2014;16(2):209. doi:10.1186/s13058-014-0492-924887180
- Van Herpen C, Banerji U, Mommers E, et al. 333 Phase I dose-escalation trial with the DNA-alkylating anti-HER2 antibody-drug conjugate SYD985. Eur J Cancer. 2015;51:S65. doi:10.1016/S0959-8049(16)30197-6
- Medina PJ, Goodin S. Lapatinib: a dual inhibitor of human epidermal growth factor receptor tyrosine kinases. Clin Ther. 2008;30(8):1426–1447. doi:10.1016/j.clinthera.2008.08.00818803986
- Tevaarwerk AJ, Kolesar JM. Lapatinib: A small-molecule inhibitor of epidermal growth factor receptor and human epidermal growth factor receptor-2 tyrosine kinases used in the treatment of breast cancer. Clin Ther. 2009;31:2332–2348. doi:10.1016/j.clinthera.2009.11.02920110044
- Konecny GE, Pegram MD, Venkatesan N, et al. Activity of the dual kinase inhibitor lapatinib (GW572016) against HER-2-overexpressing and trastuzumab-treated breast cancer cells. Cancer Res. 2006;66(3):1630–1639. doi:10.1158/0008-5472.CAN-05-118216452222
- Vicario R, Peg V, Morancho B, et al. Patterns of HER2 gene amplification and response to anti-HER2 therapies. PLoS One. 2015;10(6):e0129876. doi:10.1371/journal.pone.012987626075403
- Hafizi S, Dahlbäck B. Signalling and functional diversity within the Axl subfamily of receptor tyrosine kinases. Cytokine Growth Factor Rev. 2006;17(4):295–304. doi:10.1016/j.cytogfr.2006.04.00416737840
- Franklin MC, Carey KD, Vajdos FF, Leahy DJ, De Vos AM, Sliwkowski MX. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell. 2004;5(4):317–328.15093539
- Agus DB, Gordon MS, Taylor C, et al. Phase I clinical study of pertuzumab, a novel HER dimerization inhibitor, in patients with advanced cancer. J clin oncol. 2005;23(11):2534–2543. doi:10.1200/JCO.2005.03.18415699478
- Leung W-Y, Roxanis I, Sheldon H, et al. Combining lapatinib and pertuzumab to overcome lapatinib resistance due to NRG1-mediated signalling in HER2-amplified breast cancer. Oncotarget. 2015;6(8):5678. doi:10.18632/oncotarget.329625691057
- Hyman D, Piha-Paul S, Rodón J, et al. editors. Neratinib for ERBB2 mutant, HER2 non-amplified, metastatic breast cancer: preliminary analysis from a multicenter, open-label, multi-histology phase II basket trial. Cancer Res. 2016. AMER ASSOC CANCER RESEARCH 615 CHESTNUT ST, 17TH FLOOR, PHILADELPHIA, PA …. doi:10.1158/1538-7445.SABCS15-PD5-05
- Subramaniam D, He A R, Hwang J, et al. Irreversible multitargeted ErbB family inhibitors for therapy of lung and breast cancer. Curr Cancer Drug Targets. 2014;14(9):775–793. doi:10.2174/1568009614666141111104643
- Chan A, Delaloge S, Holmes FA, et al. Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2016;17(3):367–377. doi:10.1016/S1470-2045(15)00551-326874901
- Li D, Ambrogio L, Shimamura T, et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene. 2008;27(34):4702. doi:10.1038/onc.2008.10918408761
- Hurvitz SA, Shatsky R, Harbeck N. Afatinib in the treatment of breast cancer. Expert Opin Investig Drugs. 2014;23(7):1039–1047. doi:10.1517/13543784.2014.924505
- Gunzer K, Joly F, Ferrero J-M, et al. A phase II study of afatinib, an irreversible ErbB family blocker, added to letrozole in patients with estrogen receptor-positive hormone-refractory metastatic breast cancer progressing on letrozole. Springerplus. 2016;5(1):45. doi:10.1186/s40064-015-1601-726835225
- Judes G, Rifaï K, Daures M, et al. High-throughput «Omics» technologies: new tools for the study of triple-negative breast cancer. Cancer Lett. 2016;382(1):77–85. doi:10.1016/j.canlet.2016.03.00126965997
- Telli ML, Hellyer J, Audeh W, et al. Homologous recombination deficiency (HRD) status predicts response to standard neoadjuvant chemotherapy in patients with triple-negative or BRCA1/2 mutation-associated breast cancer. Breast Cancer Res Treat. 2018;168(3):625–630. doi:10.1007/s10549-017-4624-729275435
- Shah SP, Roth A, Goya R, et al. The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature. 2012;486(7403):395. doi:10.1038/nature1093322495314
- Foedermayr M, Sebesta M, Rudas M, et al. BRCA-1 methylation and TP53 mutation in triple-negative breast cancer patients without pathological complete response to taxane-based neoadjuvant chemotherapy. Cancer Chemother Pharmacol. 2014;73(4):771–778. doi:10.1007/s00280-014-2404-124526178
- Birgisdottir V, Stefansson OA, Bodvarsdottir SK, Hilmarsdottir H, Jonasson JG, Eyfjord JE. Epigenetic silencing and deletion of the BRCA1 gene in sporadic breast cancer. Breast Cancer Res. 2006;8(4):R38. doi:10.1186/bcr152216846527
- Gudmundsdottir K, Ashworth A. The roles of BRCA1 and BRCA2 and associated proteins in the maintenance of genomic stability. Oncogene. 2006;25(43):5864. doi:10.1038/sj.onc.120987416998501
- O‘Shaughnessy J, Schwartzberg L, Danso M, et al. A randomized phase III study of iniparib (BSI-201) in combination with gemcitabine/carboplatin (G/C) in metastatic triple-negative breast cancer (TNBC). J Clin Oncol. 2011;29(15_suppl):1007. doi:10.1200/jco.2011.29.15_suppl.100721205758
- Patel AG, De Lorenzo SB, Flatten KS, Poirier GG, Kaufmann SH. Failure of iniparib to inhibit poly (ADP-Ribose) polymerase in vitro. Clin Cancer Res. 2012;15;18(6):1655–1662.
- Reles A, Wen WH, Schmider A, et al. Correlation of p53 mutations with resistance to platinum-based chemotherapy and shortened survival in ovarian cancer. Clin Cancer Res. 2001;7(10):2984–2997.11595686
- Gonzalez-Angulo AM, Morales-Vasquez F, Hortobagyi GN. Overview of resistance to systemic therapy in patients with breast cancer. Breast Cancer Chemosensitivity. 2007;608:1–22.
- Gewirtz D. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol. 1999;57(7):727–741. doi:10.1016/S0006-2952(98)00307-410075079
- Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev. 2004;56(2):185–229. doi:10.1124/pr.56.2.615169927
- Senchenkov A, Litvak DA, Cabot MC. Targeting ceramide metabolism—a strategy for overcoming drug resistance. J Natl Cancer Inst. 2001;93(5):347–357.11238696
- Chen G, J-P J, Fleming WH, Durán GE, Sikic BI. Prevalence of multidrug resistance related to activation of the mdr1 gene in human sarcoma mutants derived by single-step doxorubicin selection. Cancer Res. 1994;54(18):4980–4987.7915196
- Larsen AK, Skladanowski A. Cellular resistance to topoisomerase-targeted drugs: from drug uptake to cell death. Biochim Biophys Acta. 1998;1400(1–3):257–274. doi:10.1016/S0167-4781(98)00140-79748618
- Withoff S, De SJ, De EV, Mulder N. Human DNA topoisomerase II: biochemistry and role in chemotherapy resistance. Anticancer Res. 1996;16(4A):1867–1880.8712715
- Finlay GJ, Baguley BC, Snow K, Judd W. Multiple patterns of resistance of human leukemia cell sublines to amsacrine analogues. Jnci. 1990;82(8):662–667. doi:10.1093/jnci/82.8.6622157028
- Seidman AD, Reichman BS, Crown J, et al. Paclitaxel as second and subsequent therapy for metastatic breast cancer: activity independent of prior anthracycline response. J Clin Oncol. 1995;13(5):1152–1159. doi:10.1200/JCO.1995.13.5.11527537798
- Wilson WH, Berg SL, Bryant G, et al. Paclitaxel in doxorubicin-refractory or mitoxantrone-refractory breast cancer: a phase I/II trial of 96 hr infusion. J Clin Oncol. 1994;12(8):1621–1629. doi:10.1200/JCO.1994.12.8.16217913721
- Anderson H, Hopwood P, Prendiville J, Radford JA, Thatcher N, Ashcroft L. A randomised study of bolus vs continuous pump infusion of ifosfamide and doxorubicin with oral etoposide for small cell lung cancer. Br J Cancer. 1993;67(6):1385. doi:10.1038/bjc.1993.2568390287
- Bristol-Myers Squibb. Taxol® (paclitaxel) [Prescribing information]. New York: Bristol-Myers Squibb; 2011.
- Greenberger L, Williams SS, Horwitz SB. Biosynthesis of heterogeneous forms of multidrug resistance-associated glycoproteins. J Biol Chem. 1987;262(28):13685–13689.2888763
- Tolcher A, Cowan K, Solomon D, et al. Phase I crossover study of paclitaxel with r-verapamil in patients with metastatic breast cancer. J clin oncol. 1996;14(4):1173–1184. doi:10.1200/JCO.1996.14.4.11738648372
- Twelves C, Jove M, Gombos A, Awada A. Cytotoxic chemotherapy: still the mainstay of clinical practice for all subtypes metastatic breast cancer. Crit Rev Oncol Hematol. 2016;100:74–87. doi:10.1016/j.critrevonc.2016.01.02126857987
- Jameson GS, Hamm JT, Weiss GJ, et al. A multicenter, phase I, dose-escalation study to assess the safety, tolerability, and pharmacokinetics of etirinotecan pegol in patients with refractory solid tumors. Clin Cancer Res. 2013;19(1):268–278. doi:10.1158/1078-0432.CCR-12-120123136196
- Hoch U, Staschen C-M, Johnson RK, Eldon MA. Nonclinical pharmacokinetics and activity of etirinotecan pegol (NKTR-102), a long-acting topoisomerase 1 inhibitor, in multiple cancer models. Cancer Chemother Pharmacol. 2014;74(6):1125–1137. doi:10.1007/s00280-014-2577-725228368
- Huennekens F. The methotrexate story: a paradigm for development of cancer chemotherapeutic agents. Adv Enzyme Regul. 1994;34:397–419.7942284
- Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3(5):330. doi:10.1038/nrc107412724731
- Grant SC, Kris MG, Young CW, Sirotnak FM. Edatrexate, an antifolate with antitumor activity: a review. Cancer Invest. 1993;11(1):36–45.8422595
- Sirotnak F, Moccio D, Kelleher L, Goutas L. Relative frequency and kinetic properties of transport-defective phenotypes among methotrexate-resistant L1210 clonal cell lines derived in vivo. Cancer Research. 1981;41(11 Pt 1):4447–4452.
- Kool M, Van Der Linden M, de Haas M, et al. MRP3, an organic anion transporter able to transport anti-cancer drugs. Proc National Acad Sci. 1999;96(12):6914–6919. doi:10.1073/pnas.96.12.6914
- Hooijberg J, Broxterman H, Scheffer G, et al. Potent interaction of flavopiridol with MRP1. Br J Cancer. 1999;81(2):269. doi:10.1038/sj.bjc.669068710496352
- Cowan KH, Jolivet J. A methotrexate-resistant human breast cancer cell line with multiple defects, including diminished formation of methotrexate polyglutamates. J Biol Chem. 1984;259(17):10793–10800.6206061
- Volk EL, Rohde K, Rhee M, et al. Methotrexate cross-resistance in a mitoxantrone-selected multidrug-resistant MCF7 breast cancer cell line is attributable to enhanced energy-dependent drug efflux. Cancer Res. 2000;60(13):3514–3521.10910063
- Ohmori T, Podack E, Nishio K, et al. Apoptosis of lung cancer cells caused by some anti-cancer agents (MMC, CPT-11, ADM) is inhibited by bcl-2. Biochem Biophys Res Commun. 1993;192(1):30–36.8476431
- Priest DG, Ledford BE, Doig MT. Increased thymidylate synthetase in 5-fluorodeoxyuridine resistant cultured hepatoma cells. Biochem Pharmacol. 1980;29(11):1549–1553.6446915
- Spears CP. Clinical resistance to antimetabolites. Hematol Oncol Clin North Am. 1995;9(2):397–414.7642470
- Klatt O, Stehlin JS, McBride C, Griffin A. The effect of nitrogen mustard treatment on the deoxyribonucleic acid of sensitive and resistant Ehrlich tumor cells. Cancer Res. 1969;29(2):286–290.5765411
- Ichiro N, Kimitoshi K, Junko K, et al. Analysis of structural features of dihydropyridine analogs needed to reverse multidrug resistance and to inhibit photoaffinity labeling of P-glycoprotein. Biochem Pharmacol. 1989;38(3):519–527.2563655
- Zamble DB, Lippard SJ. Cisplatin and DNA repair in cancer chemotherapy. Trends Biochem Sci. 1995;20(10):435–439.8533159
- Perez R. Cellular and molecular determinants of cisplatin resistance. Eur J Cancer. 1998;34(10):1535–1542.9893624
- Moudi M, Go R, Yien CYS, Nazre M. Vinca alkaloids. Int J Prev Med. 2013;4(11):1231.24404355
- Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev. 2013;65(1):36–48. doi:10.1016/j.addr.2012.09.03723036225
- Coyle C, Cafferty F, Vale C, Langley R. Metformin as an adjuvant treatment for cancer: a systematic review and meta-analysis. Ann Oncol. 2016;27(12):2184–2195. doi:10.1093/annonc/mdw41027681864
- Zhang J, Li G, Chen Y, et al. Metformin Inhibits tumorigenesis and tumor growth of breast cancer cells by upregulating miR-200c but downregulating AKT2 expression. J Cancer. 2017;8(10):1849. doi:10.7150/jca.1985828819383
- Al-Zaidan L, Ruz E, Abu R, Malki AM. Screening novel molecular targets of metformin in breast cancer by proteomic approach. Front Public Health. 2017;5:277. doi:10.3389/fpubh.2017.0008129085821
- Obaidi J, Musallam E, Al-Ghzawi HM, Azzeghaiby SN, Alzoghaibi IN. Vitamin D and its relationship with breast cancer: an evidence based practice paper. Glob J Health Sci. 2015;7(1):261.
- Gall TL, Kristjansson E, Charbonneau C, Florack P. A longitudinal study on the role of spirituality in response to the diagnosis and treatment of breast cancer. J Behav Med. 2009;32(2):174–186. doi:10.1007/s10865-008-9182-318982441
- Chida Y, Hamer M, Wardle J, Steptoe A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat Rev Clin Onco. 2008;5(8):466. doi:10.1038/ncponc1134
- MacArthur AC, Le ND, Abanto ZU, Gallagher RP. Occupational female breast and reproductive cancer mortality in British Columbia, Canada, 1950–94. Occup Med (Chic Ill). 2007;57(4):246–253. doi:10.1093/occmed/kqm002
- Akbari ME, Kashani FL, Ahangari G, et al. The effects of spiritual intervention and changes in dopamine receptor gene expression in breast cancer patients. Breast Cancer. 2016;23(6):893–900. doi:10.1007/s12282-015-0658-z26597879
- Hejazi SH, Ahangari G, Pornour M, et al. Evaluation of gene expression changes of serotonin receptors, 5-HT3AR and 5-HT2AR as main stress factors in breast cancer patients. Asian Pac J Cancer Prev. 2013;15(11):4455–4458. doi:10.7314/APJCP.2014.15.11.4455
- Urbaniak C, Gloor GB, Brackstone M, Scott L, Tangney M, Reid G. The microbiota of breast tissue and its association with tumours. Appl Environ Microbiol. 2016;AEM: 01235–16.
- Toga AW, Foster I, Kesselman C, et al. Big biomedical data as the key resource for discovery science. JAMIA. 2015;22(6):1126–1131. doi:10.1093/jamia/ocv07726198305
- Kanehisa M, Goto S. KEGG: Breast cancer - Reference pathway; 2018 Available at: https://www.genome.jp/kegg-bin/show_pathway?map05224. Accessed May 15, 2019
